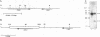Abstract
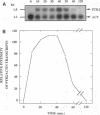
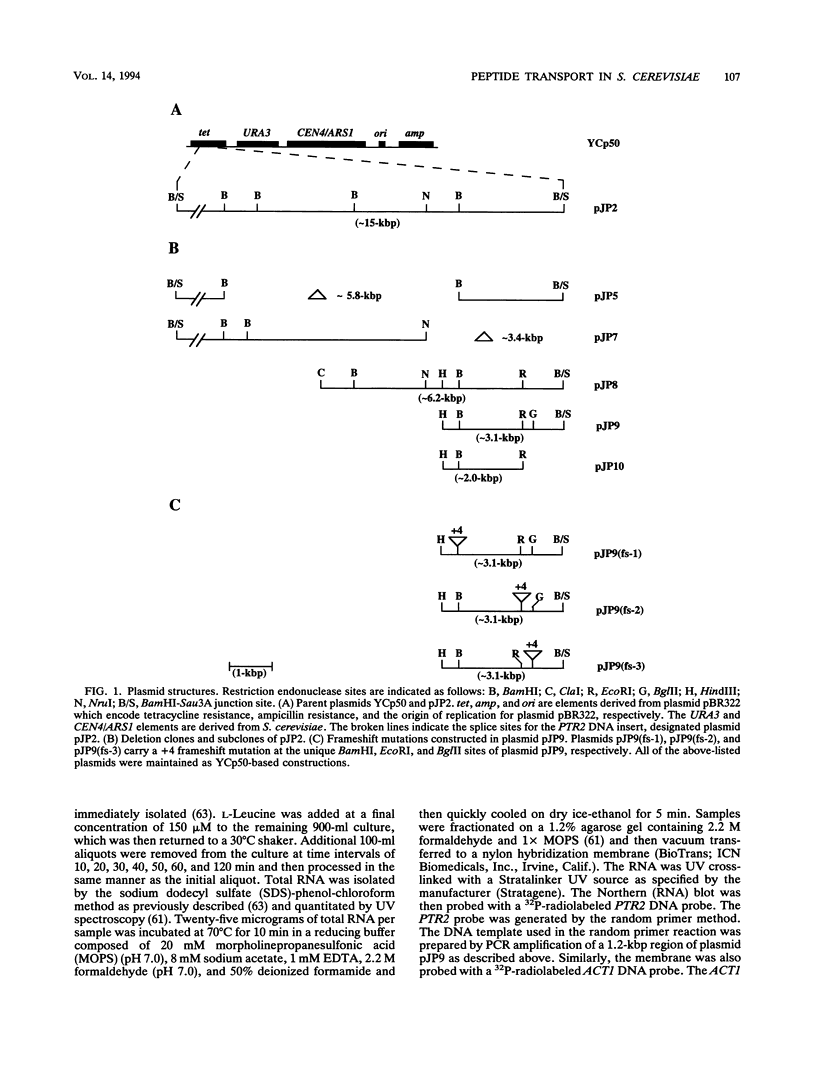
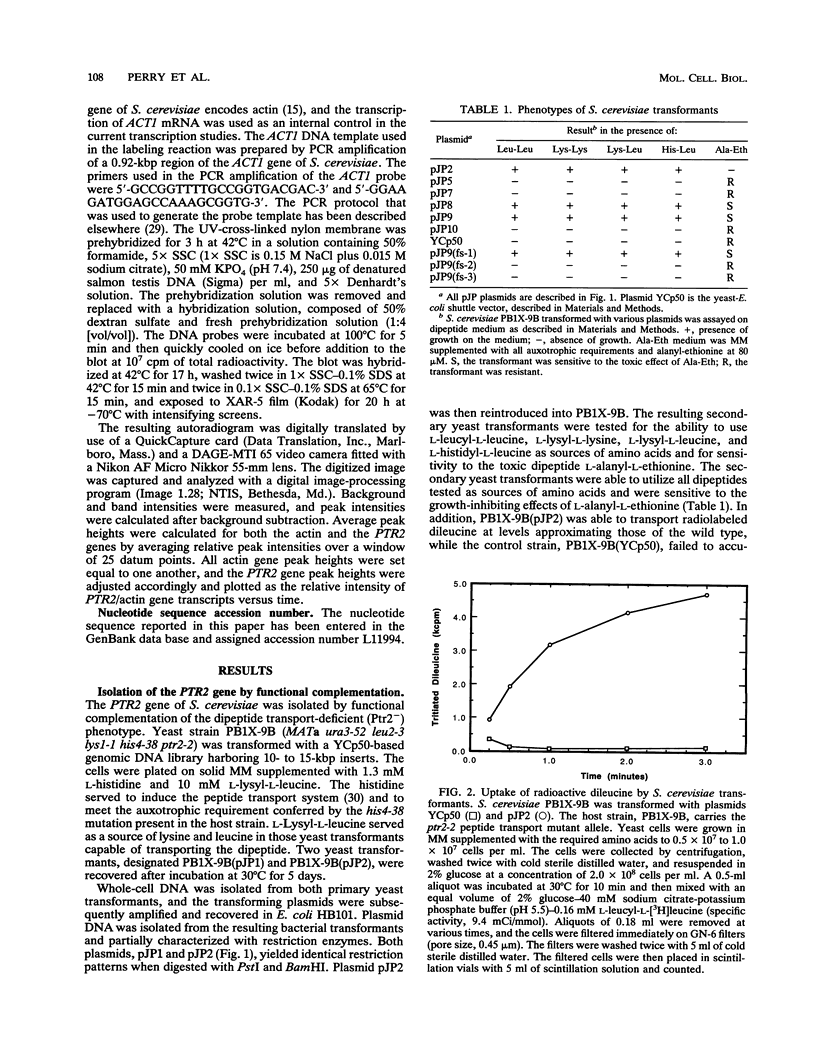
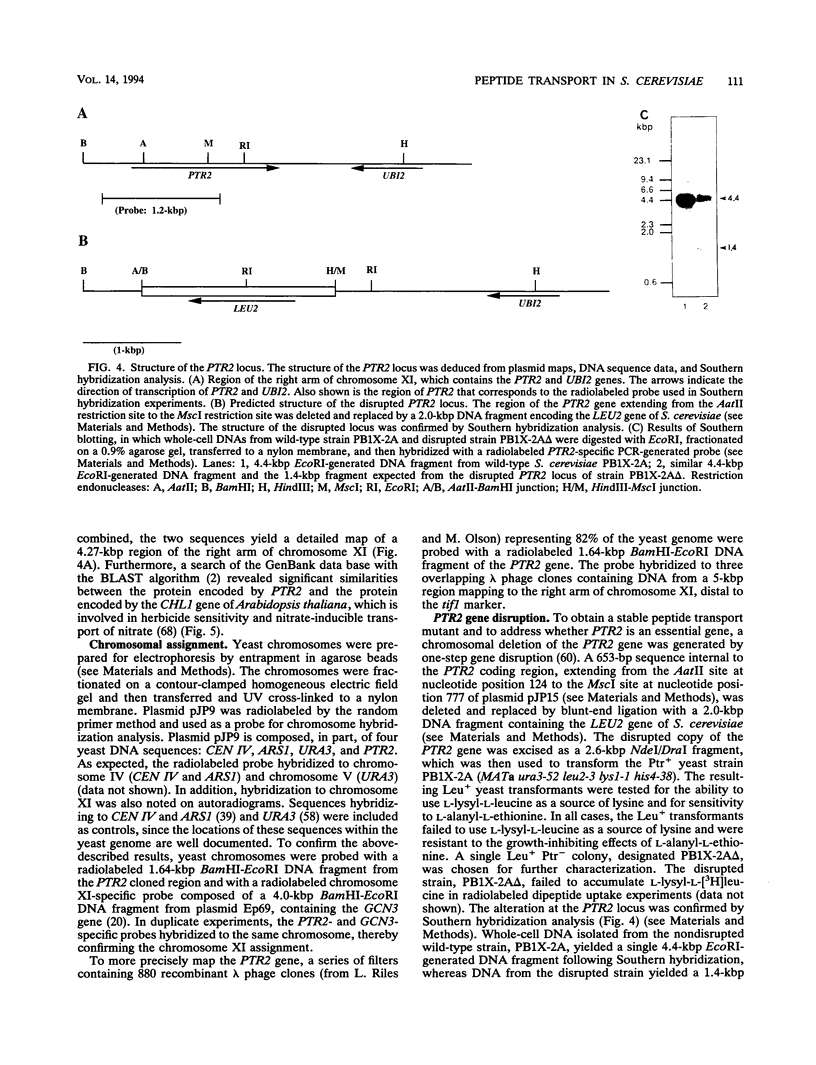
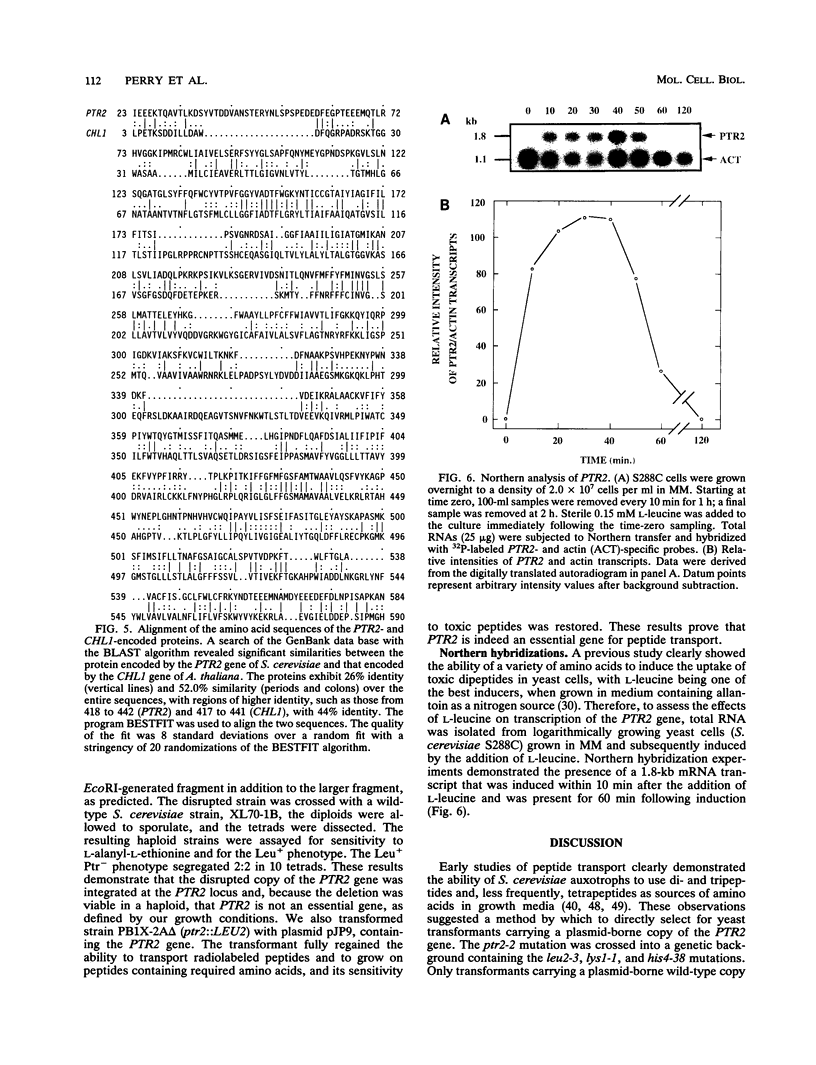
We have cloned and characterized a Saccharomyces cerevisiae peptide transport gene (PTR2) isolated from a genomic DNA library by directly selecting for functional complementation of a peptide transport-deficient mutant. Deletion and frameshift mutageneses were used to localize the complementing activity to a 3.1-kbp region on the transforming plasmid. DNA sequencing of the complementing region identified an open reading frame spanning 1,803 bp. The deduced amino acid sequence predicts a hydrophobic peptide consisting of 601 amino acids, having a molecular mass of 68.1 kDa, composed in part of 12 hydrophobic segments, and sharing significant similarities with a nitrate transport protein encoded by the CHL1 gene of Arabidopsis thaliana. Northern (RNA) hybridization experiments demonstrated a single transcript that was 1.8 kb in length and that was transiently induced by the addition of L-leucine to the growth medium. The PTR2 gene was localized to the right arm of chromosome XI by contour-clamped homogeneous electric field gel chromosome blotting and by hybridization to known chromosome XI lambda phage clones of S. cerevisiae DNA. PTR2 was tightly linked to the UBI2 gene, with the coding sequences being separated by a 466-bp region and oriented so that the genes were transcribed convergently. A chromosomal disruption of the PTR2 gene in a haploid strain was not lethal under standard growth conditions. The cloning of PTR2 represents the first example of the molecular genetic characterization of a eucaryotic peptide transport gene.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abouhamad W. N., Manson M., Gibson M. M., Higgins C. F. Peptide transport and chemotaxis in Escherichia coli and Salmonella typhimurium: characterization of the dipeptide permease (Dpp) and the dipeptide-binding protein. Mol Microbiol. 1991 May;5(5):1035–1047. doi: 10.1111/j.1365-2958.1991.tb01876.x. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Ames G. F., Young J. D., Tsuchiya D., Lecocq J. Illicit transport: the oligopeptide permease. Proc Natl Acad Sci U S A. 1973 Feb;70(2):456–458. doi: 10.1073/pnas.70.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J. C., Short S. A. Genetic analysis of Escherichia coli oligopeptide transport mutants. J Bacteriol. 1985 Feb;161(2):484–492. doi: 10.1128/jb.161.2.484-492.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basrai M. A., Zhang H. L., Miller D., Naider F., Becker J. M. Toxicity of oxalysine and oxalysine-containing peptides against Candida albicans: regulation of peptide transport by amino acids. J Gen Microbiol. 1992 Nov;138(11):2353–2362. doi: 10.1099/00221287-138-11-2353. [DOI] [PubMed] [Google Scholar]
- Becker J. M., Covert N. L., Shenbagamurthi P., Steinfeld A. S., Naider F. Polyoxin D inhibits growth of zoopathogenic fungi. Antimicrob Agents Chemother. 1983 Jun;23(6):926–929. doi: 10.1128/aac.23.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekcer J. M., Naider F. Peptide transport in yeast: uptake of radioactive trimethionine in Saccharomyces cerevisiae. Arch Biochem Biophys. 1977 Jan 15;178(1):245–255. doi: 10.1016/0003-9861(77)90189-8. [DOI] [PubMed] [Google Scholar]
- Bysani N., Daugherty J. R., Cooper T. G. Saturation mutagenesis of the UASNTR (GATAA) responsible for nitrogen catabolite repression-sensitive transcriptional activation of the allantoin pathway genes in Saccharomyces cerevisiae. J Bacteriol. 1991 Aug;173(16):4977–4982. doi: 10.1128/jb.173.16.4977-4982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Davies M. B. Peptide uptake in Candida albicans. J Gen Microbiol. 1980 Nov;121(1):181–186. doi: 10.1099/00221287-121-1-181. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickel T. E., Gilvarg C. Transport of impermeant substances in E. coli by way of oligopeptide permease. Nat New Biol. 1973 Feb 7;241(110):161–163. doi: 10.1038/newbio241161a0. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Sures I. Structure of a split yeast gene: complete nucleotide sequence of the actin gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 May;77(5):2546–2550. doi: 10.1073/pnas.77.5.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. M., Price M., Higgins C. F. Genetic characterization and molecular cloning of the tripeptide permease (tpp) genes of Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):122–130. doi: 10.1128/jb.160.1.122-130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Higgins C. F. Uptake of cell wall peptides by Salmonella typhimurium and Escherichia coli. J Bacteriol. 1987 Aug;169(8):3861–3865. doi: 10.1128/jb.169.8.3861-3865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S. M., Claesson A., Jansson A. M., Larsson L. G., Pring B. G., Town C. M., Ekström B. A new class of synthetic antibacterials acting on lipopolysaccharide biosynthesis. 1987 Jun 25-Jul 1Nature. 327(6124):730–732. doi: 10.1038/327730a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hannig E. M., Hinnebusch A. G. Molecular analysis of GCN3, a translational activator of GCN4: evidence for posttranslational control of GCN3 regulatory function. Mol Cell Biol. 1988 Nov;8(11):4808–4820. doi: 10.1128/mcb.8.11.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Higgins C. Microbiology. Synthesizing designer drugs. 1987 Jun 25-Jul 1Nature. 327(6124):655–656. doi: 10.1038/327655a0. [DOI] [PubMed] [Google Scholar]
- Hiles I. D., Gallagher M. P., Jamieson D. J., Higgins C. F. Molecular characterization of the oligopeptide permease of Salmonella typhimurium. J Mol Biol. 1987 May 5;195(1):125–142. doi: 10.1016/0022-2836(87)90332-9. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G. The general control of amino acid biosynthetic genes in the yeast Saccharomyces cerevisiae. CRC Crit Rev Biochem. 1986;21(3):277–317. doi: 10.3109/10409238609113614. [DOI] [PubMed] [Google Scholar]
- Hogarth B. G., Higgins C. F. Genetic organization of the oligopeptide permease (opp) locus of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Mar;153(3):1548–1551. doi: 10.1128/jb.153.3.1548-1551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C., Meeks-Wagner D. W., Fangman W. L., Botstein D. A rapid, efficient method for isolating DNA from yeast. Gene. 1986;42(2):169–173. doi: 10.1016/0378-1119(86)90293-3. [DOI] [PubMed] [Google Scholar]
- Island M. D., Naider F., Becker J. M. Regulation of dipeptide transport in Saccharomyces cerevisiae by micromolar amino acid concentrations. J Bacteriol. 1987 May;169(5):2132–2136. doi: 10.1128/jb.169.5.2132-2136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Island M. D., Perry J. R., Naider F., Becker J. M. Isolation and characterization of S. cerevisiae mutants deficient in amino acid-inducible peptide transport. Curr Genet. 1991 Dec;20(6):457–463. doi: 10.1007/BF00334772. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Two genetically distinct pathways for transcriptional regulation of anaerobic gene expression in Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):389–397. doi: 10.1128/jb.168.1.389-397.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. S., Prakash L. Yeast Saccharomyces cerevisiae selectable markers in pUC18 polylinkers. Yeast. 1990 Sep-Oct;6(5):363–366. doi: 10.1002/yea.320060502. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Sterne R. E., Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989 Dec 20;8(13):3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lichliter W. D., Naider F., Becker J. M. Basis for the design of anticandidal agents from studies of peptide utilization in Canadida albicans. Antimicrob Agents Chemother. 1976 Sep;10(3):483–490. doi: 10.1128/aac.10.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan D. A., Becker J. M., Naider F. Peptide transport in Candida albicans. J Gen Microbiol. 1979 Sep;114(1):179–186. doi: 10.1099/00221287-114-1-179. [DOI] [PubMed] [Google Scholar]
- Mann C., Davis R. W. Structure and sequence of the centromeric DNA of chromosome 4 in Saccharomyces cerevisiae. Mol Cell Biol. 1986 Jan;6(1):241–245. doi: 10.1128/mcb.6.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder R., Becker J. M., Naider F. Peptide transport in yeast: utilization of leucine- and lysine-containing peptides by Saccharomyces cerevisiae. J Bacteriol. 1977 Sep;131(3):906–916. doi: 10.1128/jb.131.3.906-916.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy P. J., Nisbet L. J., Boehm J. C., Kingsbury W. D. Multiplicity of peptide permeases in Candida albicans: evidence from novel chromophoric peptides. J Bacteriol. 1985 Jun;162(3):1024–1029. doi: 10.1128/jb.162.3.1024-1029.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989 Aug 3;340(6232):400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Mehta R. J., Kingsbury W. D., Valenta J., Actor P. Anti-Candida activity of polyoxin: example of peptide transport in yeasts. Antimicrob Agents Chemother. 1984 Mar;25(3):373–374. doi: 10.1128/aac.25.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naider F., Becker J. M., Katzir-Katchalski E. Utilization of methionine-containing peptides and their derivatives by a methionine-requiring auxotroph of Saccharomyces cerevisiae. J Biol Chem. 1974 Jan 10;249(1):9–20. [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Genetic applications of yeast transformation with linear and gapped plasmids. Methods Enzymol. 1983;101:228–245. doi: 10.1016/0076-6879(83)01017-4. [DOI] [PubMed] [Google Scholar]
- Ozkaynak E., Finley D., Solomon M. J., Varshavsky A. The yeast ubiquitin genes: a family of natural gene fusions. EMBO J. 1987 May;6(5):1429–1439. doi: 10.1002/j.1460-2075.1987.tb02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W., Gilvarg C. Size restriction on peptide utilization in Escherichia coli. J Biol Chem. 1968 Dec 10;243(23):6291–6299. [PubMed] [Google Scholar]
- Payne J. W. Oligopeptide transport in Escherichia coli. Specificity with respect to side chain and distinction from dipeptide transport. J Biol Chem. 1968 Jun 25;243(12):3395–3403. [PubMed] [Google Scholar]
- Pikielny C. W., Teem J. L., Rosbash M. Evidence for the biochemical role of an internal sequence in yeast nuclear mRNA introns: implications for U1 RNA and metazoan mRNA splicing. Cell. 1983 Sep;34(2):395–403. doi: 10.1016/0092-8674(83)90373-2. [DOI] [PubMed] [Google Scholar]
- Rai R., Genbauffe F. S., Cooper T. G. Structure and transcription of the allantoate permease gene (DAL5) from Saccharomyces cerevisiae. J Bacteriol. 1988 Jan;170(1):266–271. doi: 10.1128/jb.170.1.266-271.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rose M., Grisafi P., Botstein D. Structure and function of the yeast URA3 gene: expression in Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):113–124. doi: 10.1016/0378-1119(84)90172-0. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. E., Brown T. A., Trumpower B. L. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990 May 25;18(10):3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Molecular mechanisms of transcriptional regulation in yeast. Annu Rev Biochem. 1989;58:1051–1077. doi: 10.1146/annurev.bi.58.070189.005155. [DOI] [PubMed] [Google Scholar]
- Tsay Y. F., Schroeder J. I., Feldmann K. A., Crawford N. M. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell. 1993 Mar 12;72(5):705–713. doi: 10.1016/0092-8674(93)90399-b. [DOI] [PubMed] [Google Scholar]
- Weber E., Chevallier M. R., Jund R. Evolutionary relationship and secondary structure predictions in four transport proteins of Saccharomyces cerevisiae. J Mol Evol. 1988;27(4):341–350. doi: 10.1007/BF02101197. [DOI] [PubMed] [Google Scholar]
- Yadan J. C., Gonneau M., Sarthou P., Le Goffic F. Sensitivity to nikkomycin Z in Candida albicans: role of peptide permeases. J Bacteriol. 1984 Dec;160(3):884–888. doi: 10.1128/jb.160.3.884-888.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos P. H., Morris L. M., Hellwig R. J. A novel method for rapid isolation of plasmid DNA. Biotechniques. 1988 Mar;6(3):238–242. [PubMed] [Google Scholar]