Abstract
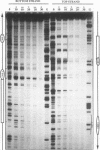
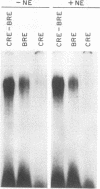
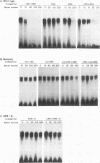
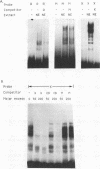
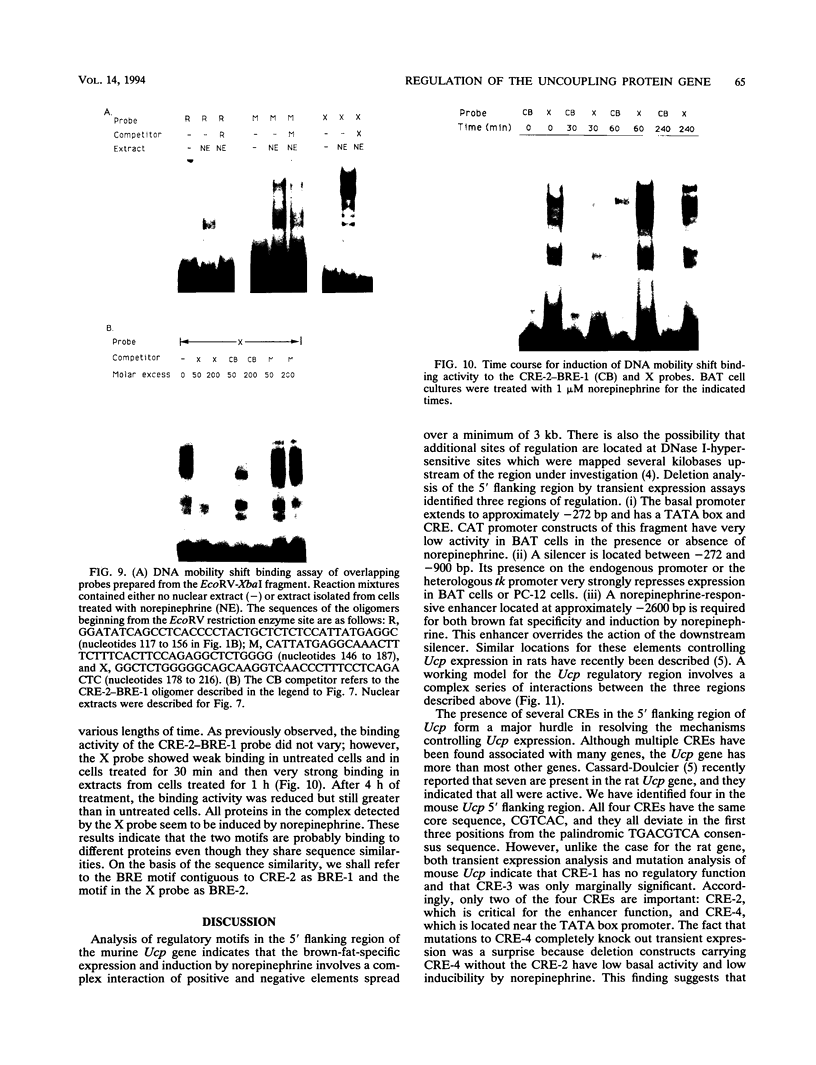
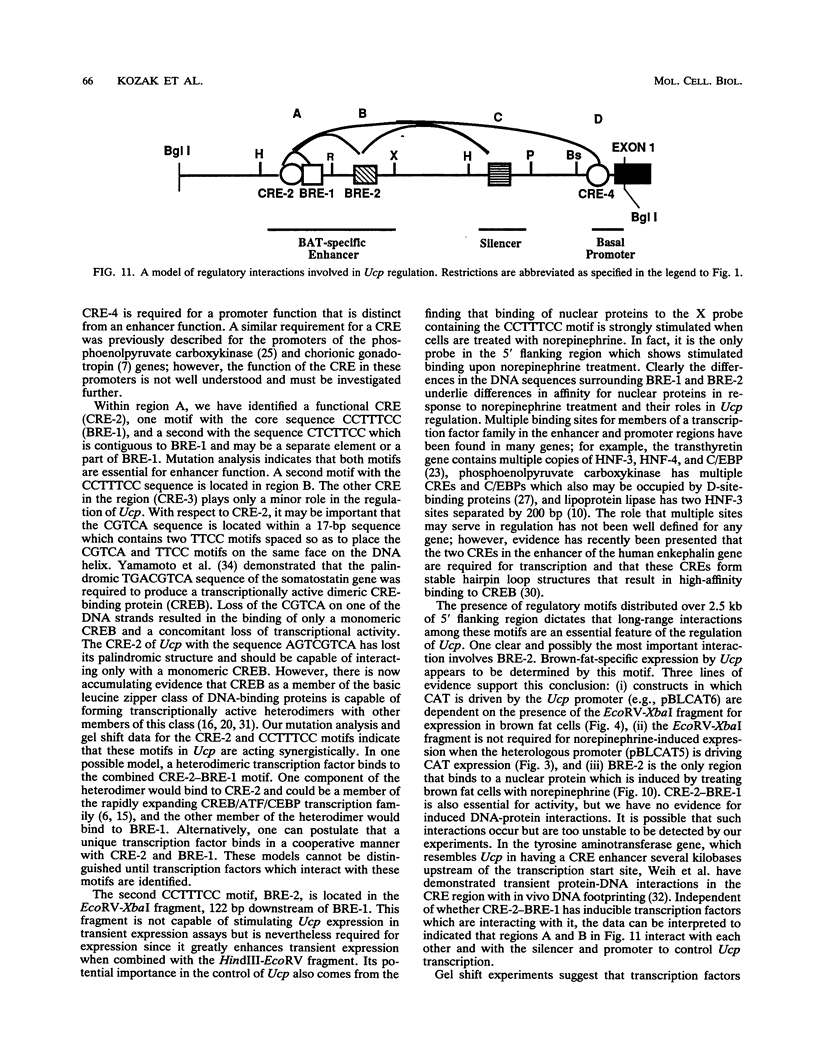
Previous studies on the regulation of a Ucp minigene in transgenic mice demonstrated that the sequences necessary for brown-fat-specific expression and inducibility by norepinephrine were located in the 5' flanking region between 1 and 2.8 kb from the transcriptional start site. We have investigated this region in more detail in cultured mouse brown adipocyte tumor cells. Deletion analysis of two types of chloramphenicol acetyltransferase reporter gene constructs under control of either the Ucp promoter or a heterologous herpes simplex virus-tk promoter defined an enhancer in a 220-bp HindIII-XbaI fragment which was essential for both brown fat specificity and norepinephrine inducibility. Site-directed mutagenesis of the reporter gene constructs established that independent mutations to a cyclic AMP-responsive element (CRE-2) or one of two TTCC motifs (BRE [brown fat regulatory element]), all within 17 bp, eliminated transient expression. Competitive DNA mobility shift assays with probes of the CRE and BRE motifs indicate that nuclear proteins interact with these motifs in a cooperative, synergistic manner. While these CRE-BRE probes do not show changes in binding which is dependent on norepinephrine treatment, a probe containing a third TTCC motif located 130 bp downstream of BRE-1 does show this dependency. The results indicate that a complex interaction of the CRE and BRE motifs, which cannot be functionally separated, control Ucp expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Ainsworth A. T., Cawthorne M. A., Piercy V., Sennitt M. V., Thody V. E., Wilson C., Wilson S. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984 May 10;309(5964):163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- Bianco A. C., Sheng X. Y., Silva J. E. Triiodothyronine amplifies norepinephrine stimulation of uncoupling protein gene transcription by a mechanism not requiring protein synthesis. J Biol Chem. 1988 Dec 5;263(34):18168–18175. [PubMed] [Google Scholar]
- Bokar J. A., Roesler W. J., Vandenbark G. R., Kaetzel D. M., Hanson R. W., Nilson J. H. Characterization of the cAMP responsive elements from the genes for the alpha-subunit of glycoprotein hormones and phosphoenolpyruvate carboxykinase (GTP). Conserved features of nuclear protein binding between tissues and species. J Biol Chem. 1988 Dec 25;263(36):19740–19747. [PubMed] [Google Scholar]
- Boyer B. B., Kozak L. P. The mitochondrial uncoupling protein gene in brown fat: correlation between DNase I hypersensitivity and expression in transgenic mice. Mol Cell Biol. 1991 Aug;11(8):4147–4156. doi: 10.1128/mcb.11.8.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassard-Doulcier A. M., Gelly C., Fox N., Schrementi J., Raimbault S., Klaus S., Forest C., Bouillaud F., Ricquier D. Tissue-specific and beta-adrenergic regulation of the mitochondrial uncoupling protein gene: control by cis-acting elements in the 5'-flanking region. Mol Endocrinol. 1993 Apr;7(4):497–506. doi: 10.1210/mend.7.4.8388995. [DOI] [PubMed] [Google Scholar]
- Delegeane A. M., Ferland L. H., Mellon P. L. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Mol Cell Biol. 1987 Nov;7(11):3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck S., Ohlsson B. G., Samuelsson L., Bjursell G. Characterization of the human lipoprotein lipase (LPL) promoter: evidence of two cis-regulatory regions, LP-alpha and LP-beta, of importance for the differentiation-linked induction of the LPL gene during adipogenesis. Mol Cell Biol. 1992 Oct;12(10):4622–4633. doi: 10.1128/mcb.12.10.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves R. A., Tontonoz P., Spiegelman B. M. Analysis of a tissue-specific enhancer: ARF6 regulates adipogenic gene expression. Mol Cell Biol. 1992 Mar;12(3):1202–1208. doi: 10.1128/mcb.12.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Habener J. F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990 Aug;4(8):1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Jacobsson A., Stadler U., Glotzer M. A., Kozak L. P. Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mRNA expression. J Biol Chem. 1985 Dec 25;260(30):16250–16254. [PubMed] [Google Scholar]
- Kozak L. P., Britton J. H., Kozak U. C., Wells J. M. The mitochondrial uncoupling protein gene. Correlation of exon structure to transmembrane domains. J Biol Chem. 1988 Sep 5;263(25):12274–12277. [PubMed] [Google Scholar]
- Kozak U. C., Held W., Kreutter D., Kozak L. P. Adrenergic regulation of the mitochondrial uncoupling protein gene in brown fat tumor cells. Mol Endocrinol. 1992 May;6(5):763–772. doi: 10.1210/mend.6.5.1603085. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod K., Leprince D., Stehelin D. The ets gene family. Trends Biochem Sci. 1992 Jul;17(7):251–256. doi: 10.1016/0968-0004(92)90404-w. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J., Darnell J. E., Jr Rapid in vivo footprinting technique identifies proteins bound to the TTR gene in the mouse liver. Genes Dev. 1991 Jan;5(1):83–93. doi: 10.1101/gad.5.1.83. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Locke R. M. Thermogenic mechanisms in brown fat. Physiol Rev. 1984 Jan;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Quinn P. G., Wong T. W., Magnuson M. A., Shabb J. B., Granner D. K. Identification of basal and cyclic AMP regulatory elements in the promoter of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1988 Aug;8(8):3467–3475. doi: 10.1128/mcb.8.8.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler W. J., McFie P. J., Dauvin C. The liver-enriched transcription factor D-site-binding protein activates the promoter of the phosphoenolpyruvate carboxykinase gene in hepatoma cells. J Biol Chem. 1992 Oct 15;267(29):21235–21243. [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979 Sep 6;281(5726):31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Horwitz B. A. Brown fat and thermogenesis. Physiol Rev. 1969 Apr;49(2):330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Spiro C., Richards J. P., Chandrasekaran S., Brennan R. G., McMurray C. T. Secondary structure creates mismatched base pairs required for high-affinity binding of cAMP response element-binding protein to the human enkephalin enhancer. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4606–4610. doi: 10.1073/pnas.90.10.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., Hai T., Boyd S. M. Dimerization specificity of the leucine zipper-containing bZIP motif on DNA binding: prediction and rational design. Genes Dev. 1993 Jun;7(6):1047–1058. doi: 10.1101/gad.7.6.1047. [DOI] [PubMed] [Google Scholar]
- Weih F., Stewart A. F., Boshart M., Nitsch D., Schütz G. In vivo monitoring of a cAMP-stimulated DNA-binding activity. Genes Dev. 1990 Aug;4(8):1437–1449. doi: 10.1101/gad.4.8.1437. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- de Groot R. P., Sassone-Corsi P. Hormonal control of gene expression: multiplicity and versatility of cyclic adenosine 3',5'-monophosphate-responsive nuclear regulators. Mol Endocrinol. 1993 Feb;7(2):145–153. doi: 10.1210/mend.7.2.8385737. [DOI] [PubMed] [Google Scholar]