Abstract
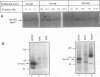
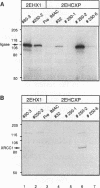
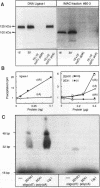
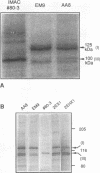
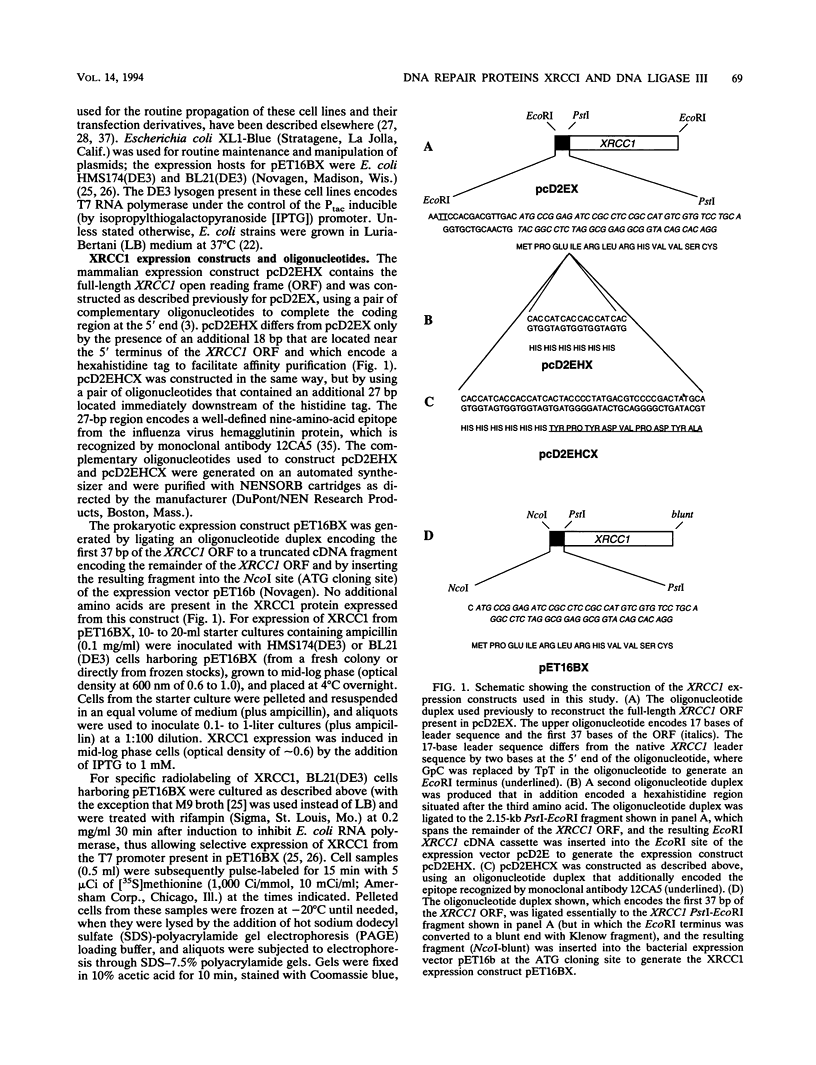
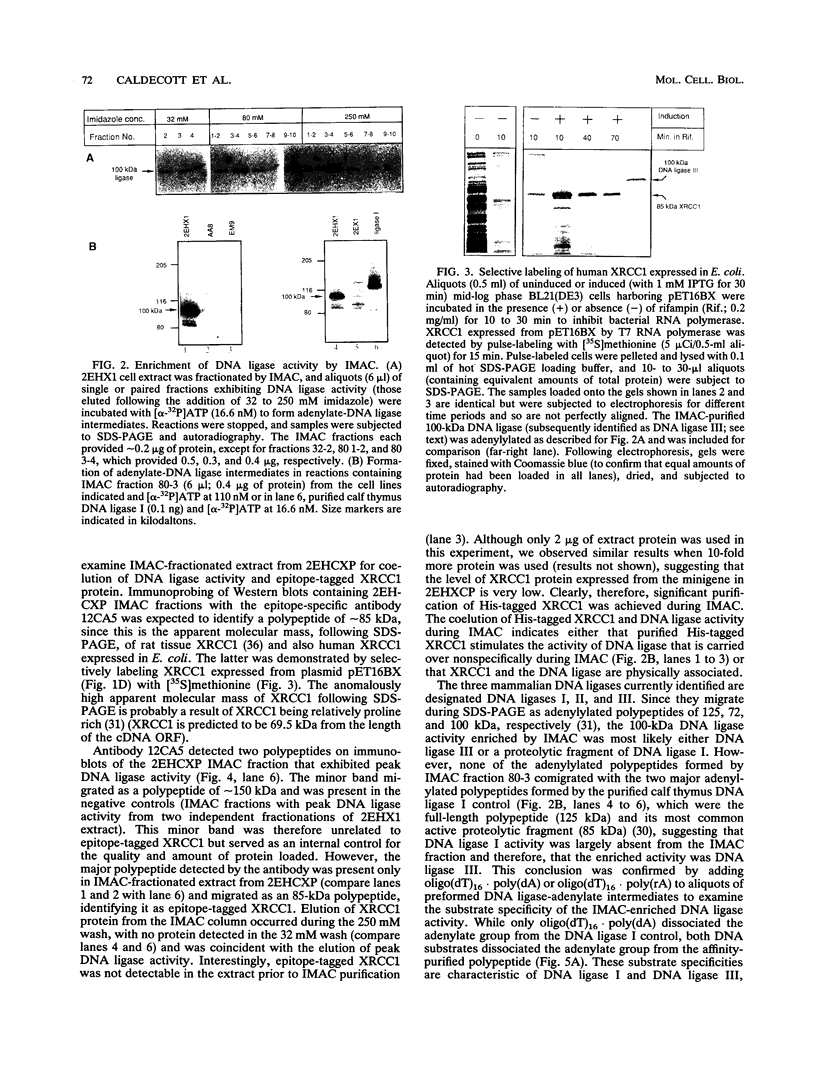
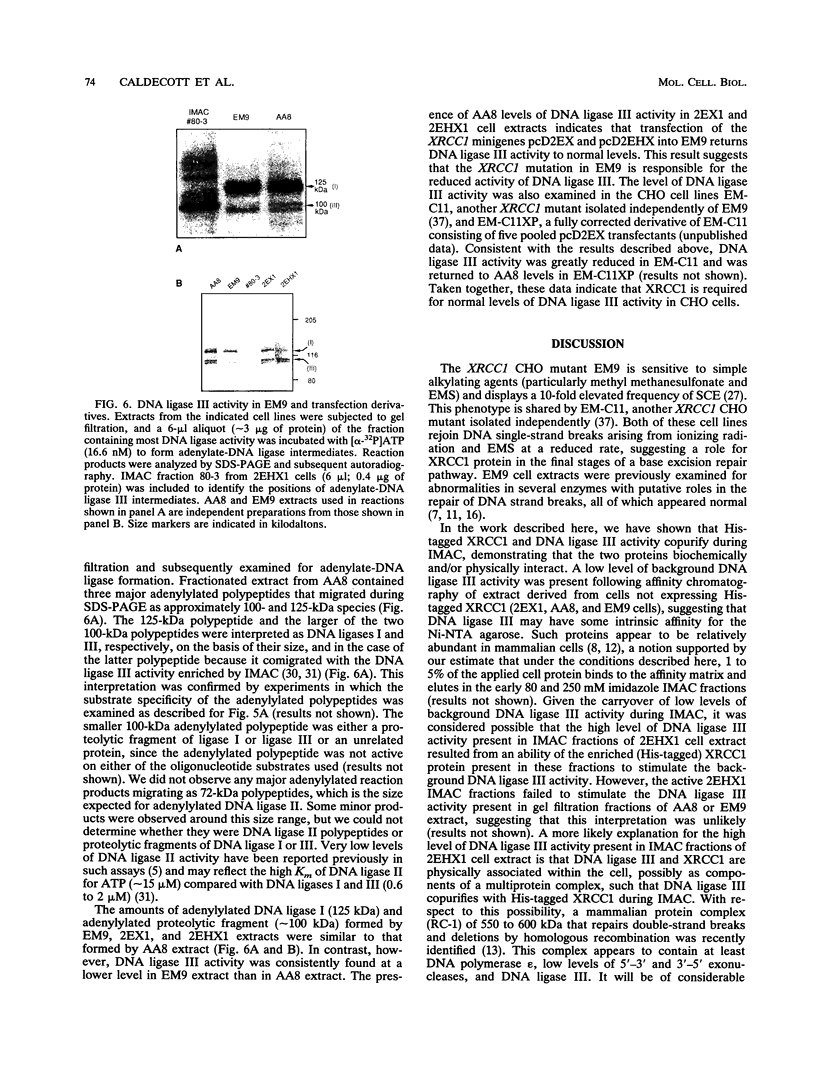
XRCC1, the human gene that fully corrects the Chinese hamster ovary DNA repair mutant EM9, encodes a protein involved in the rejoining of DNA single-strand breaks that arise following treatment with alkylating agents or ionizing radiation. In this study, a cDNA minigene encoding oligohistidine-tagged XRCC1 was constructed to facilitate affinity purification of the recombinant protein. This construct, designated pcD2EHX, fully corrected the EM9 phenotype of high sister chromatid exchange, indicating that the histidine tag was not detrimental to XRCC1 activity. Affinity chromatography of extract from EM9 cells transfected with pcD2EHX resulted in the copurification of histidine-tagged XRCC1 and DNA ligase III activity. Neither XRCC1 or DNA ligase III activity was purified during affinity chromatography of extract from EM9 cells transfected with pcD2EX, a cDNA minigene that encodes untagged XRCC1, or extract from wild-type AA8 or untransfected EM9 cells. The copurification of DNA ligase III activity with histidine-tagged XRCC1 suggests that the two proteins are present in the cell as a complex. Furthermore, DNA ligase III activity was present at lower levels in EM9 cells than in AA8 cells and was returned to normal levels in EM9 cells transfected with pcD2EHX or pcD2EX. These findings indicate that XRCC1 is required for normal levels of DNA ligase III activity, and they implicate a major role for this DNA ligase in DNA base excision repair in mammalian cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D. E., Tomkinson A. E., Lehmann A. R., Webster A. D., Lindahl T. Mutations in the DNA ligase I gene of an individual with immunodeficiencies and cellular hypersensitivity to DNA-damaging agents. Cell. 1992 May 1;69(3):495–503. doi: 10.1016/0092-8674(92)90450-q. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caldecott K. W., Tucker J. D., Thompson L. H. Construction of human XRCC1 minigenes that fully correct the CHO DNA repair mutant EM9. Nucleic Acids Res. 1992 Sep 11;20(17):4575–4579. doi: 10.1093/nar/20.17.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Becker F. F. Defective DNA ligase I in Bloom's syndrome cells. Simultaneous analysis using immunoblotting and the ligase-[32P]AMP adduct assay. J Biol Chem. 1988 Dec 5;263(34):18231–18235. [PubMed] [Google Scholar]
- Chan J. Y., Becker F. F., German J., Ray J. H. Altered DNA ligase I activity in Bloom's syndrome cells. Nature. 1987 Jan 22;325(6102):357–359. doi: 10.1038/325357a0. [DOI] [PubMed] [Google Scholar]
- Chan J. Y., Thompson L. H., Becker F. F. DNA-ligase activities appear normal in the CHO mutant EM9. Mutat Res. 1984 May-Jun;131(5-6):209–214. doi: 10.1016/0167-8817(84)90027-0. [DOI] [PubMed] [Google Scholar]
- Dillehay L. E., Thompson L. H., Carrano A. V. DNA-strand breaks associated with halogenated pyrimidine incorporation. Mutat Res. 1984 Mar-Apr;131(3-4):129–136. doi: 10.1016/0167-8817(84)90052-x. [DOI] [PubMed] [Google Scholar]
- Franke C. A., Hruby D. E. Expression and single-step purification of enzymatically active vaccinia virus thymidine kinase containing an engineered oligohistidine domain by immobilized metal affinity chromatography. Protein Expr Purif. 1993 Apr;4(2):101–109. doi: 10.1006/prep.1993.1015. [DOI] [PubMed] [Google Scholar]
- Ikejima M., Bohannon D., Gill D. M., Thompson L. H. Poly(ADP-ribose) metabolism appears normal in EM9, a mutagen-sensitive mutant of CHO cells. Mutat Res. 1984 Sep;128(2):213–220. doi: 10.1016/0027-5107(84)90109-x. [DOI] [PubMed] [Google Scholar]
- Janknecht R., de Martynoff G., Lou J., Hipskind R. A., Nordheim A., Stunnenberg H. G. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger R., Podust V., Hübscher U., Berg P. A mammalian protein complex that repairs double-strand breaks and deletions by recombination. J Biol Chem. 1993 Jul 15;268(20):15070–15079. [PubMed] [Google Scholar]
- Krepinsky A. B., Heddle J. A., German J. Sensitivity of Bloom's syndrome lymphocytes to ethyl methanesulfonate. Hum Genet. 1979;50(2):151–156. doi: 10.1007/BF00390236. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Inoue M., Tatsumi K. Hypersensitivity of Bloom's syndrome fibroblasts to N-ethyl-N-nitrosourea. Mutat Res. 1987 Sep;184(2):147–151. doi: 10.1016/0167-8817(87)90071-x. [DOI] [PubMed] [Google Scholar]
- La Belle M., Linn S., Thompson L. H. Apurinic/apyrimidinic endonuclease activities appear normal in the CHO-cell ethyl methanesulfonate-sensitive mutant, EM9. Mutat Res. 1984 Sep;141(1):41–44. doi: 10.1016/0165-7992(84)90035-6. [DOI] [PubMed] [Google Scholar]
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993 Apr 22;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- McDaniel L. D., Schultz R. A. Elevated sister chromatid exchange phenotype of Bloom syndrome cells is complemented by human chromosome 15. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7968–7972. doi: 10.1073/pnas.89.17.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler J., Stetka D., Jr, Carrano A. V. An ultraviolet light source for consistent differential staining of sister chromatids. Stain Technol. 1978 Nov;53(6):359–360. [PubMed] [Google Scholar]
- Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974 Sep 13;251(5471):156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Ray J. H., Louie E., German J. Different mutations are responsible for the elevated sister-chromatid exchange frequencies characteristic of Bloom's syndrome and hamster EM9 cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2368–2371. doi: 10.1073/pnas.84.8.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano M. J., Carrano A. V., Thompson L. H. Assignment of a human DNA-repair gene associated with sister-chromatid exchange to chromosome 19. Mutat Res. 1986 Aug;174(4):303–308. doi: 10.1016/0165-7992(86)90051-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Söderhäll S., Lindahl T. Mammalian DNA ligases. Serological evidence for two separate enzymes. J Biol Chem. 1975 Nov 10;250(21):8438–8444. [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Dillehay L. E., Carrano A. V., Mazrimas J. A., Mooney C. L., Minkler J. L. A CHO-cell strain having hypersensitivity to mutagens, a defect in DNA strand-break repair, and an extraordinary baseline frequency of sister-chromatid exchange. Mutat Res. 1982 Aug;95(2-3):427–440. doi: 10.1016/0027-5107(82)90276-7. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Jones N. J., Allen S. A., Carrano A. V. Molecular cloning of the human XRCC1 gene, which corrects defective DNA strand break repair and sister chromatid exchange. Mol Cell Biol. 1990 Dec;10(12):6160–6171. doi: 10.1128/mcb.10.12.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Minkler J. L., Fuscoe J. C., Henning K. A., Carrano A. V. DNA-mediated transfer of a human DNA repair gene that controls sister chromatid exchange. Mol Cell Biol. 1985 Apr;5(4):881–884. doi: 10.1128/mcb.5.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson A. E., Lasko D. D., Daly G., Lindahl T. Mammalian DNA ligases. Catalytic domain and size of DNA ligase I. J Biol Chem. 1990 Jul 25;265(21):12611–12617. [PubMed] [Google Scholar]
- Tomkinson A. E., Roberts E., Daly G., Totty N. F., Lindahl T. Three distinct DNA ligases in mammalian cells. J Biol Chem. 1991 Nov 15;266(32):21728–21735. [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Willis A. E., Lindahl T. DNA ligase I deficiency in Bloom's syndrome. Nature. 1987 Jan 22;325(6102):355–357. doi: 10.1038/325355a0. [DOI] [PubMed] [Google Scholar]
- Willis A. E., Weksberg R., Tomlinson S., Lindahl T. Structural alterations of DNA ligase I in Bloom syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8016–8020. doi: 10.1073/pnas.84.22.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Yoo H., Li L., Sacks P. G., Thompson L. H., Becker F. F., Chan J. Y. Alterations in expression and structure of the DNA repair gene XRCC1. Biochem Biophys Res Commun. 1992 Jul 31;186(2):900–910. doi: 10.1016/0006-291x(92)90831-5. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., van der Schans G. P., Natarajan A. T., Thompson L. H., Neuteboom I., Simons J. W. A Chinese hamster ovary cell mutant (EM-C11) with sensitivity to simple alkylating agents and a very high level of sister chromatid exchanges. Mutagenesis. 1992 Jul;7(4):265–269. doi: 10.1093/mutage/7.4.265. [DOI] [PubMed] [Google Scholar]