Abstract
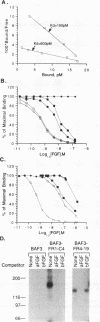
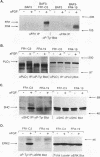
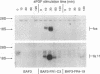
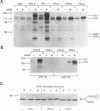
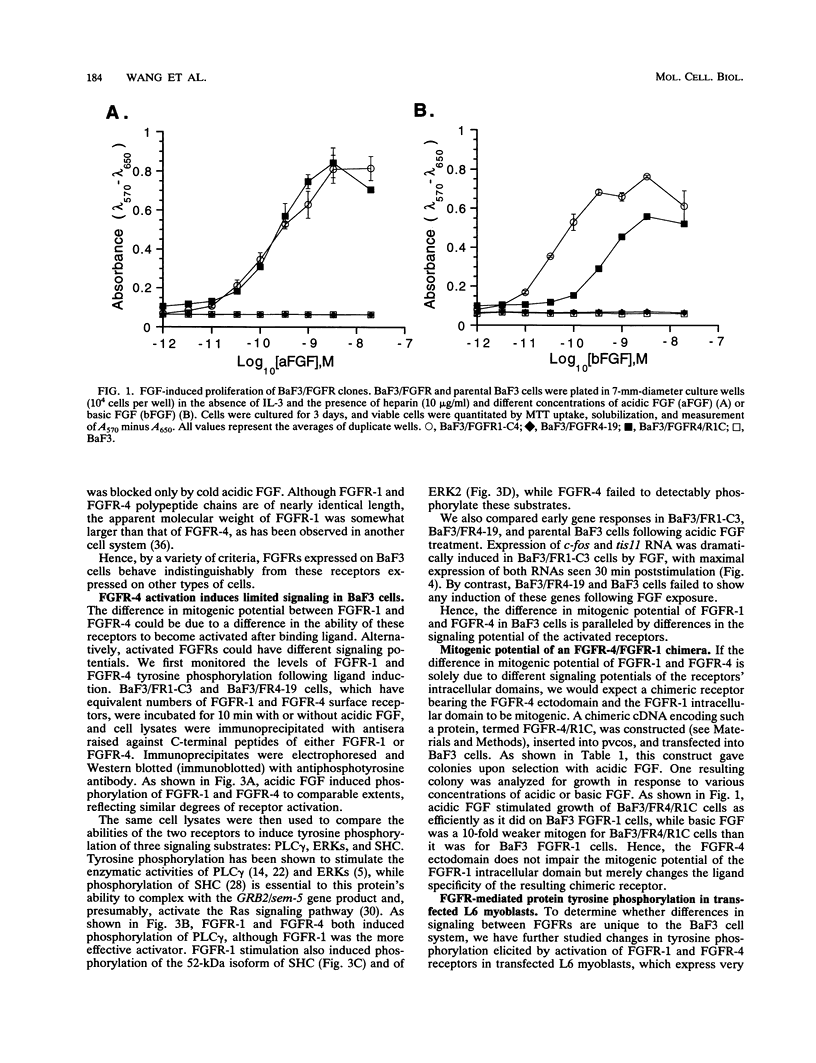
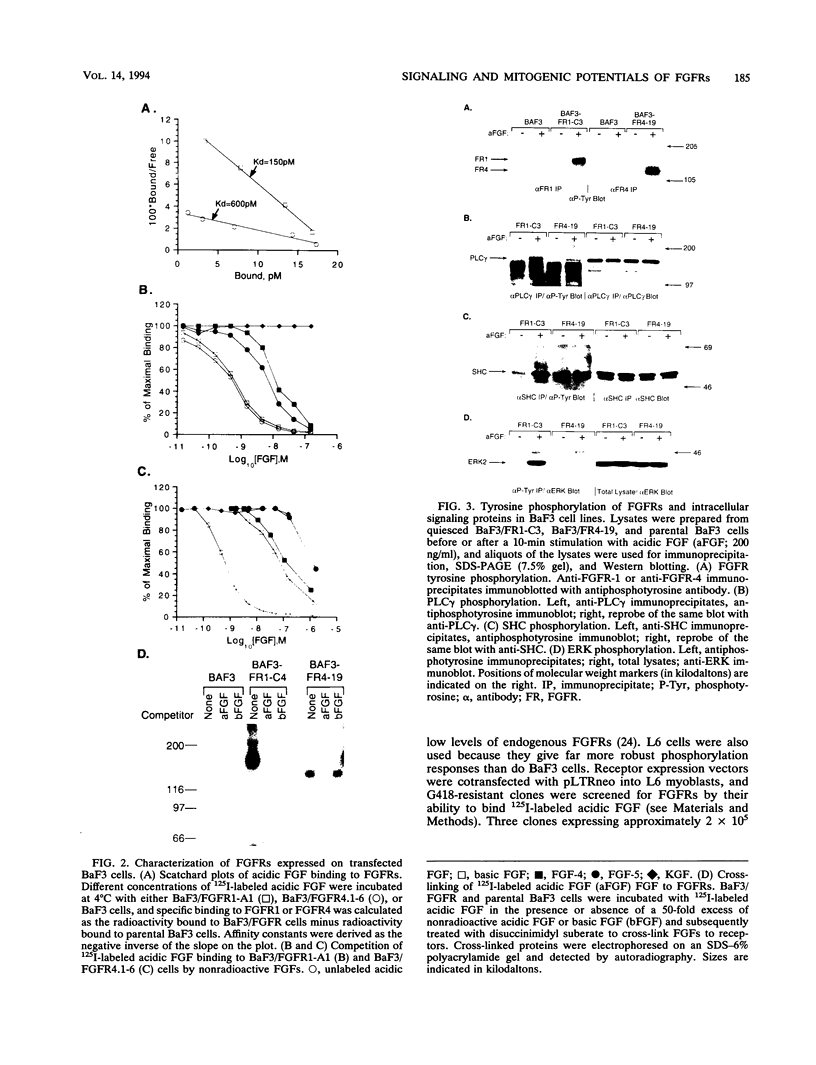
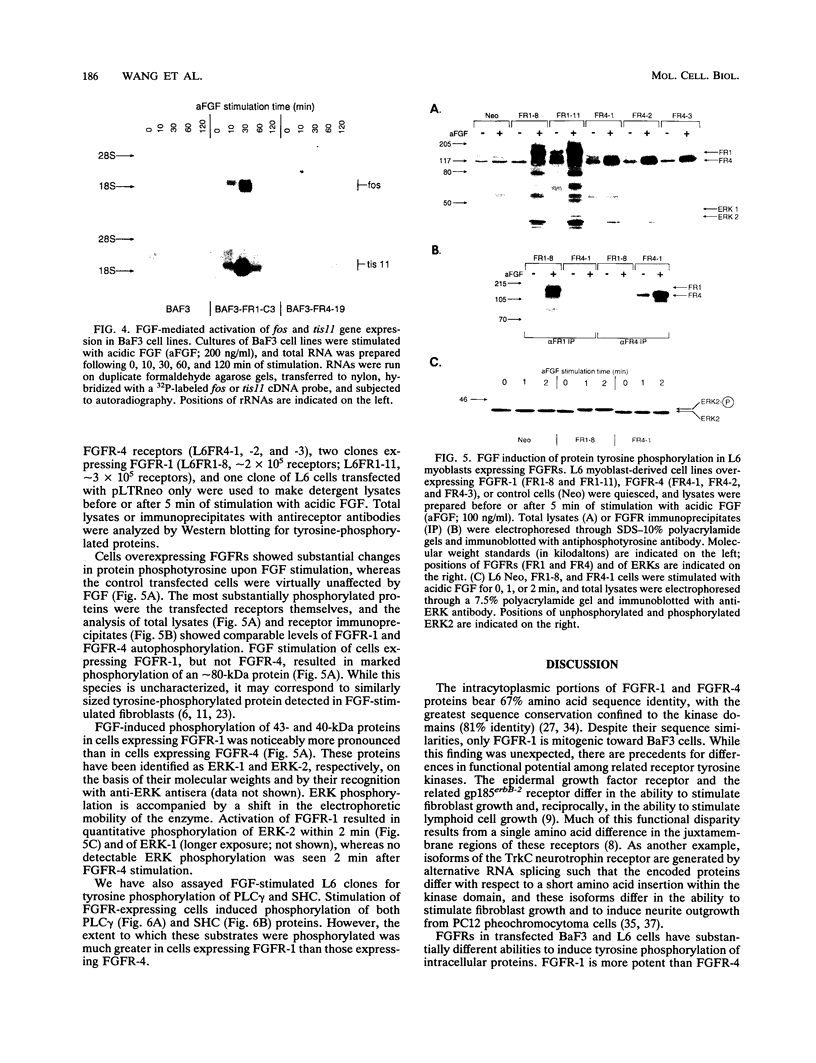
Fibroblast growth factor (FGF) receptors (FGFRs) are structurally related receptor protein tyrosine kinases encoded by four distinct genes. Activation of FGFR-1, -2, and -3 by FGFs induces mitogenic responses in various cell types, but the mitogenic potential of FGFR-4 has not been previously explored. We have compared the properties of BaF3 murine lymphoid cells and L6 rat myoblast cells engineered to express FGFR-1 or FGFR-4. Acidic FGF binds with high affinity to and elicits tyrosine phosphorylation of FGFR-1 or FGFR-4 receptors displayed on BaF3 cells, but only FGFR-1 activation leads to cell survival and growth. FGFR-4 activation also fails to elicit detectable signals characteristic of the FGFR-1 response: tyrosine phosphorylation of SHC and extracellular signal-related kinase (ERK) proteins and induction of fos and tis11 RNA expression. The only detected response to FGFR-4 activation was weak phosphorylation of phospholipase C gamma. A chimeric receptor containing the extracellular domain of FGFR-4 and the intracellular domain of FGFR-1 confers FGF-dependent growth upon transfected BaF3 cells, demonstrating that the intracellular domains of the receptors dictate their functional capacity. Activation of FGFR-1 in transfected L6 myoblasts induced far stronger phosphorylation of phospholipase C gamma, SHC, and ERK proteins than could activation of FGFR-4 in L6 cells, and only FGFR-1 activation induced tyrosine phosphorylation of a characteristic 80-kD protein. Hence, the signaling and biological responses elicited by different FGF receptors substantially differ.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess W. H., Dionne C. A., Kaplow J., Mudd R., Friesel R., Zilberstein A., Schlessinger J., Jaye M. Characterization and cDNA cloning of phospholipase C-gamma, a major substrate for heparin-binding growth factor 1 (acidic fibroblast growth factor)-activated tyrosine kinase. Mol Cell Biol. 1990 Sep;10(9):4770–4777. doi: 10.1128/mcb.10.9.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clements D. A., Wang J. K., Dionne C. A., Goldfarb M. Activation of fibroblast growth factor (FGF) receptors by recombinant human FGF-5. Oncogene. 1993 May;8(5):1311–1316. [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. R., Barr P. J., Cousens L. S., Fretto L. J., Williams L. T. Acidic and basic fibroblast growth factors stimulate tyrosine kinase activity in vivo. J Biol Chem. 1988 Jan 15;263(2):988–993. [PubMed] [Google Scholar]
- Dell K. R., Williams L. T. A novel form of fibroblast growth factor receptor 2. Alternative splicing of the third immunoglobulin-like domain confers ligand binding specificity. J Biol Chem. 1992 Oct 15;267(29):21225–21229. [PubMed] [Google Scholar]
- Di Fiore P. P., Helin K., Kraus M. H., Pierce J. H., Artrip J., Segatto O., Bottaro D. P. A single amino acid substitution is sufficient to modify the mitogenic properties of the epidermal growth factor receptor to resemble that of gp185erbB-2. EMBO J. 1992 Nov;11(11):3927–3933. doi: 10.1002/j.1460-2075.1992.tb05486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne C. A., Crumley G., Bellot F., Kaplow J. M., Searfoss G., Ruta M., Burgess W. H., Jaye M., Schlessinger J. Cloning and expression of two distinct high-affinity receptors cross-reacting with acidic and basic fibroblast growth factors. EMBO J. 1990 Sep;9(9):2685–2692. doi: 10.1002/j.1460-2075.1990.tb07454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesel R., Burgess W. H., Maciag T. Heparin-binding growth factor 1 stimulates tyrosine phosphorylation in NIH 3T3 cells. Mol Cell Biol. 1989 May;9(5):1857–1865. doi: 10.1128/mcb.9.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. E., Lee P. L., Lu J., Williams L. T. Diverse forms of a receptor for acidic and basic fibroblast growth factors. Mol Cell Biol. 1990 Sep;10(9):4728–4736. doi: 10.1128/mcb.10.9.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan K., Johnson D. E., Williams L. T., Hayman M. J. Isolation of an additional member of the fibroblast growth factor receptor family, FGFR-3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1095–1099. doi: 10.1073/pnas.88.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livant D. L., Hough-Evans B. R., Moore J. G., Britten R. J., Davidson E. H. Differential stability of expression of similarly specified endogenous and exogenous genes in the sea urchin embryo. Development. 1991 Oct;113(2):385–398. doi: 10.1242/dev.113.2.385. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Goldfarb M., Yancopoulos G. D., Gao G. Distinct rat genes with related profiles of expression define a TIE receptor tyrosine kinase family. Oncogene. 1993 Jun;8(6):1631–1637. [PubMed] [Google Scholar]
- Mansukhani A., Moscatelli D., Talarico D., Levytska V., Basilico C. A murine fibroblast growth factor (FGF) receptor expressed in CHO cells is activated by basic FGF and Kaposi FGF. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4378–4382. doi: 10.1073/pnas.87.11.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey-Prevot B., Nabel G., Palacios R., Baltimore D. Abelson virus abrogation of interleukin-3 dependence in a lymphoid cell line. Mol Cell Biol. 1986 Nov;6(11):4133–4135. doi: 10.1128/mcb.6.11.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Fleming T. P., Bottaro D. P., Rubin J. S., Ron D., Aaronson S. A. Expression cDNA cloning of the KGF receptor by creation of a transforming autocrine loop. Science. 1991 Jan 4;251(4989):72–75. doi: 10.1126/science.1846048. [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Honegger A. M., Rotin D., Fischer R., Bellot F., Li W., Dionne C. A., Jaye M., Rubinstein M., Schlessinger J. A tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the SH2 domain of phospholipase C-gamma 1. Mol Cell Biol. 1991 Oct;11(10):5068–5078. doi: 10.1128/mcb.11.10.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Nye S. H., Squinto S. P., Glass D. J., Stitt T. N., Hantzopoulos P., Macchi M. J., Lindsay N. S., Ip N. Y., Yancopoulos G. D. K-252a and staurosporine selectively block autophosphorylation of neurotrophin receptors and neurotrophin-mediated responses. Mol Biol Cell. 1992 Jun;3(6):677–686. doi: 10.1091/mbc.3.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olwin B. B., Hauschka S. D. Cell type and tissue distribution of the fibroblast growth factor receptor. J Cell Biochem. 1989 Apr;39(4):443–454. doi: 10.1002/jcb.240390410. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Leder P. Ligand specificity and heparin dependence of fibroblast growth factor receptors 1 and 3. J Biol Chem. 1992 Aug 15;267(23):16305–16311. [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Partanen J., Mäkelä T. P., Eerola E., Korhonen J., Hirvonen H., Claesson-Welsh L., Alitalo K. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991 Jun;10(6):1347–1354. doi: 10.1002/j.1460-2075.1991.tb07654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Peters K. G., Werner S., Chen G., Williams L. T. Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development. 1992 Jan;114(1):233–243. doi: 10.1242/dev.114.1.233. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Squinto S. P., Aldrich T. H., Lindsay R. M., Morrissey D. M., Panayotatos N., Bianco S. M., Furth M. E., Yancopoulos G. D. Identification of functional receptors for ciliary neurotrophic factor on neuronal cell lines and primary neurons. Neuron. 1990 Dec;5(6):757–766. doi: 10.1016/0896-6273(90)90334-c. [DOI] [PubMed] [Google Scholar]
- Stahl N., Davis S., Wong V., Taga T., Kishimoto T., Ip N. Y., Yancopoulos G. D. Cross-linking identifies leukemia inhibitory factor-binding protein as a ciliary neurotrophic factor receptor component. J Biol Chem. 1993 Apr 15;268(11):7628–7631. [PubMed] [Google Scholar]
- Tsoulfas P., Soppet D., Escandon E., Tessarollo L., Mendoza-Ramirez J. L., Rosenthal A., Nikolics K., Parada L. F. The rat trkC locus encodes multiple neurogenic receptors that exhibit differential response to neurotrophin-3 in PC12 cells. Neuron. 1993 May;10(5):975–990. doi: 10.1016/0896-6273(93)90212-a. [DOI] [PubMed] [Google Scholar]
- Vainikka S., Partanen J., Bellosta P., Coulier F., Birnbaum D., Basilico C., Jaye M., Alitalo K. Fibroblast growth factor receptor-4 shows novel features in genomic structure, ligand binding and signal transduction. EMBO J. 1992 Dec;11(12):4273–4280. doi: 10.1002/j.1460-2075.1992.tb05526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela D. M., Maisonpierre P. C., Glass D. J., Rojas E., Nuñez L., Kong Y., Gies D. R., Stitt T. N., Ip N. Y., Yancopoulos G. D. Alternative forms of rat TrkC with different functional capabilities. Neuron. 1993 May;10(5):963–974. doi: 10.1016/0896-6273(93)90211-9. [DOI] [PubMed] [Google Scholar]
- Vallés A. M., Boyer B., Badet J., Tucker G. C., Barritault D., Thiery J. P. Acidic fibroblast growth factor is a modulator of epithelial plasticity in a rat bladder carcinoma cell line. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1124–1128. doi: 10.1073/pnas.87.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Duan D. S., de Vries C., Peters K. G., Johnson D. E., Williams L. T. Differential splicing in the extracellular region of fibroblast growth factor receptor 1 generates receptor variants with different ligand-binding specificities. Mol Cell Biol. 1992 Jan;12(1):82–88. doi: 10.1128/mcb.12.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Yayon A., Zimmer Y., Shen G. H., Avivi A., Yarden Y., Givol D. A confined variable region confers ligand specificity on fibroblast growth factor receptors: implications for the origin of the immunoglobulin fold. EMBO J. 1992 May;11(5):1885–1890. doi: 10.1002/j.1460-2075.1992.tb05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerlin M., Julius M. A., Goldfarb M. NEP: a novel receptor-like tyrosine kinase expressed in proliferating neuroepithelia. Oncogene. 1993 Oct;8(10):2731–2739. [PubMed] [Google Scholar]
- Zhan X., Culpepper A., Reddy M., Loveless J., Goldfarb M. Human oncogenes detected by a defined medium culture assay. Oncogene. 1987;1(4):369–376. [PubMed] [Google Scholar]