Abstract
Necrosis is commonly found in the core region of solid tumours due to metabolic stress such as hypoxia and glucose deprivation (GD) resulting from insufficient vascularization. Necrosis promotes tumour growth and development by releasing the tumour-promoting cytokine high mobility group box 1 (HMGB1); however, the molecular mechanism underlying necrotic cell death remains largely unknown. In this study, we show that early growth response 1 (Egr-1) is induced in a reactive oxygen species (ROS)-dependent manner by GD in several cell lines such as A549, MDA-MB-231 and HepG2 cells that exhibit necrosis upon GD. We found that Egr-1 short hairpin RNA (shRNA) prevented GD-induced necrosis and HMGB1 release. Necrosis-inhibiting activity of Egr-1 shRNA was also seen in multicellular tumour spheroids (MTSs), an in vitro tumour model system. In contrast, Egr-1 overexpression appeared to make tumour cells more susceptible to GD-induced necrosis. Finally, Egr-1 shRNA suppressed the growth of MTSs. These findings demonstrate that Egr-1 is implicated in GD-induced necrosis and tumour progression.
Keywords: early growth response 1, glucose deprivation, reactive oxygen species, necrosis, multicellular tumour spheroids
Introduction
Unlike tumour suppressive apoptosis and autophagic cell death, necrosis promotes tumour growth, progression, and aggressiveness (1,2). The tumour promoting activity of necrosis is thought to be mediated by a nuclear protein high mobility group box 1 (HMGB1), which exerts pro-inflammatory and tumour-promoting cytokine activities when released into the extracellular spaces due to the rupture of the plasma membrane by necrosis (1–4). In solid tumours, the cells in the inner regions experience metabolic stress such as hypoxia and glucose deprivation (GD) resulting from insufficient vascularization. Although most cells adapt to this environment to obtain more aggressive properties, those in the core region die by necrosis, thereby forming the necrotic core (5). Because necrosis is linked to tumour growth and development, it is an important cell death type in tumour cell biology; however, the molecular mechanism underlying necrotic cell death remains largely unknown.
Early growth response 1 (Egr-1) is a 3 Cys2-His2 type COOH-terminal zinc-finger transcription factor that binds to GC-rich recognition motifs (5′-GCGT/GGGGCG-3′ or 5′-TCCT/ACCTCCTCC-3′) (6,7). Egr-1 is induced by a number of different stimuli, such as anti-cancer drugs and growth factors and inhibits or stimulates tumour growth depending the cellular context and the duration of Egr-1 induction (6–13). Egr-1 was able to directly regulate multiple tumour suppressors including p53, TGF-β1, and PTEN to induce apoptotic cell death (14). In addition, Egr-1 is induced by hypoxia and plays a critical role(s) in hypoxia-induced tumour progression, survival, and angiogenesis (15–18). Furthermore, Egr-1 is involved in hepatocyte growth factor (HGF)-induced cell scattering, migration, and invasion via Snail activation (19). While transient induction of Egr-1 is known to activate angiogenesis, sustained Egr-1 expression induces antiangiogenesis, growth arrest, and apoptosis (20). Thus, Egr-1 is thought to act as a crucial regulator of tumour cell death, growth, invasion, and angiogenesis.
In this study, we tried to identify the mechanism underlying metabolic stress-induced necrosis. Previously, we showed that GD induced necrosis in several tumour cell types including A549, MDA-MB-231, and HepG2 cells and activation of protein kinase C by treatment of phorbol 12-myristate 13-acetate (PMA) switched GD-induced necrosis to apoptosis in A549 cells (21). By cDNA microarray analysis, we found that Egr-1 expression was increased by GD, but not by GD+PMA. In this study, we evaluated the possible role(s) of Egr-1 in necrosis. We found that Egr-1 shRNA prevented necrosis, whereas Egr-1 overexpression made tumour cells more sensitive to GD, thereby leading to necrosis. In addition, Egr-1 shRNA suppressed the growth of multicellular tumour spheroids (MTSs), an in vitro tumour model system. Taken together, these results demonstrate that Egr-1 plays an important role(s) in GD-induced necrosis and tumour progression.
Materials and methods
Cell culture, chemical treatment, and multicellular tumour spheroid (MTS) culture
A549, MDA-MB-231, HepG2, HCT116, and HeLa cells were obtained from American Type Culture Collection, and maintained in RPMI-1640 or DMEM supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Hyclone, Logan, UT, USA) and 1% penicillin-streptomycin (Hyclone) in a 37°C humidified incubator with 5% CO2. The cells were treated with GD, reactive oxygen species [ROS, including H2O2 and menadione (an O2- generator)], or other chemicals as described previously (22). Multicellular tumour spheroid culture was performed using MCF-7 cells (provided by Dr J.I. Yook, University of Yonsei, Korea) as described previously (22) and MTSs dissociation into subpopulations of cells from four different locations was conducted as described by LaRue and colleagues (23).
Microarray
Microarray was performed to screen the differentially expressed genes using Operon Human Whole 35K Oligo chips (GenoCheck, Korea) (22). The Affymetrix microarray data have been deposited in the Gene Expression Omnibus (GEO) database (GEO accession no. GSE24271).
Western blotting, HMGB1 release assay, RT-PCR, and real-time PCR
Western blotting were performed using the following antibodies: Egr-1 (Santa Cruz, CA); α-tubulin (Biogenex, CA); HMGB1 (BD Pharmingen, CA); CuZnSOD (Santa Cruz, CA); ERK1/2 (Cell Signaling, MA). The HMGB1 release assay was carried out as described previously (21,22). Transcript levels were assessed with reverse transcription-polymerase chain reaction (RT-PCR) with primers for Egr-1 and GAPDH (Table I). Quantitative real-time PCR was conducted in a LightCycler (Roche Diagnostics, Mannheim, Germany) using a SYBR Green kit (Roche Diagnostics) with primers for Egr-1 and β-actin (Table I).
Table I.
Sequences used in this study for RT-PCR, real-time PCR, and shRNA interference.
| Sequence 5′→3′ | Annealing temperature (°C) | ||
|---|---|---|---|
| RT-PCR | |||
| GAPDH | |||
| NM_002046.3 | Sense | GTGGTCTCCTCTGACTTCAAC | |
| Antisense | TCTCTTCCTCTTGTGCTCTTG | ||
| Egr-1 | |||
| NM_001964.2 | Sense | ATTCTGAGGCCTCGCAAGTA | 54 |
| Antisense | CACTGCTTTTCCGCTCTTTC | ||
| Real-time PCR | |||
| β-actin | |||
| NM_001101.3 | Sense | ACTCTTCCAGCCTTCCTTCC | |
| Antisense | TGTTGGCGTACAGGTCTTTG | ||
| Egr-1 | Sense | AGGACAGGAGGAGGAGATGG | 62 |
| Antisense | GGAAGTGGGCAGAAAGGATTG | ||
| shRNA interference | |||
| Con shRNA | AATTCTCCGAACGTGTCACGT | ||
| Egr-1 shRNA | AAGTTACTACCTCTTATCCAT | ||
Hoechst 33342 (HO)/propidium iodide (PI) staining and ROS staining
To determine the cell death mode, Hoechst 33342 (HO) and propidium iodide (PI) double staining was performed (21,22). In 2D culture, cells were seeded at a density of 2.5×105 cells/ml in 35-mm dishes. After 24 h, the cells were treated with GD for the indicated times and then stained with HO (1 μg/ml) and PI (5 μg/ml) for 15 min. In 3D culture, equal numbers of spheroids were transferred to 1.2% agarose-coated 60-mm dishes and trypsinized and then stained with HO/PI, The stained cells were observed under a fluorescence microscope and apoptotic and necrotic cells were scored. Intracellular H2O2, O2- and mitochondrial ROS measurement were conducted as described previously (21,22).
Egr-1 transfection and short hairpin RNA (shRNA) interference
pcDNA3.1-Egr-1, constructed by inserting the Egr-1 open reading frame into plasmid pcDNA3.1/NEO expression vector (Invitrogen), was provided by Dr Thomas E. Eling (Laboratory of Molecular Carcinogenesis, National Institute of Environmental Health Sciences, USA). The vectors pcDNA3.1 and pcDNA3.1-Egr-1 were transfected into MCF-7 cells using jetPEI (Polyplus transfection) according to manufacturer’s protocol. Egr-1 shRNA target sequences were designed and verified as specific for Egr-1 by Blast search against the human genome and real-time PCR, respectively (Table I). The vectors pSUPER-control shRNA and pSUPER-Egr-1 shRNA were transfected using jetPEI and stable cell lines were selected using 1–2 mg/ml G418. Several stable clones were isolated after shRNA transfection and individually characterized.
Statistical analysis
Data were analyzed by the Student’s t-test and P<0.05 was considered statistically significant.
Results and Discussion
Induction of Egr-1 by metabolic stress and ROS
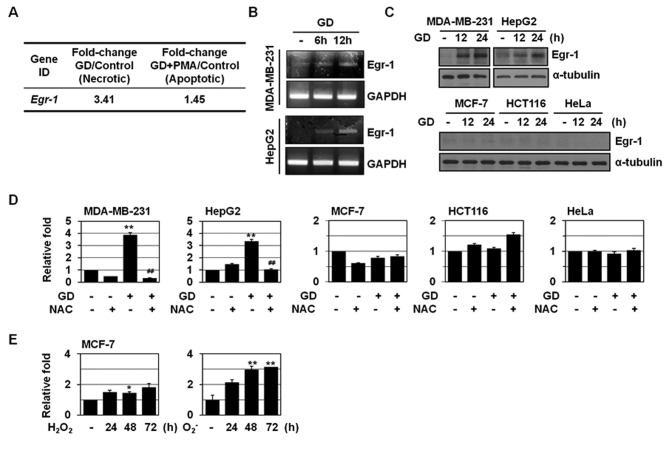
We analyzed the gene expression profiling of A549 cells that were treated with GD or GD+PMA by cDNA microarrays (21). One of GD-induced genes was Egr-1 (Fig. 1A); Egr-1 level was increased 3.4-fold during necrosis, whereas its level was not significantly changed during apoptosis. The induction of Egr-1 by GD was also observed in MDA-MB-231 and HepG2 cells that underwent necrosis upon GD, as revealed by RT-PCR (Fig. 1B). We further examined GD induction of Egr-1 in other cancer cells. Western blot analysis showed the induction of Egr-1 by GD in MDA-MB-231 and HepG2 cells, but not in MCF-7 cells that exhibit necrosis to a much lower degree than MDA-MB-231 and HepG2 cells upon GD and in HCT116 and HeLa cells that exhibit apoptosis upon exposure to GD (Fig. 1C). Real-time PCR confirmed the induction of Egr-1 by GD in MDA-MB-231 and HepG2 cells (3- to 4-fold), and but not in MCF-7, HCT116, and HeLa cells (Fig. 1D). GD is known to induce necrosis by increasing mitochondrial ROS production. Thus, we examined the possible role(s) of ROS in GD-induced Egr-1 expression. Egr-1 induction by GD was inhibited by treatment with the antioxidant N-acetylcysteine (NAC) in MDA-MB-231 and HepG2 cells (Fig. 1D). In addition, H2O2 and menadione (an O2- generator) increased Egr-1 mRNA expression in MCF-7 cells as determined by real-time PCR (Fig. 1E), indicating the redox-sensitivity of Egr-1 expression. H2O2 at non-toxic doses has been shown to induce the accumulation of mRNA for Egr-1 gene in mammalian cells (24).
Figure 1.
Induction of Egr-1 during GD-induced necrosis. (A) A549 cells were pretreated with PMA and treated with GD for 12 h and microarray analysis was performed. The numbers mean fold increase in expression as compared with GD-untreated control cells. (B) MDA-MB-231 and HepG2 cells were exposed to GD medium for the indicated times, and then analyzed by RT-PCR for Egr-1 and GAPDH. (C) Several cancer cells including MDA-MB-231, HepG2, MCF-7, HCT116, and HeLa were exposed to GD for the indicated times, and then analyzed by western blotting for Egr-1 and α-tubulin. (D) Cancer cells were pretreated with NAC (10 mM) for 1 h, exposed to GD medium for 12 h, and then analyzed by real-time PCR for Egr-1 and β-actin. Results are expressed as mean ± SE. **P<0.01 versus untreated; ##P<0.01 versus GD-treated cells. (E) MCF-7 cells were treated with H2O2 (300 μM) or menadione (O2-, 10 μM) for the indicated times and then analyzed by real-time PCR for Egr-1 and β-actin. Results are expressed as mean ± SE. *P<0.05, **P<0.01 versus untreated.
Egr-1 shRNA prevented metabolic stress-induced necrosis and HMGB1 release
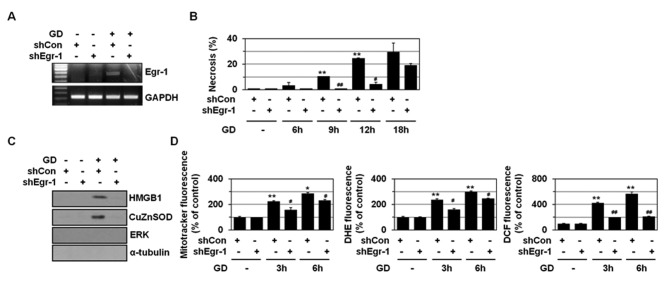
To explore the role(s) of Egr-1 in necrosis, we examined the effects of Egr-1 shRNA, directed to the C-terminal region of human Egr-1 mRNA sequences (position from 1630 to 1648 in human cDNA, Table I), on GD-induced necrosis. HO/PI double staining method was used to identify apoptosis as well as necrosis. While HO penetrates non-selectively plasma membrane of both damaged and intact cells and binds to DNA, causing a blue nuclear fluorescence, PI penetrates only cells with damaged-membranes, causing red nuclear fluorescence. Thus, the cell death mode could be discriminated morphologically by nuclear fluorescence images: intact blue nuclei, condensed/fragmented blue nuclei, condensed/fragmented pink nuclei, and intact pink nuclei indicated viable, early apoptotic, late apoptotic (secondary necrotic), and necrotic cells, respectively. Egr-1 shRNA appeared to effectively knock down Egr-1 mRNA levels in MDA-MB-231 cells, as determined by RT-PCR (Fig. 2A). Egr-1 shRNA significantly inhibited GD-induced cell rounding (data not shown) and increase in cell population that had intact pink nuclei in HO/PI staining in MDA-MB-231 cells (Fig. 2B), without increasing the population of the cells with condensed/fragmented blue nuclei and apoptotic bodies (data not shown). Thus, knockdown of Egr-1 appeared to inhibit GD-induced necrosis without switching to apoptotic cell death as an alternative death mechanism. We also observed that Egr-1 shRNA suppressed GD-induced release of HMGB1 into the extracellular space (Fig. 2C). Previously, we showed that in contrast to a general concept that necrotic cell death causes the release of most cellular proteins due to cell membrane rupture, only a restricted set of cellular proteins such as HMGB1 and CuZnSOD were selectively released during GD-induced necrosis (25). Egr-1 shRNA appeared to suppress GD-induced release of CuZnSOD into the extracellular space (Fig. 2C). These results indicate that Egr-1 may be involved in metabolic stress-induced necrosis.
Figure 2.
Egr-1 plays a role(s) in GD-induced necrosis. (A) MDA-MB-231 cells were stably transfected with control or Egr-1 shRNA and exposed to GD for 12 h and then analyzed by RT-PCR using primers for Egr-1. (B) MDA-MB-231 cells stably transfected with control or Egr-1 shRNA were exposed to GD for the indicated times, and stained with HO/PI, and observed by fluorescence microscopy, and apoptotic and necrotic cells were scored. Results are expressed as mean ± SE from 500 to 800 cells per treatment group and from three independent experiments. **P<0.01 versus control; #P<0.05, ##P<0.01 versus control shRNA. (C) MDA-MB-231 cells stably transfected with control or Egr-1 shRNA were exposed to GD for 12 h and the media were analyzed by western blotting with antibodies against HMGB1, CuZnSOD, ERK, and α-tubulin. (D) MDA-MB-231 cells stably transfected with control or Egr-1 shRNA were exposed to GD for 3 or 6 h, and mitochondrial ROS and O2- and intracellular H2O2 production was measured using the MitoTracker Red CM-H2XRos, DHE, and DCFH-DA, respectively, under a fluorescent microscope (X200, Carl Zeiss). Results are expressed as mean ± SE. *P<0.05, **P<0.01 versus untreated; #P<0.05, ##P<0.01 versus control shRNA.
Mitochondrial O2- is induced upon GD and mediates GD-induced necrosis and cytotoxicity (26–28). As shown in Fig. 2D, GD significantly enhanced the production of mitochondrial ROS, O2-, and intracellular H2O2, as revealed by staining with three different fluorogenic probes including MitoTracker Red CM-H2XRos, HE, and DCFH-DA, respectively. Egr-1 interference blocked GD-induced production of mitochondrial ROS, O2-, and intracellular H2O2 (Fig. 2D), indicating that Egr-1 may control necrosis through regulating GD-induced mitochondrial ROS production.
Egr-1 overexpression facilitates GD-induced necrosis
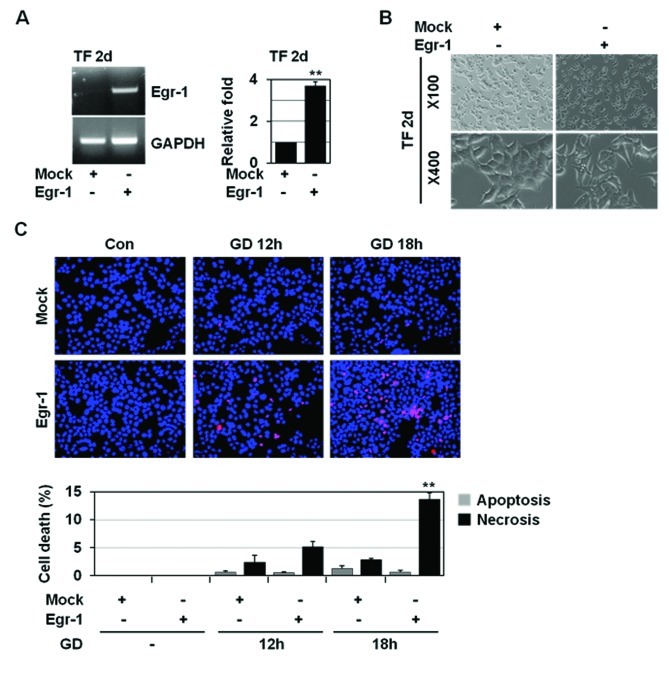
To further examine the role of Egr-1 in necrosis, we overexpressed Egr-1 in MCF-7 cells. Egr-1 is involved in HGF-induced cell scattering, migration, and invasion via Snail activation (19). Egr-1 overexpression in MCF-7 cells caused the morphological changes including loss of intercellular adhesion and formation of a spindle-like cell shape and pseudopodia, which represent the morphology typical of mesenchymal cells (Fig. 3A and B). However, it did not induce necrosis (data not shown). These results demonstrate that Egr-1 is necessary but not sufficient to trigger necrosis. Necrosis is closely linked to excess ROS production, mitochondrial dysfunction, and decreased ATP production (29,30). Thus, Egr-1 may trigger necrosis if tumour cells are under metabolic stress. Therefore, we examined whether Egr-1 overexpression could facilitate GD-inducible necrosis in MCF-7 cells that exhibit a much lower degree (approximately 2–3%) of necrosis upon GD. In MCF-7 cells, Egr-1 overexpression slightly, but to a statistically significant extent (12–13%), increased necrosis upon GD (Fig. 3C), indicating the necrosis-facilitating activity of Egr-1.
Figure 3.
Egr-1 overexpression triggers GD-induced necrosis in MCF-7. (A and B) MCF-7 cells were transiently transfected with a pcDNA3.1-Egr-1 expression vector for 48 h and Egr-1 expression was analyzed using RT-PCR and real-time PCR (n=3) for Egr-1 (A) and the cell morphology was examined using phase-contrast microscopy and photographed under magnification ×100–400 (B). (C) MCF-7 cells transiently transfected with pcDNA3.1-Egr-1 plasmid for 5 days were exposed to GD for 12 and 18 h and then stained with HO/PI and observed under a fluorescence microscope and apoptotic and necrotic cells were scored. Results are expressed as mean ± SE from 500 to 800 cells per treatment group and from three independent experiments. Results are expressed as mean ± SE. **P<0.01 versus Mock.
Egr-1 shRNA prevents metabolic stress-induced necrosis in MTSs and suppresses MTS growth
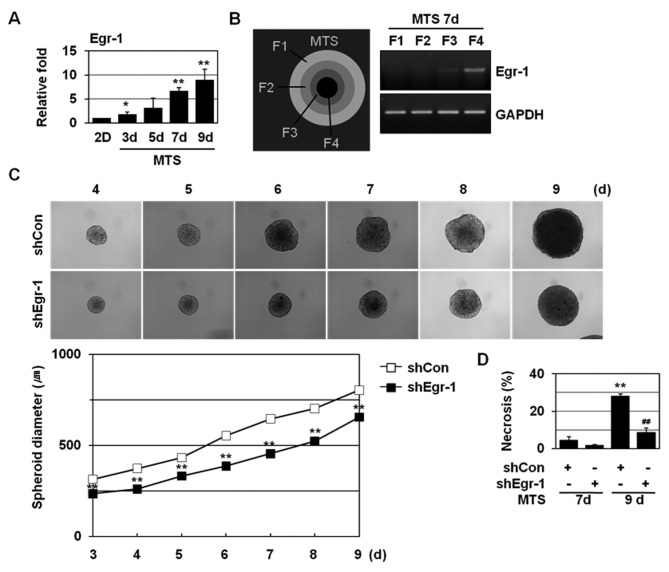
We examined the effects of Egr-1 shRNA on necrosis using MTSs. MTSs closely resemble poorly vascularized solid tumours, and thus are used for an in vitro model of solid tumours. MCF-7 cells formed tightly packed spheroids of a homogeneous size and as the MCF-7 MTSs grow, a proliferation gradient is observed, with proliferating cells at the periphery, cell cycle arrested cells in the inner regions, and necrotizing cells in the core regions (31,32). Continued MTS growth leads to the formation of necrotic core due to microenvironmental stresses including deprivation of oxygen and nutrients. H&E and HO/PI double staining revealed the necrotic core formation at 8–9 days of MTS culture (22,33). Although monolayer-cultured MCF-7 cells exhibit limited levels of necrosis upon GD, they showed prominent necrotic cell death in the core region when cultured as MTSs. An increased expression of Egr-1 was detected with extended MTS culture (Fig. 4A); Egr-1 induction was observed at 7 and 9 day MTSs. To determine the expression of Egr-1 in MTSs, the spheroids were selectively dissociated to yield cells from four discrete regions within the spheroid. Enhanced Egr-1 expression was detected in the innermost F4 fraction (Fig. 4B), indicating that Egr-1 expression is closely related to microenvironmental stresses, such as hypoxia and GD. As shown in Fig. 4C, Egr-1 shRNA prevented MTS growth. We further found that Egr-1 shRNA caused a prominent reduction in the population of cells that had pink nuclei with HO/PI staining at 9 days in MCF-7 MTS culture (Fig. 4D). These findings demonstrate that Egr-1 is involved in GD-induced necrosis and tumour progression.
Figure 4.
Egr-1 shRNA inhibits the growth of multicellular tumour spheroids (MTSs). (A) MCF-7 spheroids were seeded into 1.2% agarose-coated 96-well plates at a density of 400 cells per well and cultured for up to 9 days. The MTS was analyzed by real-time PCR for Egr-1 expression. The values are expressed as mean ± SE (n=3). *P<0.05, **P<0.01 versus two-dimensional cultured cells. (B) After 7 days of MCF-7 MTS culture, the MTSs were dissociated into subpopulations of cells from different locations in the spheroids, as described in Materials and methods. The isolated cells were analyzed by RT-PCR using primers for Egr-1 and GAPDH. (C) MTS of MCF-7 cells stably transfected with control or Egr-1 shRNA were cultured for up to 9 days. To calculate MTS size, diameters of five spheroids were measured every day. Results are expressed as mean ± SE. **P<0.01 versus control shRNA. (D) MTS of MCF-7 cells stably transfected with control or Egr-1 shRNA were cultured for 7 and 9 days and the cells were isolated and stained with HO/PI and apoptotic and necrotic cells were scored. Results are expressed as mean ± SE from 500 to 800 cells per treatment group and from three independent experiments. Results are expressed as mean ± SE. **P<0.01 versus 7 days MTS; **P<0.01 versus control shRNA.
Biological relevance of this study
An immediate early gene Egr-1 is implicated in tumour cell biology. While Egr-1 is known to induce apoptosis in tumour cells, it also promotes tumour progression, survival, and angiogenesis (6–13). In this study, we showed that Egr-1 is induced upon GD and is implicated in GD-induced necrosis. Egr-1 appeared to be induced by GD-triggered mitochondrial ROS production and to exert a positive effect on mitochondrial ROS production, in a forward feeding ROS-producing fashion. ROS induced under stressful conditions are known to move from a mitochondrion to neighboring mitochondria to enhance ROS production in a process known as ROS-induced ROS release (RIRR) (34,35). Thus, Egr-1 may be implicated in the mechanisms for RIRR, which is responsible for GD-induced necrosis. How does Egr-1 affect mitochondrial ROS production upon GD? Mitochondrial dysfunction has been shown to be linked to increased ROS production and necrosis induction. For instance, tumour cells with dysregulated mitochondria undergo necrosis in response to a glycolysis inhibitor 2-deoxyglucose or alkylating DNA damage that causes rapid ATP depletion (36). Thus, Egr-1 may regulate genes linked to mitochondrial dysfunction. Previously, we showed that Snail (22) and Dlx-2 (37) are also implicated in GD-induced necrosis; thus, Snail, Dlx-2 and Egr-1 may cooperate to induce necrosis. We further showed that Snail suppressed mitochondrial respiration and cytochrome C oxidase (COX) activity by inhibiting the expression of 3 COX subunits, including COXVIc, COXVIIa, and COXVIIc (38). Because Egr-1 is able to induce Snail (data not shown), Snail may be responsible for Egr-1-triggered necrosis. The mechanism whereby Egr-1 and Snail enhance mitochondrial depression, mitochondrial ROS production, and necrosis is under investigation.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2009-0072912). We thank Dr Thomas E. Eling (Laboratory of Molecular Carcinogenesis, National Institute of Environmental Health Sciences, USA) and Dr J.I. Yook (University of Yonsei, Korea) for providing pcDNA3.1-Egr-1 and MCF-7 cells, respectively.
References
- 1.Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–648. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 2.Jin S, White E. Role of autophagy in cancer: management of metabolic stress. Autophagy. 2007;3:28–31. doi: 10.4161/auto.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 4.Schlueter C, Weber H, Meyer B, Rogalla P, Roser K, Hauke S, Bullerdiek J. Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic switch molecule. Am J Pathol. 2005;166:1259–1263. doi: 10.1016/S0002-9440(10)62344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 6.Adamson ED, Mercola D. Egr1 transcription factor: multiple roles in prostate tumor cell growth and survival. Tumour Biol. 2002;23:93–102. doi: 10.1159/000059711. [DOI] [PubMed] [Google Scholar]
- 7.Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol. 2002;193:287–292. doi: 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- 8.Xie B, Wang C, Zheng Z, Song B, Ma C, Thiel G, Li M. Egr-1 transactivates Bim gene expression to promote neuronal apoptosis. J Neurosci. 2011;31:5032–5044. doi: 10.1523/JNEUROSCI.5504-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi H, Chen CT, Chou CK, Pal A, Bornmann W, Hortobagyi GN, Hung MC. Adenovirus 5 E1A enhances histone deacetylase inhibitors-induced apoptosis through Egr-1-mediated Bim upregulation. Oncogene. 2010;29:5619–5629. doi: 10.1038/onc.2010.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahalingam D, Natoni A, Keane M, Samali A, Szegezdi E. Early growth response-1 is a regulator of DR5-induced apoptosis in colon cancer cells. Br J Cancer. 2010;102:754–764. doi: 10.1038/sj.bjc.6605545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner M, Schmelz K, Dorken B, Tamm I. Transcriptional regulation of human survivin by early growth response (Egr)-1 transcription factor. Int J Cancer. 2008;122:1278–1287. doi: 10.1002/ijc.23183. [DOI] [PubMed] [Google Scholar]
- 12.Zagurovskaya M, Shareef MM, Das A, Reeves A, Gupta S, Sudol M, Bedford MT, Prichard J, Mohiuddin M, Ahmed MM. EGR-1 forms a complex with YAP-1 and upregulates Bax expresion in irradiated prostate carcinoma cells. Oncogene. 2009;28:1121–1131. doi: 10.1038/onc.2008.461. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed MM. Regulation of radiation-induced apoptosis by early growth response-1 gene in solid tumors. Curr Cancer Drug Targets. 2004;4:43–52. doi: 10.2174/1568009043481704. [DOI] [PubMed] [Google Scholar]
- 14.Baron V, Adamson ED, Calogero A, Ragona G, Mercola D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther. 2006;13:115–124. doi: 10.1038/sj.cgt.7700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rong Y, Hu F, Huang R, Mackman N, Horowitz JM, Jensen RL, Durden DL, Van Meir EG, Brat DJ. Early growth response gene-1 regulates hypoxia-induced expression of tissue factor in glioblastoma multiforme through hypoxia-inducible factor-1-independent mechanisms. Cancer Res. 2006;66:7067–7074. doi: 10.1158/0008-5472.CAN-06-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao H, Hyman MC, Lawrence DA, Pinsky DJ. Molecular regulation of the PAI-1 gene by hypoxia: contributions of Egr-1, HIF-1alpha, and C/EBPalpha. FASEB J. 2007;21:935–949. doi: 10.1096/fj.06-6285com. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P, Tchou-Wong KM, Costa M. Egr-1 mediates hypoxia-inducible transcription of the NDRG1 gene through an overlapping Egr-1/Sp1 binding site in the promoter. Cancer Res. 2007;67:9125–9133. doi: 10.1158/0008-5472.CAN-07-1525. [DOI] [PubMed] [Google Scholar]
- 18.Nishi H, Nishi KH, Johnson AC. Early growth response-1 gene mediates up-regulation of epidermal growth factor receptor expression during hypoxia. Cancer Res. 2002;62:827–834. [PubMed] [Google Scholar]
- 19.Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J. 2006;25:3534–3545. doi: 10.1038/sj.emboj.7601213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucerna M, Pomyje J, Mechtcheriakova D, Kadl A, Gruber F, Bilban M, Sobanov Y, Schabbauer G, Breuss J, Wagner O, Bischoff M, Clauss M, Binder BR, Hofer E. Sustained expression of early growth response protein-1 blocks angiogenesis and tumor growth. Cancer Res. 2006;66:6708–6713. doi: 10.1158/0008-5472.CAN-05-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CH, Han SI, Lee SY, Youk HS, Moon JY, Duong HQ, Park MJ, Joo YM, Park HG, Kim YJ, Yoo MA, Lim SC, Kang HS. Protein kinase C-ERK1/2 signal pathway switches glucose depletion-induced necrosis to apoptosis by regulating superoxide dismutases and suppressing reactive oxygen species production in A549 lung cancer cells. J Cell Physiol. 2007;211:371–385. doi: 10.1002/jcp.20941. [DOI] [PubMed] [Google Scholar]
- 22.Kim CH, Jeon HM, Lee SY, Ju MK, Moon JY, Park HG, Yoo MA, Choi BT, Yook JI, Lim SC, Han SI, Kang HS. Implication of snail in metabolic stress-induced necrosis. PLoS One. 2011;6:e18000. doi: 10.1371/journal.pone.0018000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaRue KE, Khalil M, Freyer JP. Microenvironmental regulation of proliferation in multicellular spheroids is mediated through differential expression of cyclin-dependent kinase inhibitors. Cancer Res. 2004;64:1621–1631. doi: 10.1158/0008-5472.can-2902-2. [DOI] [PubMed] [Google Scholar]
- 24.Nose K, Ohba M. Functional activation of the egr-1 (early growth response-1) gene by hydrogen peroxide. Biochem J. 1996;316:381–383. doi: 10.1042/bj3160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han SI, Duong HQ, Choi JE, Lee TB, Kim CH, Lee SY, Jeon HM, Shin SH, Lim SC, Kang HS. Hyperthermia switches glucose depletion-induced necrosis to apoptosis in A549 lung adenocarcinoma cells. Int J Oncol. 2008;32:851–860. [PubMed] [Google Scholar]
- 26.Ahmad IM, Aykin-Burns N, Sim JE, Walsh SA, Higashikubo R, Buettner GR, Venkataraman S, Mackey MA, Flanagan SW, Oberley LW, Spitz DR. Mitochondrial O2*- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem. 2005;280:4254–4263. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 27.Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann NY Acad Sci. 2000;899:349–362. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 28.Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418:29–37. doi: 10.1042/BJ20081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 30.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Horning JL, Sahoo SK, Vijayaraghavalu S, Dimitrijevic S, Vasir JK, Jain TK, Panda AK, Labhasetwar V. 3-D tumor model for in vitro evaluation of anticancer drugs. Mol Pharm. 2008;5:849–862. doi: 10.1021/mp800047v. [DOI] [PubMed] [Google Scholar]
- 32.Ivascu A, Kubbies M. Diversity of cell-mediated adhesions in breast cancer spheroids. Int J Oncol. 2007;31:1403–1413. [PubMed] [Google Scholar]
- 33.Jeong EK, Lee SY, Jeon HM, Ju MK, Kim CH, Kang HS. Role of extracellular signal-regulated kinase (ERK)1/2 in multicellular resistance to docetaxel in MCF-7 cells. Int J Oncol. 2010;37:655–661. doi: 10.3892/ijo_00000714. [DOI] [PubMed] [Google Scholar]
- 34.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 36.Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SY, Jeon HM, Kim CH, Ju MK, Bae HS, Park HG, Lim SC, Han SI, Kang HS. Homeobox gene Dlx-2 is implicated in metabolic stress-induced necrosis. Mol Cancer. 2011;10:113. doi: 10.1186/1476-4598-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SY, Jeon HM, Ju MK, Kim CH, Yoon G, Han SI, Park HG, Kang HS. Wnt/Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Res. 2012;72:3607–3617. doi: 10.1158/0008-5472.CAN-12-0006. [DOI] [PubMed] [Google Scholar]