Abstract
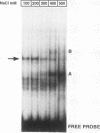
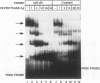
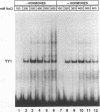
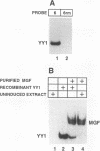
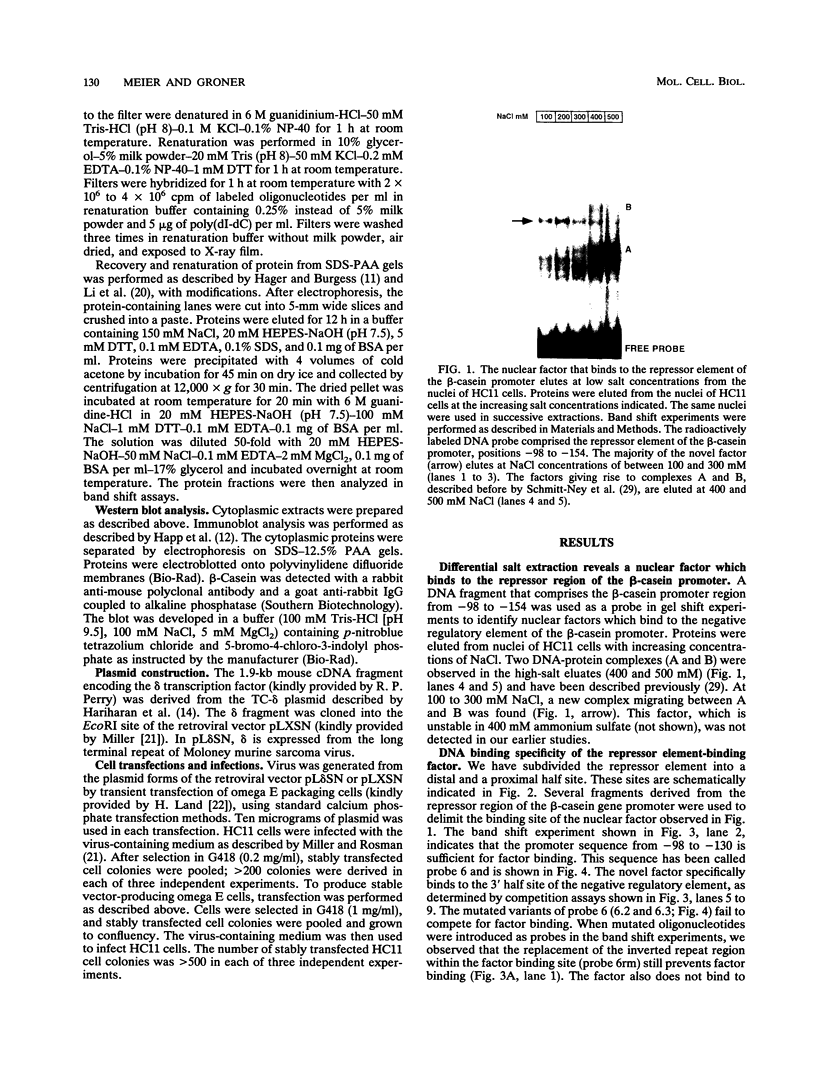
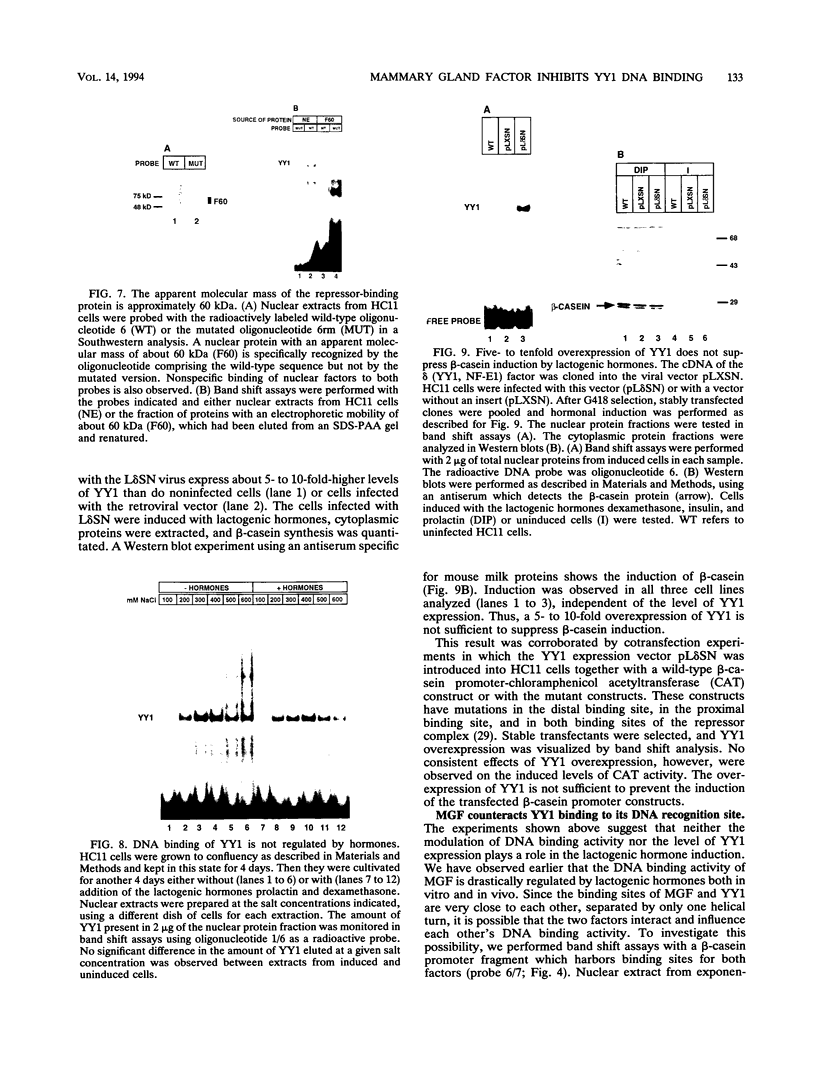
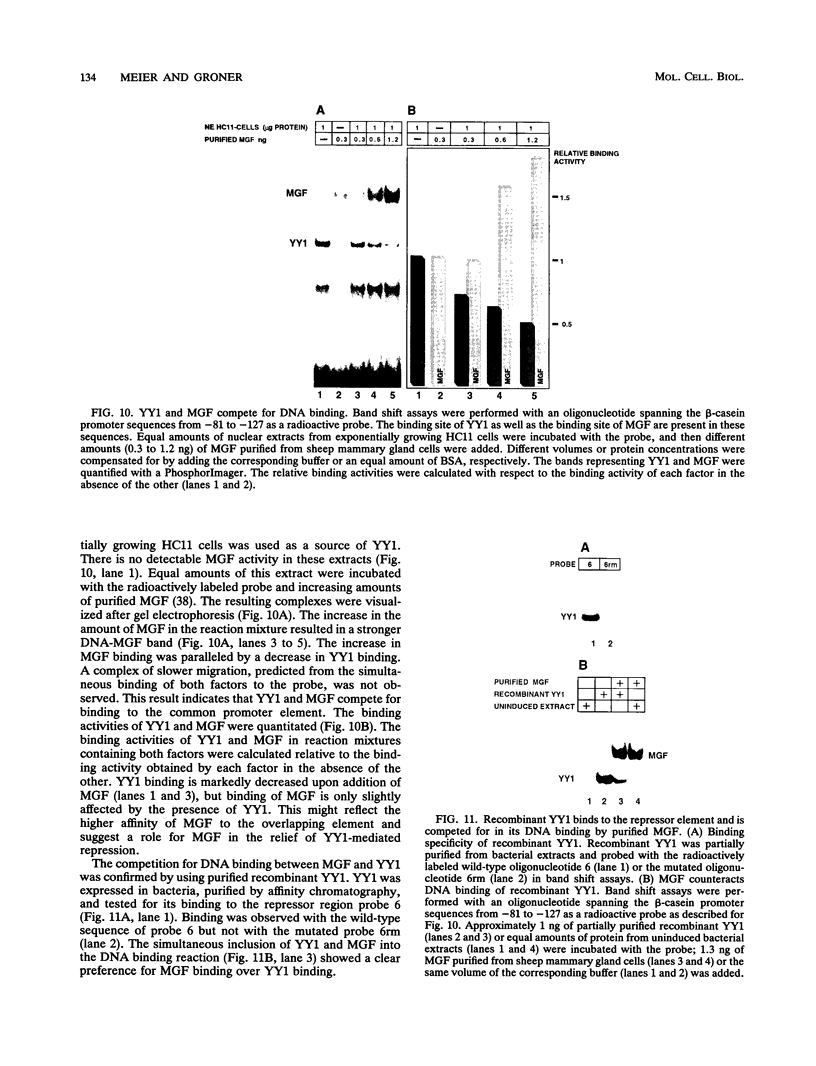
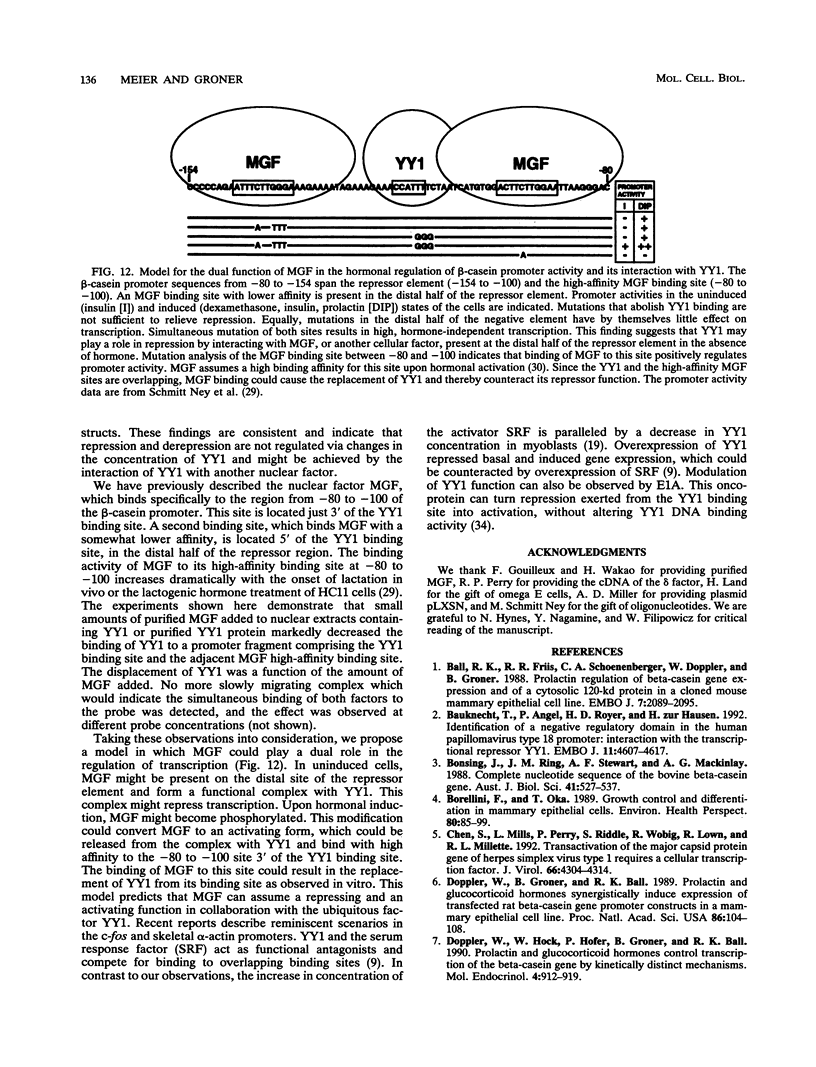
Expression of the beta-casein milk protein gene in the mammary epithelial cell line HC11 is primarily regulated at the transcriptional level. A 338-bp segment of promoter sequence 5' of the transcription start site is sufficient to confer inducibility by the lactogenic hormones insulin, glucocorticoid hormone, and prolactin. Positively and negatively acting promoter elements and specific DNA binding proteins have been identified. The binding of the mammary gland factor MGF to a site between -80 and -100 is indispensable for hormonal induction of transcription. Binding of MGF activity to DNA is greatly enhanced by the action of the lactogenic hormones. Repression of transcription in the uninduced state is mediated by a promoter element located adjacent to the MGF binding site at positions -110 to -150. This repressor element consists of two interacting protein binding sites. A nuclear factor that binds specifically to the proximal site between positions -110 and -120 has been characterized and found to be identical with the nuclear factor YY1 (delta, NF-E1). YY1 does not bind to the distal site. The simultaneous mutation in the proximal and the distal sites results in high, hormone-independent transcription. This finding suggests that YY1 plays a functional role in the repression and acts in conjunction with a second DNA binding protein. Comparison of YY1 DNA binding activity in uninduced and hormone-induced cells showed that relief of repression is not mediated by changes in the concentration or binding affinity of YY1. Infection of HC11 cells with a YY1-expressing recombinant retrovirus resulted in overexpression of YY1 but did not suppress hormonal induction. The addition of purified MGF decreased YY1 binding to its DNA recognition site in vitro. This finding indicates that MGF regulates the DNA binding activity of YY1 and thereby may cause the relief of transcriptional repression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball R. K., Friis R. R., Schoenenberger C. A., Doppler W., Groner B. Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988 Jul;7(7):2089–2095. doi: 10.1002/j.1460-2075.1988.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauknecht T., Angel P., Royer H. D., zur Hausen H. Identification of a negative regulatory domain in the human papillomavirus type 18 promoter: interaction with the transcriptional repressor YY1. EMBO J. 1992 Dec;11(12):4607–4617. doi: 10.1002/j.1460-2075.1992.tb05563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsing J., Ring J. M., Stewart A. F., Mackinlay A. G. Complete nucleotide sequence of the bovine beta-casein gene. Aust J Biol Sci. 1988;41(4):527–537. doi: 10.1071/bi9880527. [DOI] [PubMed] [Google Scholar]
- Borellini F., Oka T. Growth control and differentiation in mammary epithelial cells. Environ Health Perspect. 1989 Mar;80:85–99. doi: 10.1289/ehp.898085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Mills L., Perry P., Riddle S., Wobig R., Lown R., Millette R. L. Transactivation of the major capsid protein gene of herpes simplex virus type 1 requires a cellular transcription factor. J Virol. 1992 Jul;66(7):4304–4314. doi: 10.1128/jvi.66.7.4304-4314.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler W., Groner B., Ball R. K. Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat beta-casein gene promoter constructs in a mammary epithelial cell line. Proc Natl Acad Sci U S A. 1989 Jan;86(1):104–108. doi: 10.1073/pnas.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler W., Höck W., Hofer P., Groner B., Ball R. K. Prolactin and glucocorticoid hormones control transcription of the beta-casein gene by kinetically distinct mechanisms. Mol Endocrinol. 1990 Jun;4(6):912–919. doi: 10.1210/mend-4-6-912. [DOI] [PubMed] [Google Scholar]
- Flanagan J. R., Becker K. G., Ennist D. L., Gleason S. L., Driggers P. H., Levi B. Z., Appella E., Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992 Jan;12(1):38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto A., LePage D., Pons G., Mader S. L., Park K., Atchison M. L., Walsh K. Functional antagonism between YY1 and the serum response factor. Mol Cell Biol. 1992 Sep;12(9):4209–4214. doi: 10.1128/mcb.12.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio D. L., Heilstedt-Williamson H., Gray T. A., Tarlé S. A., Shelton D. A., Tagle D. A., Slightom J. L., Goodman M., Collins F. S. Phylogenetic footprinting reveals a nuclear protein which binds to silencer sequences in the human gamma and epsilon globin genes. Mol Cell Biol. 1992 Nov;12(11):4919–4929. doi: 10.1128/mcb.12.11.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Happ B., Hynes N. E., Groner B. Ha-ras and v-raf oncogenes, but not int-2 and c-myc, interfere with the lactogenic hormone dependent activation of the mammary gland specific transcription factor. Cell Growth Differ. 1993 Jan;4(1):9–15. [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Delta, a transcription factor that binds to downstream elements in several polymerase II promoters, is a functionally versatile zinc finger protein. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9799–9803. doi: 10.1073/pnas.88.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Equipotent mouse ribosomal protein promoters have a similar architecture that includes internal sequence elements. Genes Dev. 1989 Nov;3(11):1789–1800. doi: 10.1101/gad.3.11.1789. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Taverna D., Harwerth I. M., Ciardiello F., Salomon D. S., Yamamoto T., Groner B. Epidermal growth factor receptor, but not c-erbB-2, activation prevents lactogenic hormone induction of the beta-casein gene in mouse mammary epithelial cells. Mol Cell Biol. 1990 Aug;10(8):4027–4034. doi: 10.1128/mcb.10.8.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U. Double replica southwestern. Nucleic Acids Res. 1987 Jul 10;15(13):5486–5486. doi: 10.1093/nar/15.13.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. K., Yu-Lee L. Y., Clift S. M., Brown T. L., Rosen J. M. The rat casein multigene family. Fine structure and evolution of the beta-casein gene. J Biol Chem. 1985 Jun 10;260(11):7042–7050. [PubMed] [Google Scholar]
- Kakkis E., Riggs K. J., Gillespie W., Calame K. A transcriptional repressor of c-myc. Nature. 1989 Jun 29;339(6227):718–721. doi: 10.1038/339718a0. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Shi Y., Schwartz R. J. Displacement of BrdUrd-induced YY1 by serum response factor activates skeletal alpha-actin transcription in embryonic myoblasts. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9814–9818. doi: 10.1073/pnas.89.20.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Morzycka-Wroblewska E., Desiderio S. V. NBP, a protein that specifically binds an enhancer of immunoglobulin gene rearrangement: purification and characterization. Genes Dev. 1989 Nov;3(11):1801–1813. doi: 10.1101/gad.3.11.1801. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990 Jun 25;18(12):3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K., Atchison M. L. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3' enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B., Merezhinskaya N., Diffley J. F., Noguchi C. T. Protein-DNA interactions in the epsilon-globin gene silencer. J Biol Chem. 1993 Feb 15;268(5):3430–3437. [PubMed] [Google Scholar]
- Riggs K. J., Merrell K. T., Wilson G., Calame K. Common factor 1 is a transcriptional activator which binds in the c-myc promoter, the skeletal alpha-actin promoter, and the immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1991 Mar;11(3):1765–1769. doi: 10.1128/mcb.11.3.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B., DiTullio P., Vitale J., Hehir K., Gordon K. Cloning of the goat beta-casein-encoding gene and expression in transgenic mice. Gene. 1992 Nov 16;121(2):255–262. doi: 10.1016/0378-1119(92)90129-d. [DOI] [PubMed] [Google Scholar]
- Schmidhauser C., Bissell M. J., Myers C. A., Casperson G. F. Extracellular matrix and hormones transcriptionally regulate bovine beta-casein 5' sequences in stably transfected mouse mammary cells. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9118–9122. doi: 10.1073/pnas.87.23.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Ney M., Doppler W., Ball R. K., Groner B. Beta-casein gene promoter activity is regulated by the hormone-mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol Cell Biol. 1991 Jul;11(7):3745–3755. doi: 10.1128/mcb.11.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Ney M., Happ B., Ball R. K., Groner B. Developmental and environmental regulation of a mammary gland-specific nuclear factor essential for transcription of the gene encoding beta-casein. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3130–3134. doi: 10.1073/pnas.89.7.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Ney M., Happ B., Hofer P., Hynes N. E., Groner B. Mammary gland-specific nuclear factor activity is positively regulated by lactogenic hormones and negatively by milk stasis. Mol Endocrinol. 1992 Dec;6(12):1988–1997. doi: 10.1210/mend.6.12.1491685. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Identification of a novel lymphoid specific octamer binding protein (OTF-2B) by proteolytic clipping bandshift assay (PCBA). EMBO J. 1988 Dec 20;7(13):4221–4229. doi: 10.1002/j.1460-2075.1988.tb03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Shi Y., Shenk T. YY1 is an initiator sequence-binding protein that directs and activates transcription in vitro. Nature. 1991 Nov 21;354(6350):241–245. doi: 10.1038/354241a0. [DOI] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Taverna D., Groner B., Hynes N. E. Epidermal growth factor receptor, platelet-derived growth factor receptor, and c-erbB-2 receptor activation all promote growth but have distinctive effects upon mouse mammary epithelial cell differentiation. Cell Growth Differ. 1991 Mar;2(3):145–154. [PubMed] [Google Scholar]
- Vincent C. K., Gualberto A., Patel C. V., Walsh K. Different regulatory sequences control creatine kinase-M gene expression in directly injected skeletal and cardiac muscle. Mol Cell Biol. 1993 Feb;13(2):1264–1272. doi: 10.1128/mcb.13.2.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H., Schmitt-Ney M., Groner B. Mammary gland-specific nuclear factor is present in lactating rodent and bovine mammary tissue and composed of a single polypeptide of 89 kDa. J Biol Chem. 1992 Aug 15;267(23):16365–16370. [PubMed] [Google Scholar]
- Weinberger J., Jat P. S., Sharp P. A. Localization of a repressive sequence contributing to B-cell specificity in the immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1988 Feb;8(2):988–992. doi: 10.1128/mcb.8.2.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M., Oka T. Isolation and structural analysis of the mouse beta-casein gene. Gene. 1989 May 30;78(2):267–275. doi: 10.1016/0378-1119(89)90229-1. [DOI] [PubMed] [Google Scholar]
- Zock C., Iselt A., Doerfler W. A unique mitigator sequence determines the species specificity of the major late promoter in adenovirus type 12 DNA. J Virol. 1993 Feb;67(2):682–693. doi: 10.1128/jvi.67.2.682-693.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]