Abstract
The prostate is a branched ductal-acinar gland that is part of the male reproductive tract. Prostate development depends upon the integration of steroid hormone signals, paracrine interactions between the stromal and epithelial tissue layers, and the actions of cell autonomous factors. Several genes and signalling pathways are known to be required for one or more steps of prostate development including epithelial budding, duct elongation, branching morphogenesis, and/or cellular differentiation. Recent progress in the field of prostate development has included the application of genome-wide technologies including serial analysis of gene expression (SAGE), expression profiling microarrays, and other large scale approaches to identify new genes and pathways that are essential for prostate development. The aggregation of experimental results into online databases by organized multi-lab projects including the Genitourinary Developmental Molecular Atlas Project (GUDMAP) has also accelerated the understanding of molecular pathways that function during prostate development and identified links between prostate anatomy and molecular signaling. Rapid progress has also recently been made in understanding the nature and role of candidate stem cells in the developing and adult prostate. This has included the identification of putative prostate stem cell markers, lineage tracing, and organ reconstitution studies. However, several issues regarding their origin, precise nature, and possible role(s) in disease remain unresolved. Nevertheless, several links between prostatic developmental mechanisms and the pathogenesis of prostatic diseases including benign prostatic hyperplasia and prostate cancer have led to recent progress on targeting developmental pathways as therapeutic strategies for these diseases.
The prostate is an exocrine gland that functions as part of the male reproductive tract in mammals. Although the prostate is present in many mammalian species, the morphology and secretory products of the prostate vary widely among mammals. The goal of this review is to highlight recent progress in research on the cellular and molecular basis for prostate development. Because the mouse has emerged as the most important model system for investigating prostate development, this review will focus primarily on development of the mouse prostate. Data from other species will be highlighted in selective fashion with a special emphasis on the role of developmental pathways in human prostatic diseases including benign prostatic hyperplasia (BPH) and prostate cancer.
Prostate Development and Anatomy
Development of the mouse prostate is initiated during late embryogenesis under control of androgens secreted from the testes of male embryos. Morphogenesis and differentiation of the prostate continue during the postnatal period with a majority of growth and branching morphogenesis occurring between birth and the attainment of sexual maturity at the completion of puberty1. The initial steps of prostate development are the male-specific molecular and morphological changes in the urogenital sinus (UGS), the embryonic precursor of the prostate in males and precursor of part of the vagina in females. The process of prostate development from the UGS can be viewed as a series of developmental steps including organ determination, epithelial budding, duct elongation, branching morphogenesis, and cellular differentiation/maturation2. Organ determination is mediated by male-specific gene expression changes in the UGS that occur in response to androgen signaling. Currently, the earliest molecular marker of prostate organ determination is expression of the transcription factor Nkx3.1 in the urogenital sinus epithelium (UGE) at e15.5 in the mouse3. Tissue recombination and allografting experiments using androgen receptor (AR) null mice have shown that prostate organ determination requires AR activation in the urogenital sinus mesenchyme (UGM)4. Consequently, currently unknown androgen-regulated gene expression changes in the UGM are also required for prostate organ determination. Epithelial budding is the first morphological step of prostate development in which cords of undifferentiated epithelial cells from the UGE invade the UGM at E16.5 in mice. Following budding, the developing prostatic buds elongate via proliferation at the distal (furthest from the urethra) bud tips. Lumen formation also occurs in proximal (adjacent to the urethra) to distal fashion to form prostatic ducts1. As development proceeds, prostatic ducts undergo multiple rounds of branching morphogenesis. In mice, the result of branching morphogenesis is a multi-lobed organ (Fig. 1A) with distinct duct branch patterns characteristic for each lobe1. The histologic appearance of the ducts in different lobes is also distinct (Fig. 1B–D) and may reflect differences in cellular differentiation and secretory protein products across the different lobes of the mouse prostate5 In contrast, the human prostate is not organized into discrete lobes and has a different tissue organization with epithelial ducts surrounded by a dense and continuous fibromuscular stroma (Fig. 1E).
Figure 1. Recent advances in the anatomy of prostate development.
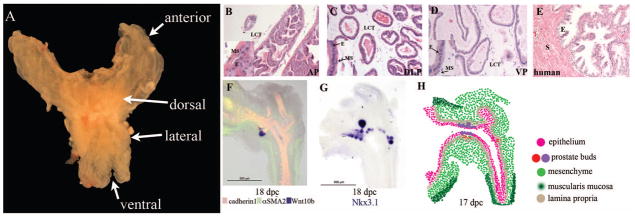
A) The mouse prostate is composed of three distinct lobes that have bilateral symmetry. The image shown is a photograph of an adult prostate. The four lobes of the prostate are labelled anterior, dorsal, lateral, and ventral. Each of lobe of the mouse prostate exhibits unique branching patterns which is highlighted in the work of Sugimura and colleagues1. Hematoxylin and eosin staining of 12 week prostate demonstrate the distinct histological appearance of the B) anterior (AP), C) dorsolateral (DLP), and D) ventral (VP) mouse prostate lobes. The loose connective tissue (LCT) is labelled in the mouse prostate and insets highlight the muscular stroma (MS) and epithelial (E) cells of the mouse prostate. E) H&E staining of adult human prostate from a biopsy specimen. In contrast to the mouse prostate there is more stroma (pink staining- S) and distinct organization of the epithelium (E). F) In situ hybridization (ISH) for Wnt10b (purple) and immunofluorescence for cadherin 1 (red) and alpha smooth muscle actin 2 (αSMA2, green) in the UGS of an E18 mouse. Wnt10b is a newly identified marker for budding prostate epithelium. Epithelial and mesenchymal cells are represented by cadherin 1 and αSMA2, respectively. G) ISH for Nkx3.1 expression that is a well-characterized marker of prostate epithelial cells. Images in F&G are from the UGS of male mice at Theiler stage 26 (18 dpc) from the GUDMAP database (images # 14307 and #14311 from the laboratory of Dr. Chad Vezina). H) Traditionally, the developing prostate is divided into the UGM and UGE, but new studies have identified markers that further divide the developing prostate into distinct compartments at 17 dpc. Epithelium is represented by cadherin 1 expression (pink) and mesenchyme is based upon expression of αSMA2 (green). Wnt 10b (red) and Nkx3.1 (violet) depict the budding prostate epithelium Mesenchymal compartments in addition general mesenchyme include Acta2 positive muscularis mucosa (dark green) and Snai1 positive lamina propria (tan). Expression data from GUDMAP ISH files deposited by Dr. Chad Vezina’s laboratory were used to determine expression pattern and demonstrates that the mesenchyme and epithelium contain distinct compartments even at the initial stages of prostate development.
Androgens, paracrine signaling, and transcription factor pathways
As the investigation of prostate development transitioned from observational studies to more mechanistic studies, several key features of prostatic development were uncovered. Early studies demonstrated a requirement for steroid hormones including testosterone and other androgens in prostate development6. These studies were also the first to demonstrate that interactions between the stromal and epithelial cells are critical for normal prostate development. Pioneering studies in this area by the Cunha group separated the UGS and other tissues in the male urogenital tract into the mesenchymal and epithelial cell types. Heterotypic recombination of the UGM with bladder epithelium or UGE in kidney capsule grafts demonstrated that either UGE or bladder epithelium formed prostate-like branched ductal structures that expressed prostatic secretory proteins7. This implicated paracrine signals from the prostatic stromal cells as both inducers of branching morphogenesis and as instructive signals for the differentiation of epithelial cells into secretory cells characteristic of the mature prostate. Additional studies showed that stromal-to-epithelial paracrine signaling also directed lobe-specific epithelial differentiation8. These studies formed the basis for further molecular studies that identified specific genes and pathways in the UGE and UGM that are required for key aspects of prostatic morphogenesis or differentiation. Early molecular studies identified several paracrine signaling pathways as playing key roles in prostatic development including components of the transforming growth factor beta (TGFβ), fibroblast growth factor (FGF), bone morphogenetic protein (BMP), insulin-like growth factor (IGF), and sonic hedgehog (Shh) pathways (reviewed in2). Other early studies implicated transcription factors in prostatic development including the androgen receptor (AR), homeobox (Hox) genes, and Nkx3.1 (reviewed in2).
Recent research has identified additional genes and paracrine signaling pathways that are required for one or more aspects of prostate development (summarized in Table 1 and Fig. 2). One of the important recent advances in the field has been the application of genome-wide technologies to obtain a more global perspective on prostatic development. Serial analysis of gene expression (SAGE) was used to identify paracrine signaling pathways in prostate development by comparing gene expression in mesenchyme and epithelium at several stages during development9, 10. SAGE studies linked several members of the Wnt signaling pathway to prostate development including secreted frizzled related protein 1 (SFRP1). SFRP1, a member of the non-canonical Wnt signaling pathway, has increased expression in the developing stromal cells of the prostate and loss of SFRP1 expression decreases prostate branching in all lobes of the prostate11, 12. SFRP1 overexpression increased proliferation of the epithelial cells, but decreased secretory gene expression11, 12. SFRP2, another soluble factor that binds to Wnt receptors, has increased expression in the UGM10. Additional gene expression profiling studies of mouse UGS/prostate development from embryonic day 15.5 (E15.5) until postnatal day 90 (P90) identified gene expression profiles that were linked to particular developmental stages13. For example, a prostate induction gene signature is composed of Wnt signaling pathway (Sfrp1, Sfrp2, and Dkk2), hemoglobin genes (Hba-a1 and Hbb1-b1), and chemokines (cklfsf3). Additional progress in this area is being made though databases that archive large volumes of data such as gene expression omnibus (GEO), which contains data from published arrays for gene, protein, and non-coding RNA studies. The Genitourinary Development Molecular Atlas Project (GUDMAP) is another developmental database that collects both images and information on gene expression studies (microarray, high throughput in situ hybridization) and provides both spatial and temporal information on gene expression6,14, 15. The analysis is performed during various developmental stages of the genitounrinary tract, on a variety of reproductive tissues including the prostate. These databases are searchable and can be used to determine the expression patterns for many genes during prostate development even though the genes were not the focus of individual published studies. In addition to the information projects like the GUDMAP provide for individual genes, they are also being used to refine our understanding of the cellular anatomy of prostate development such as lobe-specific developmental pathways and early differentiation events that sub-divide developing tissues into separate functional domains. As part of the GUDMAP project, mRNAs with lobe specific expression have been identified. In situ hybridization of the lower urinary tract was used to identify specific markers for the prostate, urethra, and other tissues as well as identification of Bmp2 as having specific ventral lobe expression16. In addition, the BMP inhibitor noggin has been shown to be critical for the budding of the ventral prostate17. These studies and the GUDMAP project have also allowed the mesenchymal and epithelial tissues (Fig. 1 F–G) to be further categorized into more precise sub-regions within the mesenchyme (muscularis mucosa and lamina propria) and epithelium (budding regions)16 (Fig. 1H). Although progress has been made, it is likely that additional genes display specific expression during development that may be responsible for determination and subsequent differentiation.
Table 1.
Recent Advances in paracrine regulation of prostate development
| Name | Process | Ref |
|---|---|---|
| Axin2 | Expressed in budding & branching epithelium | 86 |
| Bmp2 | Marker of the ventral prostate | 16 |
| Bmp7 | Mesenchymal expression inhibits Notch and restricts budding | 84 |
| Lef1 | Expressed in budding & branching epithelium | 86 |
| FGF10 | Stromal expression promotes branching | 78, 87 |
| FGFR2 | Epithelial expression is required for proper branching and optimal androgen responsiveness | 78, 88 |
| MMP2 | Epithelial expression required for branching and reducing collagen deposition of stroma | 81 |
| Notch | Required for terminal differentiation of epithelium | 61 |
| SFRP1 | Prostate initiation gene signature, branching | 12, 13 |
| Shh | Required for epithelial growth | 71 |
| SOX9 | Promotes prostate budding (particularly VP and AP), deletion reduces FGFR2 expression | 19 |
| Sulf1 | Inhibits ductal branching and FGFR signaling | 80 |
| Wnt4 | Prostate epithelium marker | 86 |
| Wnt7a | Prostate epithelium marker | 86 |
| Wnt9b | Prostate epithelium marker | 86 |
| Wnt10b | Marker for prostate buds and epithelium | 16, 86 |
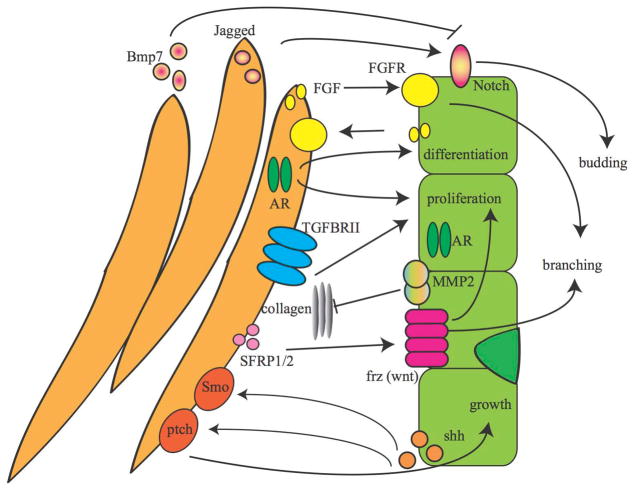
Figure 2. Recent advances in paracrine signaling pathways in prostate development.
Communication between the prostate mesenchymal (orange) and epithelial cells (green) is essential for normal prostate development. Here recent progress in paracrine signaling involved in prostate development is highlighted. Arrows indicate the directionality of the communication between the mesenchyme and epithelium. Pathways including the Wnt, sonic hedgehog (Shh), androgen receptor (AR)46, and fibroblast growth factor (FGF) pathways are critical for the normal growth, differentiation, and development of the prostate. Receptor and ligand locations in the mesenchyme and epithelium are depicted in the model. FGF 7, 13, and 14 are secreted by the mesenchyme and bind to epithelial FGFR1 and FGFR233. The FGF pathway also works the opposite direction where the UGM expresses FGFR1 and the UGE secretes FGF1. Studies using TGFβRII knockout in the epithelium or mesenchyme demonstrated that TGFβRII receptor on the UGM was important for androgen response20. SFRP1 and SFRP2 are expressed in the developing UGM and activate non-canonical Wnt signaling pathways by binding to Wnt receptors such as Wnt4 that activate epithelial proliferation10, 12. Shh secretion by the UGE to activate stromal smoothened (Smo) and patched (Ptch) is critical for normal prostate development, but this same pathway can also activate inhibitors of prostate development such as Bmp771. Notch expression in the epithelium is required for budding of the prostate epithelium, but is restricted to specific locations by mesenchymal Bmp784. This complex network of signaling between the UGM and UGE is critical normal prostate development.
In addition to advances in the understanding the contribution of particular genes and pathways to prostate development, there have been recent technical advances that have facilitated mechanism-based research on prostate development. A variety of model systems are typically used to investigate prostate development (see sidebar). Some of the most important models are genetically modified mice that can add or remove particular genes as a means of testing their contribution to prostate development. An important factor limiting the application of knockout mice for investigating prostate development is embryonic or perinatal lethality for global gene knockouts due to failure of developmental events outside the prostate. Since much of prostate development occurs postnatally, the prostate developmental phenotypes often cannot be determined for global knockout models. A strategy that overcomes this limitation is the use of conditional knockout models where genes are removed by the activity of CRE recombinase acting as a transgene with tissue-specific expression18. Recent progress in prostate research has included the development of the Nkx3.1-CRE mouse that can be used to achieve deletion of genes in the developing prostatic epithelium18. Nkx3.1-CRE is expressed early in prostate development from E17.5 and persists through adulthood in epithelial cells. In this model, a cDNA encoding CRE recombinase was inserted into the Nkx3.1 locus so that it is expressed under the control of the Nkx3.1 promoter providing prostate epithelial selective CRE expression. This new transgenic model has been used to evaluate the prostate developmental roles for genes that would be embryonic lethal in whole animal knockouts including FGFR2 and SOX9. Utilizing the Nkx3.1-CRE mouse model and a floxed (fl) FRGFR2 gene Lin and colleagues demonstrated that FGFR2 expression in the prostate epithelium is required for normal branching and optimal androgen responsiveness11. Another study in the Nkx3.1-CRE mouse using SOX9fl/+ allele model demonstrated an essential role for SOX9 in budding and the formation of the ventral and anterior prostate lobes19. The Nkx3.1-CRE model has also been used to clarify the role of epithelial and mesenchymal signaling in prostate development. For example, a floxed TGFβRII crossed with either Nkx3.1-CRE (epithelial) or fsp-CRE (mesenchymal) determined that mesenchymal TGFβRII was important for castration-induced prostate regression20.
Prostate Stem Cells
Recent advances have also included a dramatically increased understanding of prostate stem cells. Several differentiated epithelial cell types are present in the adult prostate including luminal, basal, and relatively rare neuroendocrine cells. Since the adult prostate is relatively growth quiescent, the possible existence of adult prostate stem cells was controversial for many years. Studies which demonstrated that the prostate could undergo cycles of castration-induced regression and regrowth supported the hypothesis that stem cells existed within the adult prostate21, 22. The seasonal regression and regrowth of the prostate in marsupials, such as brushtail possum and wallaby, provided additional support for the existence of prostate stem cells23. Studies suggesting that the basal and luminal cells could develop independently also led to an unresolved controversy about whether there was a single prostate stem cell, but recent studies using in vivo lineage tracking of prostate stem cells suggest that multiple adult epithelial cell types arise from a common progenitor cell24, 25. In the rodent prostate stem cell markers are enriched in the proximal region of the prostatic ducts near the urethra26. Prostate stem cells have been identified in both the mouse and human prostates and are defined by the criteria of forming all epithelial cell types in a reconstituted organ when the putative stem cells are implanted with rat UGM under the kidney capsule26–28(Fig. 3). However, the candidate stem cells identified by different studies expressed markers from distinct epithelial lineages: basal-like (p63+CD44+CD49f+Ck5+Ck8−AR−PSA−28), luminal-like (Nkx3.1+AR+Ck18+p63−Ck14−Ck5−29), multi-lineage (both luminal and basal markers30), and uncommitted (lack luminal or epithelial markers, Sca1+CD44+CD117+CD133+LIN−27 or Sca1+CD49f+CD45−CD31−Ter119−26). Despite these discrepancies, a few common markers emerged among the studies, Stem Cell Antigen1 (Sca1) in mouse31 and CD49f, in both mouse and human. This suggests several possibilities for the origin of prostate stem cells: 1) basal or luminal epithelial-like stem cells, 2) uncommitted stem cells that differentiate to form semi-committed progenitors, or 3) multiple populations of stem cells. New tools to study prostate progenitor cells that may aid in the identification of stem cell markers include two recently characterized spontaneously immortalized human prostate epithelial cells lines which exhibit self-renewing potential and when implanted with rat UGM can generate prostate epithelium32. Profiles of differential gene expression in the developing UGE and UGM were used to identify signaling pathways that may be linked to self-renewal in the embryonic stem cell population are dependent upon stromal to epithelial signaling33, but further studies are required to determine which pathways are important for the adult stem cell niche. Many pathways identified as critical for prostate epithelial stem cell self-renewal are paracrine-signaling pathways including the Wnt, sonic hedgehog (Shh), and transforming growth factor β (TGFβ) pathways33.
Figure 3. Recent advances in understanding prostate stem cells.
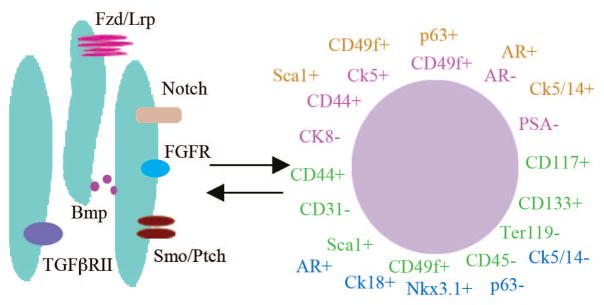
The prostate stem cells identified in mouse and human prostate have distinct markers. The illustration depicts recently identified cellular markers for prostate stem cells. Several studies have identified distinct markers for prostate stem cells including human basal-like stem cells with, CD44+CD49f+Ck5+p63+Ck8−AR−PSA−28(shown in pink). Several populations of murine stem cells with distinct makers form prostate tissue with containing all three types of differentiated prostate epithelial cells when combined with UGM and implanted in the kidney capsule of a nude mouse. These putative murine stem cells can be categorized as luminal epithelial-like (depicted in blue Nkx3.1+, AR+ CK18+ p63−CK14− and CK5−Sca1+CD44+CD117+CD133+29), uncommitted (shown in green LIN− cells27 and Sca129+ CD49f+ CD45− CD31− Ter119− cells26), and multi-lineage (shown in orange, Sca1+ CD49f+ p63+ Ck5+ Ck14+ AR+ Ck8+ Ck18+30). Makers for luminal-like are shown in blue. Recent studies have demonstrated that the stem cell niche, particularly the stromal cells, is critical for maintaining the undifferentiated state of the prostate stem cells. Signaling pathways in the niche that have differential expression in the UGM33 are thought to contribute to the maintenance of the stem cells are depicted. Receptors with increased expression include components of the Wnt (Lrp 8, Lrp11, and Fzd4), Notch (Notch4), TGFβ (TGFβ RII), FGF (FGFR1), Shh (Ptch1 & Ptch2), and Bmp (Bmp4, Bmp5, and Bmp6) signaling pathways. In the majority of these pathways the corresponding receptors or ligands are upregulated in the UGE. This suggests that interactions reciprocal signaling between the stromal cells and epithelial cells is critical for maintaining the stem cell niche.
Developmental Mechanisms in BPH and Prostate Cancer
One of the striking features of the human prostate is the high incidence of diseases including BPH and prostate cancer. These diseases potentially recapitulate aspects of prostate development through the activation of common genetic pathways and paracrine signalling mechanisms. While this section focuses on common pathways involved in development and disease, it is also important to note that some pathways are involved in disease or development but are not common between the two. Gene expression profiling studies have demonstrated that many genes expressed during prostate development are re-activated in prostate cancer models13 (Fig. 4). For example, human prostate cancers that exhibited a gene expression signature associated with branching morphogenesis had a decreased relapse free survival suggesting a critical role for developmental pathways during prostate cancer progression. Other expression profiles of pubertal prostates, prostate cancer, and BPH34 further demonstrated common gene expression features for the developing prostate, BPH, and prostate cancer. These observations highlight the importance of examining developmental pathways in BPH and prostate cancer, and they also suggest that several developmental pathways may be useful targets for BPH and/or prostate cancer therapies (Table 2).
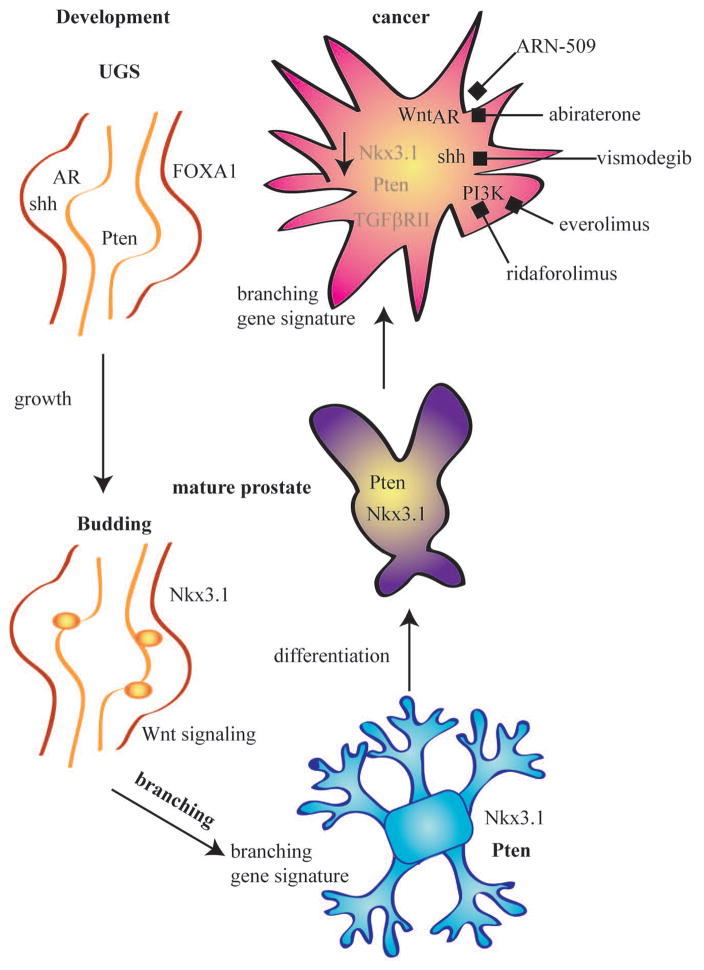
Figure 4. Advances in signaling pathways involved prostate development and disease.
Prostate development progresses from the undifferentiated UGS epithelium and stromal cells to the invasion of the mesenchyme by the developing epithelium. The budding and branching of the epithelium form the ductal structures observed in the mature prostate. Although the prostate can remain healthy, over the lifetime of a man there is 1:6 chance of developing prostate cancer. Interestingly, pathways that are essential for prostate development are often re-expressed in prostatic diseases. Although, many different signaling pathways have been implicated in disease and development, here we highlight those pathway where recent progress has been made in links to prostate cancer, particularly those that are target of novel therapeutics that are in clinical trials. The pathways that increase in prostate cancer are in black and those that are repressed or have a loss of expression are shown in grey. Nkx3.1 and Pten, markers of early epithelium and differentiation, are completely absent or have reduced expression in prostate cancer3, 56, 62. In addition TGFβRII is frequently downregulated in cancer85. Pathways such as AR, Shh, PI3K, and Wnt signaling are upregulated in prostate cancer and promote the proliferation and tumor formation46, 58, 68. AR, Shh, and PI3K pathway are the targets for several drugs that are in clinical trials for prostate cancer48, 50, 63, 64, 73. In addition to signaling pathways, prostate cancer also displays a gene expression profile that is more similar to the developing prostate than the healthy adult prostate13. The return to a less differentiated state is a hallmark in many cancers, and the current studies in prostate cancer support the re-expression of many pathways that are important in development.
Table 2.
Drugs Targeting Prostate Development Pathways
| Drug Name | Target | Phase of Development | Ref. |
|---|---|---|---|
| abiraterone | CYP17A1 inhibitor | Approved for castration resistant prostate cancer, current trials with various drug combinations | 50 |
| ARN-509 | AR antagonist | Phase I (NCT01171898) and Phase II (NCT01709734) trials in castration resistant prostate cancer | 48 |
| bicalutamide | AR antagonist | Approved for prostate cancer, current trials in combination with other drugs | 47 |
| dutasteride | 5α reductase type II inhibitor | Approved BPH, current trials for combination therapy for BPH and prostate cancer | 39 |
| enzalutamide | AR antagonist | Approved for castration resistant prostate cancer, current trials in in combination with abiraterone, leuprolide, and bicalutamide | 49 |
| everolimus | PI3 kinase/mTOR inhibitor | Phase I (NCT01642732) and II (NCT01313559) clinical trials for advanced prostate cancer | 63 |
| finasteride | 5α reductase type II inhibitor | Approved for BPH | 39 |
| flutamide | AR antagonist | Approved for prostate cancer, current trials in combination therapies | 47 |
| leuprolide | GnRH antagonist | Approved for prostate cancer, current trials in various combination therapies | 47 |
| ridaforolimus | mTor inhibitor | Phase II prostate cancer trial (NCT00777959 and NCT00110188) | 64 |
| vismodegib | Shh inhibitor | Phase I/II prostate cancer trial (NCT01163084) FDA approved for metastatic basal cell carcinoma |
73 |
BPH is the benign growth of the prostate gland from its normal size of approximately 30g in young adult men to a larger size that can be >150g. BPH is also commonly associated with lower urinary tract symptoms that require medical intervention. While the etiology of BPH is poorly understood, it has been postulated that estrogens and/or a changing ratio of androgens to estrogens in aging men play an important role in the pathogenesis of BPH. This hypothesis is based on two main observations. First, there is a steadily declining ratio of testosterone to estradiol in aging men35. Second, experimental manipulation of androgen and estrogen levels in animal models such as the dog and mouse can cause benign prostatic enlargement and lower urinary tract symptoms36, 37. In addition, the histological appearance of stromal tissue in BPH nodules resembles developmental mesenchyme, and the glandular growth in BPH is often oriented toward areas of altered stromal histology. These facts led pathologist John McNeal to hypothesize that BPH is caused by “embryonic processes reawakened in a distorted form in adult life”38. These observations link BPH with developmental signaling pathways and suggest that as the etiology of BPH is uncovered, more links to development will be discovered. Currently, one option for BPH treatment targets androgen metabolism. The enzyme 5 alpha reductase type 2, which converts testosterone to its active form dihydroxytestosterone, is required for normal prostate development and is also the target of the drugs finasteride and dutasteride that are commonly used to treat BPH39.
Prostate cancers are predominantly adenocarcinomas of the prostatic epithelium. Although the cell of origin for prostate cancers is uncertain, prostate cancers have glandular features and produce significant amounts of glandular secretory proteins including prostate specific antigen (PSA) that is measured in serum as a diagnostic marker that can indicate the possible presence or recurrence of prostate cancer40. Although the vast majority of prostate cancers are adenocarcinomas that are driven by genetic changes within the epithelium, abnormal paracrine interactions with cells in the tumor microenvironment are also known to be critical determinants of prostate cancer progression 41. The stromal-epithelial interactions during prostate cancer progression are similar to the interactions that occur during prostate development, and at least some of the same molecular pathways are involved9.
Androgens and the AR are critical for normal prostate development, and they function in the normal adult prostate to maintain organ integrity and the expression of prostate-specific secretory proteins. This is shown by surgical or chemical castration that leads to organ apoptosis and loss of secretory protein expression42. Surgical or chemical castration (a.k.a. androgen deprivation therapy or ADT) has also been used to treat advanced prostate cancers since the pioneering work of Huggins in the 1930’s43. Many years of research have identified several androgen-regulated mechanisms that likely contribute to prostate cancer progression. For example, translocations and deletions present in human prostate epithelial tumor cells have been shown to bring oncogenes such as ETS-type transcription factors under direct control of androgen-regulated promoters44. Other studies using animal models for prostate cancer have shown that androgens can promote prostate cancer progression via actions on stromal androgen receptors that induce stromal-to-epithelial paracrine signaling molecules45. These data support the model that androgens promote growth and survival of prostate cancers via actions on both the epithelial and stromal compartments in the tumor. It is also notable that the androgen gene expression signature in prostate cancer resembles an early prostate developmental gene expression signature46. Several drugs are currently available for ADT including Gonadotropin Releasing Hormone (GnRH) agonists such as leuprolide as well as AR antagonists flutamide and bicalutamide, which are most commonly used for metastatic prostate cancer. Although most advanced prostate cancers respond to these drugs, many patients treated with ADT eventually progress to castration-resistant disease47. Once castration-resistant disease arises, it cannot be effectively treated with available therapies so new treatments options are needed. Recent advances in this area include the development of new AR antagonists such as ARN-509, which is currently in phase I clinical trials (NCT01171898)48 and enzalutamide, which was recently FDA-approved for castration resistant prostate cancer49. In addition, new strategies for targeting local androgen synthesis have been developed including the CYP17A1 inhibitor abiraterone that has recently approved for advanced prostate cancer treatment50.
The successful development of prostate cancer treatments based on targeting the AR and androgen signaling suggest that additional links may exist between androgen-related developmental pathways and the progression of prostate cancer. FOXA1 was first implicated in the prostate due to its role in promoting ductal morphogenesis and differentiation51. It also interacts with nuclear receptors such as estrogen receptor and AR to form transcriptional complexes. However, there has been a recent interest in the role of FOXA1 in cancer since it acts as a pioneer factor that enhances gene expression during development via interaction with nuclear receptors, such as AR, and it displays increased expression in several types of cancer including prostate cancer52, 53. While there is interest is FOXA1 inhibition as a clinical treatment, thus far no drugs targeting FOXA1 have been developed 54. In contrast to AR and FOXA1, Nkx3.1, which negatively regulates AR signaling, is often lost in prostate cancer55. Expression of Nkx3.1 is the first marker of the prostate epithelial cells and requires both intact AR signaling and mesenchymal cells, suggesting it may be regulated in part by the stromal cells3. Interestingly in a recent study, Nkx3.1 was recently shown to function as a paracrine transcription factor that is secreted into the extracellular environment both in vitro in human prostate cell lines and in vivo in mouse prostatic secretions and urine56. Co-culture of cells transfected with HA-Nkx3.1 and untransfected cells demonstrated that HA-tagged Nkx3.1 translocates and binds to target promoters in untransfected cells and decreased their growth. In the normal prostate, Nkx3.1 limits cell proliferation, but in prostate cancer it has reduced function through mutation or its expression is reduced or ablated via promoter methylation57. The loss of Nkx3.1 in mouse models of prostate cancer results in epithelial hyperplasia and dysplasia. The Nkx3.1 knockout mouse is a critical model for understanding the early stages of prostate cancer development because the lesions that develop resemble prostate intraepithelial neoplasia (PIN), a precursor to prostate cancer.
While therapies targeting AR signaling have been used for decades, additional pathways that are involved in both prostate development and cancer are being actively explored as potential therapeutic targets for prostate cancer. PI3K signaling is critical in many developing organs including the prostate where it is increased in the budding epithelial prostate cells, and PI3K inhibition interferes with prostate branching morphogenesis58. Increased PI3K pathway activity is also associated with prostate cancer progression and participates in pathogenesis by promoting proliferation and decreasing apoptosis. The PI3K pathway can be activated in cancers by multiple mechanisms including activating kinase mutations and loss-of-function mutations in the pathway-inhibitory phosphatase Pten59, 60. Prostate-specific Pten deletions have also been created in mice where they have been reported to cause several prostate cancer related phenotypes including high grade PIN, invasive cancer and metastasis56, 57. Pten is also critical for the differentiation of ductal epithelial cells61 and its deletion in prostate cells leads to expansion of stem and progenitor cells in the prostate62. Recently, drugs including everolimus and ridaforolimus that inhibit the PI3K pathway and/or its downstream effector mTOR have been developed for use in cancer therapy and are currently being tested pre-clinical and clinical trials for prostate cancer (NCT01313559, and NCT01295632)63, 64.
Shh signaling plays a critical role in embryonic development and its disruption leads to defects in patterning of multiple tissues in the mouse including the brain, spinal cord, and skeleton as well as perinatal lethality65. During prostate development, Shh is expressed in the UGE and binds to receptors in the UGM, and treatment with a hedgehog inhibitor disrupts growth, branching, and epithelial differentiation66, 67. In addition, activation of Shh signaling correlates with prostate cancers that have higher Gleason scores and overexpression of Shh in LNCaP human prostate cancer xenografts increases their growth65, 68, 69. Recently, Shh signaling also has been shown to play a role in maintenance of progenitor cells thought to be critical for prostate development through the activation of target gene Gli270. Additional studies have also implicated Shh in regulation of noggin and BMPs that are linked to the promotion and prevention of prostate budding, respectively71. In mouse prostate cancer models, Shh overexpression increases the incidence of PIN and prostate cancer and also leads to metastatic tumors that have prostate-like structures72. The Shh inhibitor, vismodegib, was recently approved for metastatic basal cell carcinoma and is in phase I/II clinical trial for prostate cancer in combination with AR inhibitors (NCT01163084)73. Available data suggest that the roles of the Shh pathway during prostate development and cancer progression are complex including stage-specific actions and possible roles for both paracrine and autocrine signalling. Future studies will likely improve our understanding of these complexities and determine the viability of targeting the Shh pathway for prostate cancer therapy.
Recent Advances in models for studying prostate development.
Cell culture
Prostate cells lines have been used to study aspects of prostate development such as branching. Recently, spontaneously immortalized human epithelial cells that retain stem cell-like characteristics and generate ductal structures in 3-D cell culture were created32. New prostate stromal cells in both human (BHPrS-1) and mouse models (UGSM2) have been isolated and will aid in the investigating the prostate microenvironment74, 75. In combination with new cell models, novel culture system designs that more closely recapitulate the microenvironment76 in tissue culture will provide tools to study the interactions between cells in vitro.
Transgenic animals
Recent advances include the development and use of prostate epithelial specific Nkx3.1 Cre mouse that allows for CRE-mediated genetic manipulations to be achieved much earlier during prostate development than was previously possible, and has been used for the assessment of particular genes in prostate development and pathology19, 77, 78.
Organ culture
UGS tissues isolated and cultured in vitro are useful for studying branching morphogenesis, but they do not undergo differentiation79. Transfection of the UGS followed by in vitro culture can be used to assess the effects of overexpression or knockdown of particular genes in development80. Recent studies used in vitro UGS culture to identify factors that promote and inhibit prostate development. However, the male and female UGS cannot be used interchangeably to study prostate budding since distinct bud numbers and gene expression are observed81, 82.
Renal capsule grafting
UGS tissue or cells in matrigel are inserted and grown as grafts under the kidney capsules of a nude mouse to study distinct stages of development including differentiation 79. Additionally UGS, isolation from transgenic mice that contain embryonic lethal alterations followed by kidney capsule grafts or organ culture has clarified the role of those genes in development 83.
Conclusion
Prostate development occurs through a series of coordinated steps that depend upon systemic steroid hormone signals, local paracrine signaling pathways, and cell autonomous factors. While the stages of prostate development have been defined on an anatomical basis and recent studies have identified several molecular mechanisms that regulate specific aspects of prostate development, many aspects of prostate development remain poorly understood such as how distinct lobes or zones of the prostate are patterned. Another area of active research is on how the spatial and temporal aspects of signaling that regulate prostate development that can be explored and shared. One main area of progress in this area is through the development of searchable datasets such as GUDMAP and NCBI GEO. These databases provide researchers with valuable information and allow for the comparison of multiple stages in development and advance the understanding of prostate development and disease. Although there are still many questions about the origin of prostate stem cells and their role in prostate development, there have been great advances in identification of those cells that have self-renewing capacity. Further studies will be required to unify the information that has emerged regarding the best markers for prostate stem cells and explore the idea of targeting prostate stem cells in cancer therapy. Increased expression of stem cell markers Sca1 and CD117 after castration suggests the possibility that the stem cell population could play a role in androgen insensitivity in prostate cancer27. This is an active area of interest in many types of cancer; therefore, it will be of interest if prostate stem cells may a be therapeutic target in cancer or BPH. Particularly interesting is the fact that the pathways involved in prostate development are often reactivated in diseases such as prostate cancer and BPH. Although it has long been known that reciprocal interactions between the stromal and epithelial cells regulate development and disease, few pharmacological agents have been available to target paracrine interactions or prostate stromal cells. Ongoing clinical trials testing the utility of Shh, PI3K, and mTor inhibitors are among the first to explore targeting stromal-epithelial interactions for prostate cancer treatment. The advances in connecting molecular and anatomical features in development, identification of stem cell markers, and links between development and disease have enhanced the understanding of prostate development and provide areas for future exploration.
Acknowledgments
Funding was provided by NIH grants DK091193 and CA140217 to PCM and postdoctoral training grant T32 CA157322 on Molecular and Cellular Mechanism of Tumor Development to GLP.
Footnotes
Contributor Information
Ginny L. Powers, Division of Pharmaceutical Sciences, School of Pharmacy, University of Wisconsin-Madison, Madison, Wisconsin
Paul C. Marker, Email: marker@wisc.edu, Division of Pharmaceutical Sciences, School of Pharmacy, University of Wisconsin-Madison, 777 Highland Ave., Madison, Wisconsin, 53705
References
- 1.Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biology of Reproduction. 1986;34:961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- 2.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation; research in biological diversity. 2008;76:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, et al. Roles for Nkx3.1 in prostate development and cancer. Genes & Development. 1999;13:966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunha GR, Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) Mice. Journal of Experimental Zoology. 1978;205:181–193. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto N, Akimoto Y, Suzuki T, Kitamura S, Ohta S. Identification of prostatic-secreted proteins in mice by mass spectrometric analysis and evaluation of lobe-specific and androgen-dependent mRNA expression. J Endocrinol. 2006;190:793–803. doi: 10.1677/joe.1.06733. [DOI] [PubMed] [Google Scholar]
- 6.Cunha GR. The role of androgens in the epithelio-mesenchymal interactions involved in prostatic morphogenesis in embryonic mice. Anat Rec. 1973;175:87–96. doi: 10.1002/ar.1091750108. [DOI] [PubMed] [Google Scholar]
- 7.Cunha GR, Fujii H, Neubauer BL, Shannon JM, Sawyer L, Reese BA. Epithelial-mesenchymal interactions in prostatic development. I. morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. The Journal of Cell Biology. 1983;96:1662–1670. doi: 10.1083/jcb.96.6.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pritchard CC, Nelson PS. Gene expression profiling in the developing prostate. Differentiation; research in biological diversity. 2008;76:624–640. doi: 10.1111/j.1432-0436.2008.00274.x. [DOI] [PubMed] [Google Scholar]
- 9.Vanpoucke G, Orr B, Grace OC, Chan R, Ashley GR, Williams K, Franco OE, Hayward SW, Thomson AA. Transcriptional profiling of inductive mesenchyme to identify molecules involved in prostate development and disease. Genome biology. 2007;8:R213. doi: 10.1186/gb-2007-8-10-r213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T-J, Hoffman BG, Ruiz de Algara T, Helgason CD. SAGE reveals expression of Wnt signalling pathway members during mouse prostate development. Gene Expression Patterns. 2006;6:310–324. doi: 10.1016/j.modgep.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Joesting MS, Perrin S, Elenbaas B, Fawell SE, Rubin JS, Franco OE, Hayward SW, Cunha GR, Marker PC. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer research. 2005;65:10423–10430. doi: 10.1158/0008-5472.CAN-05-0824. [DOI] [PubMed] [Google Scholar]
- 12.Joesting MS, Cheever TR, Volzing KG, Yamaguchi TP, Wolf V, Naf D, Rubin JS, Marker PC. Secreted frizzled related protein 1 is a paracrine modulator of epithelial branching morphogenesis, proliferation, and secretory gene expression in the prostate. Developmental biology. 2008;317:161–173. doi: 10.1016/j.ydbio.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pritchard C, Mecham B, Dumpit R, Coleman I, Bhattacharjee M, Chen Q, Sikes RA, Nelson PS. Conserved Gene Expression Programs Integrate Mammalian Prostate Development and Tumorigenesis. Cancer research. 2009;69:1739–1747. doi: 10.1158/0008-5472.CAN-07-6817. [DOI] [PubMed] [Google Scholar]
- 14.Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, Houghton D, Lloyd-MacGilp S, Pi X, Roochun Y, et al. The GUDMAP database – an online resource for genitourinary research. Development. 2011;138:2845–2853. doi: 10.1242/dev.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, Lessard JL, Little MH, Potter SS, Wilder EL, et al. GUDMAP: The Genitourinary Developmental Molecular Anatomy Project. Journal of the American Society of Nephrology. 2008;19:667–671. doi: 10.1681/ASN.2007101078. [DOI] [PubMed] [Google Scholar]
- 16.Abler LL, Keil KP, Mehta V, Joshi PS, Schmitz CT, Vezina CM. A high-resolution molecular atlas of the fetal mouse lower urogenital tract. Developmental Dynamics. 2011;240:2364–2377. doi: 10.1002/dvdy.22730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook C, Vezina CM, Allgeier SH, Shaw A, Yu M, Peterson RE, Bushman W. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Developmental biology. 2007;312:217–230. doi: 10.1016/j.ydbio.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanfel MN, Moses KA, Carson JA, Zimmer DB, DeMayo F, Schwartz RJ, Zimmer WE. Expression of an Nkx3.1-CRE gene using ROSA26 reporter mice. genesis. 2006;44:550–555. doi: 10.1002/dvg.20250. [DOI] [PubMed] [Google Scholar]
- 19.Thomsen MK, Butler CM, Shen MM, Swain A. Sox9 is required for prostate development. Developmental biology. 2008;316:302–311. doi: 10.1016/j.ydbio.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Placencio VR, Sharif-Afshar AR, Li X, Huang H, Uwamariya C, Neilson EG, Shen MM, Matusik RJ, Hayward SW, Bhowmick NA. Stromal transforming growth factor-beta signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer research. 2008;68:4709–4718. doi: 10.1158/0008-5472.CAN-07-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11:229–242. doi: 10.1002/pros.2990110304. [DOI] [PubMed] [Google Scholar]
- 22.Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, Shapiro E, Lepor H, Sun TT, Wilson EL. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol. 2002;157:1257–1265. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink JW, McLeod BJ, Assinder SJ, Parry LJ, Nicholson HD. Seasonal changes in mesotocin and localization of its receptor in the prostate of the brushtail possum (Trichosurus vulpecula) Biol Reprod. 2005;72:470–478. doi: 10.1095/biolreprod.104.035006. [DOI] [PubMed] [Google Scholar]
- 24.Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development. 2004;131:4955–4964. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- 25.Blackwood JK, Williamson SC, Greaves LC, Wilson L, Rigas AC, Sandher R, Pickard RS, Robson CN, Turnbull DM, Taylor RW, et al. In situ lineage tracking of human prostatic epithelial stem cell fate reveals a common clonal origin for basal and luminal cells. The Journal of Pathology. 2011;225:181–188. doi: 10.1002/path.2965. [DOI] [PubMed] [Google Scholar]
- 26.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proceedings of the National Academy of Sciences. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leong KG, Wang B-E, Johnson L, Gao W-Q. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- 28.Garraway IP, Sun W, Tran CP, Perner S, Zhang B, Goldstein AS, Hahm SA, Haider M, Head CS, Reiter RE, et al. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. The Prostate. 2010;70:491–501. doi: 10.1002/pros.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barclay WW, Axanova LS, Chen W, Romero L, Maund SL, Soker S, Lees CJ, Cramer SD. Characterization of adult prostatic progenitor/stem cells exhibiting self-renewal and multilineage differentiation. STEM CELLS. 2008;26:600–610. doi: 10.1634/stemcells.2007-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci U S A. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, Qiu Q, Schmid J, Tang DG, Hayward SW. Functional Remodeling of Benign Human Prostatic Tissues In Vivo by Spontaneously Immortalized Progenitor and Intermediate Cells. STEM CELLS. 2010;28:344–356. doi: 10.1002/stem.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blum R, Gupta R, Burger PE, Ontiveros CS, Salm SN, Xiong X, Kamb A, Wesche H, Marshall L, Cutler G, et al. Molecular Signatures of the Primitive Prostate Stem Cell Niche Reveal Novel Mesenchymal-Epithelial Signaling Pathways. PLoS ONE. 2010;5:e13024. doi: 10.1371/journal.pone.0013024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhanasekaran SM, Dash A, Yu J, Maine IP, Laxman B, Tomlins SA, Creighton CJ, Menon A, Rubin MA, Chinnaiyan AM. Molecular profiling of human prostate tissues: insights into gene expression patterns of prostate development during puberty. The FASEB Journal. 2004 doi: 10.1096/fj.04-2415fje. [DOI] [PubMed] [Google Scholar]
- 35.Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086–1090. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- 36.Coffey DS, Walsh PC. Clinical and experimental studies of benign prostatic hyperplasia. Urol Clin North Am. 1990;17:461–475. [PubMed] [Google Scholar]
- 37.Nicholson TM, Ricke EA, Marker PC, Miano JM, Mayer RD, Timms BG, Vom Saal FS, Wood RW, Ricke WA. Testosterone and 17beta-Estradiol Induce Glandular Prostatic Growth, Bladder Outlet Obstruction, and Voiding Dysfunction in Male Mice. Endocrinology. 2012 doi: 10.1210/en.2012-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNeal J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol Clin North Am. 1990;17:477–486. [PubMed] [Google Scholar]
- 39.Kramer BS, Hagerty KL, Justman S, Somerfield MR, Albertsen PC, Blot WJ, Ballentine Carter H, Costantino JP, Epstein JI, Godley PA, et al. Use of 5-alpha-reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. J Clin Oncol. 2009;27:1502–1516. doi: 10.1200/JCO.2008.16.9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 41.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–2483. [PubMed] [Google Scholar]
- 42.Sugimura Y, Cunha GR, Donjacour AA. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol Reprod. 1986;34:973–983. doi: 10.1095/biolreprod34.5.973. [DOI] [PubMed] [Google Scholar]
- 43.Fluchter SH, Weiser R, Gamper C. The role of hormonal treatment in prostate cancer. Recent Results Cancer Res. 2007;175:211–237. doi: 10.1007/978-3-540-40901-4_13. [DOI] [PubMed] [Google Scholar]
- 44.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 45.Ricke EA, Williams K, Lee YF, Couto S, Wang Y, Hayward SW, Cunha GR, Ricke WA. Androgen hormone action in prostatic carcinogenesis: stromal androgen receptors mediate prostate cancer progression, malignant transformation and metastasis. Carcinogenesis. 2012;33:1391–1398. doi: 10.1093/carcin/bgs153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaeffer EM, Marchionni L, Huang Z, Simons B, Blackman A, Yu W, Parmigiani G, Berman DM. Androgen-induced programs for prostate epithelial growth and invasion arise in embryogenesis and are reactivated in cancer. Oncogene. 2008;27:7180–7191. doi: 10.1038/onc.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 48.Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, Dilhas A, Chen Y, Grillot K, Bischoff ED, Cai L, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer research. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beckett RD, Rodeffer KM, Snodgrass R. Abiraterone for the treatment of metastatic castrate-resistant prostate cancer. Ann Pharmacother. 2012;46:1016–1024. doi: 10.1345/aph.1Q758. [DOI] [PubMed] [Google Scholar]
- 51.Gao N, Ishii K, Mirosevich J, Kuwajima S, Oppenheimer SR, Roberts RL, Jiang M, Yu X, Shappell SB, Caprioli RM, et al. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development. 2005;132:3431–3443. doi: 10.1242/dev.01917. [DOI] [PubMed] [Google Scholar]
- 52.Mirosevich J, Gao N, Gupta A, Shappell SB, Jove R, Matusik RJ. Expression and role of Foxa proteins in prostate cancer. The Prostate. 2006;66:1013–1028. doi: 10.1002/pros.20299. [DOI] [PubMed] [Google Scholar]
- 53.Shah S, Prasad S, Knudsen KE. Targeting Pioneering Factor and Hormone Receptor Cooperative Pathways to Suppress Tumor Progression. Cancer research. 2012;72:1248–1259. doi: 10.1158/0008-5472.CAN-11-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jozwik KM, Carroll JS. Pioneer factors in hormone-dependent cancers. Nat Rev Cancer. 2012;12:381–385. doi: 10.1038/nrc3263. [DOI] [PubMed] [Google Scholar]
- 55.Lei Q, Jiao J, Xin L, Chang C-J, Wang S, Gao J, Gleave ME, Witte ON, Liu X, Wu H. NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell. 2006;9:367–378. doi: 10.1016/j.ccr.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 56.Zhou J, Qin L, Gao L, Chen X, Tien JC-Y, Wang F, Hsieh J-T, Xu J. Nkx3.1 functions as a para-transcription factor to regulate gene expression and cell proliferation in a non-cell-autonomous manner. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.M111.336909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asatiani E, Huang W-X, Wang A, Rodriguez Ortner E, Cavalli LR, Haddad BR, Gelmann EP. Deletion, Methylation, and Expression of the NKX3.1 Suppressor Gene in Primary Human Prostate Cancer. Cancer research. 2005;65:1164–1173. doi: 10.1158/0008-5472.CAN-04-2688. [DOI] [PubMed] [Google Scholar]
- 58.Ghosh S, Lau H, Simons BW, Powell JD, Meyers DJ, De Marzo AM, Berman DM, Lotan TL. PI3K/mTOR signaling regulates prostatic branching morphogenesis. Developmental biology. 2011;360:329–342. doi: 10.1016/j.ydbio.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedlander TW, Roy R, Tomlins SA, Ngo VT, Kobayashi Y, Azameera A, Rubin MA, Pienta KJ, Chinnaiyan A, Ittmann MM, et al. Common structural and epigenetic changes in the genome of castration-resistant prostate cancer. Cancer research. 2012;72:616–625. doi: 10.1158/0008-5472.CAN-11-2079. [DOI] [PubMed] [Google Scholar]
- 60.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, Sboner A, Esgueva R, Pflueger D, Sougnez C, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu X, Xu K, Zhang L, Deng Y, Lee P, Shapiro E, Monaco M, Makarenkova HP, Li J, Lepor H, et al. Differentiation of the ductal epithelium and smooth muscle in the prostate gland are regulated by the Notch/PTEN-dependent mechanism. Developmental biology. 2011;356:337–349. doi: 10.1016/j.ydbio.2011.05.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1480–1485. doi: 10.1073/pnas.0510652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan TM, Pitts TE, Gross TS, Poliachik SL, Vessella RL, Corey E. RAD001 (Everolimus) inhibits growth of prostate cancer in the bone and the inhibitory effects are increased by combination with docetaxel and zoledronic acid. Prostate. 2008;68:861–871. doi: 10.1002/pros.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Floc’h N, Kinkade CW, Kobayashi T, Aytes A, Lefebvre C, Mitrofanova A, Cardiff RD, Califano A, Shen MM, Abate-Shen C. Dual targeting of the Akt/mTOR signaling pathway inhibits castration-resistant prostate cancer in a genetically engineered mouse model. Cancer research. 2012 doi: 10.1158/0008-5472.CAN-12-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 66.Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W. Prostate Development Requires Sonic Hedgehog Expressed by the Urogenital Sinus Epithelium. Developmental biology. 1999;209:28–39. doi: 10.1006/dbio.1999.9229. [DOI] [PubMed] [Google Scholar]
- 67.Freestone SH, Marker P, Grace OC, Tomlinson DC, Cunha GR, Harnden P, Thomson AA. Sonic hedgehog regulates prostatic growth and epithelial differentiation. Developmental biology. 2003;264:352–362. doi: 10.1016/j.ydbio.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 68.Fan L, Pepicelli CV, Dibble CC, Catbagan W, Zarycki JL, Laciak R, Gipp J, Shaw A, Lamm MLG, Munoz A, et al. Hedgehog Signaling Promotes Prostate Xenograft Tumor Growth. Endocrinology. 2004;145:3961–3970. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 69.Sheng T, Li C, Zhang X, Chi S, He N, Chen K, McCormick F, Gatalica Z, Xie J. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doles J, Cook C, Shi X, Valosky J, Lipinski R, Bushman W. Functional compensation in Hedgehog signaling during mouse prostate development. Developmental biology. 2006;295:13–25. doi: 10.1016/j.ydbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Shaw A, Gipp J, Bushman W. Exploration of Shh and BMP paracrine signaling in a prostate cancer xenograft. Differentiation; research in biological diversity. 2010;79:41–47. doi: 10.1016/j.diff.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang H-H, Chen B-Y, Wu C-Y, Tsao Z-J, Chen Y-Y, Chang C-P, Yang C-R, Lin D. Hedgehog overexpression leads to the formation of prostate cancer stem cells with metastatic property irrespective of androgen receptor expression in the mouse model. Journal of Biomedical Science. 2011;18:6. doi: 10.1186/1423-0127-18-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS, Revelo MP, Bhowmick NA, Hayward SW. Altered TGF-β Signaling in a Subpopulation of Human Stromal Cells Promotes Prostatic Carcinogenesis. Cancer research. 2011;71:1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shaw A, Papadopoulos J, Johnson C, Bushman W. Isolation and characterization of an immortalized mouse urogenital sinus mesenchyme cell line. The Prostate. 2006;66:1347–1358. doi: 10.1002/pros.20357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Domenech M, Yu H, Warrick J, Badders NM, Meyvantsson I, Alexander CM, Beebe DJ. Cellular observations enabled by microculture: paracrine signaling and population demographics. Integr Biol (Camb) 2009;1:267–274. doi: 10.1039/b823059e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 78.Lin Y, Liu G, Zhang Y, Hu YP, Yu K, Lin C, McKeehan K, Xuan JW, Ornitz DM, Shen MM, et al. Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development. 2007;134:723–734. doi: 10.1242/dev.02765. [DOI] [PubMed] [Google Scholar]
- 79.Doles JD, Vezina CM, Lipinski RJ, Peterson RE, Bushman W. Growth, morphogenesis, and differentiation during mouse prostate development in situ, in renal grafts, and in vitro. The Prostate. 2005;65:390–399. doi: 10.1002/pros.20321. [DOI] [PubMed] [Google Scholar]
- 80.Buresh RA, Kuslak SL, Rusch MA, Vezina CM, Selleck SB, Marker PC. Sulfatase 1 is an inhibitor of ductal morphogenesis with sexually dimorphic expression in the urogenital sinus. Endocrinology. 2010;151:3420–3431. doi: 10.1210/en.2009-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruni-Cardoso A, Rosa-Ribeiro R, Pascoal VDB, De Thomaz AA, Cesar CL, Carvalho HF. MMP-2 regulates rat ventral prostate development in vitro. Developmental Dynamics. 2010;239:737–746. doi: 10.1002/dvdy.22222. [DOI] [PubMed] [Google Scholar]
- 82.Keil KP, Mehta V, Abler LL, Joshi PS, Schmitz CT, Vezina CM. Visualization and quantification of mouse prostate development by in situ hybridization. Differentiation; research in biological diversity. 2012;84:232–239. doi: 10.1016/j.diff.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayward SW, Wang Y, Day ML. Rescue and isolation of Rb-deficient prostate epithelium by tissue recombination. Methods Mol Biol. 2003;218:17–33. doi: 10.1385/1-59259-356-9:17. [DOI] [PubMed] [Google Scholar]
- 84.Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD. BMP7 inhibits branching morphogenesis in the prostate gland and interferes with Notch signaling. Developmental biology. 2005;288:334–347. doi: 10.1016/j.ydbio.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ashida S, Orloff MS, Bebek G, Zhang L, Zheng P, Peehl DM, Eng C. Integrated Analysis Reveals Critical Genomic Regions in Prostate Tumor Microenvironment Associated with Clinicopathologic Phenotypes. Clinical Cancer Research. 2012;18:1578–1587. doi: 10.1158/1078-0432.CCR-11-2535. [DOI] [PubMed] [Google Scholar]
- 86.Mehta V, Abler LL, Keil KP, Schmitz CT, Joshi PS, Vezina CM. Atlas of Wnt and R-spondin gene expression in the developing male mouse lower urogenital tract. Developmental Dynamics. 2011;240:2548–2560. doi: 10.1002/dvdy.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuslak SL, Marker PC. Fibroblast growth factor receptor signaling through MEK-ERK is required for prostate bud induction. Differentiation; research in biological diversity. 2007;75:638–651. doi: 10.1111/j.1432-0436.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 88.Kuslak SL, Thielen JL, Marker PC. The mouse seminal vesicle shape mutation is allelic with Fgfr2. Development. 2007;134:557–565. doi: 10.1242/dev.02741. [DOI] [PubMed] [Google Scholar]