Abstract
The β subunits of voltage-gated sodium (Nav) channels possess an extracellular immunoglobulin (Ig) domain that is related to the L1 family of cell-adhesion molecules (CAMs). Here we show that in HEK293 cells, secretion of the free Ig domain of the β3 subunit is reduced significantly when it is coexpressed with the full-length β3 and β1 subunits but not with the β2 subunit. Using immunoprecipitation, we show that the β3 subunit can mediate trans homophilic-binding via its Ig domain and that the β3-Ig domain can associate heterophilically with the β1 subunit. Evolutionary tracing analysis and structural modeling identified a cluster of surface-localized amino acids fully conserved between the Ig domains of all known β3 and β1 sequences. A notable feature of this conserved surface cluster is the presence of two adjacent cysteine residues that previously we have suggested may form a disulfide bond. We now confirm the presence of the disulfide bond in β3 using mass spectrometry, and we show that its integrity is essential for the association of the full-length, membrane-anchored β3 subunit with itself. However, selective reduction of this surface disulfide bond did not inhibit homophilic binding of the purified β3-Ig domain in free solution. Hence, the disulfide bond itself is unlikely to be part of the homophilic binding site. Rather, we suggest that its integrity ensures the Ig domain of the membrane-tethered β3 subunit adopts the correct orientation for productive association to occur in vivo.—Yereddi, N. R., Cusdin, F. S., Namadurai, S., Packman, L. C., Monie, T. P., Slavny, P., Clare, J. C., Powell, A. J., Jackson, A. P. The immunoglobulin domain of the sodium channel β3 subunit contains a surface-localized disulfide bond that is required for homophilic binding.
Keywords: cell adhesion molecule, evolutionary trace analysis, mass spectrometry
The β subunits of voltage-gated sodium (Nav) channels were first isolated in association with the pore-forming Nav α subunit. Four mammalian Nav β-subunit genes (β1-4) are expressed in different tissue-specific patterns (1). The β1-4 proteins each possess an N-terminal extracellular immunoglobulin (Ig) domain, a single transmembrane α helix, and an intracellular C-terminal region. In addition, alternative mRNA splicing of the β1 gene generates a secreted form (β1B) that lacks a transmembrane and cytoplasmic domain (2–4). All five of the β-subunit protein isoforms influence the electrophysiological properties of the Nav channel, including the kinetics of channel activation and inactivation and the voltage sensitivity of channel gating. Furthermore, in at least some heterologous expression systems, the β subunits increase the efficiency of channel surface expression and thus increase peak current density (5). Consistent with this role in regulating Nav-channel behavior, mice lacking individual β subunits exhibit a variety of neurophysiological and/or cardiac defects (6–8).
The extracellular β-subunit Ig domain is most closely related to the L1 family of cell-adhesion molecules (CAMs; ref. 9), and good evidence indicates that the Nav β subunits play additional roles as CAMs. For example, both the β1 and β2 subunits can bind homophilically in trans (10), and they bind in cis to other CAM-like molecules, such as contactins, NrCAMs, and neurofascins (11–15). The β1 subunit can also bind in trans to the CAMs NF-155 and NrCAM (12). These interactions may facilitate the enhanced surface density and restricted surface clustering of Nav channels within the plasma membranes of electrically excitable cells (4). However, at least some of the CAM-like behavior shown by the Nav β subunits is likely to be independent of any effects they have on Nav α subunits (3). For example, both β1 and β2 subunits act as trans-binding CAMs in cells lacking Nav α-subunit expression (10). The β1-induced cell adhesion initiates changes in cytoskeletal assembly at the sites of cell contact (16). In neurons, such interactions activate cell–cell signaling, stimulate neurite outgrowth, and influence growth cone guidance (17, 18). Hence, the β subunits are multifunctional.
We are particularly interested in the structure and function of the Nav β3 subunit (19–22). The β3-subunit sequence is most similar to that of β1 (19), and like β1, it binds neurofascin (11). However, unlike β1, it has been reported that β3 does not self-associate (23). Here we examine the CAM-like behavior of the β3 subunit. We show that the β3 subunit does bind homophilically in trans when expressed in a mammalian cell line. We use structural modeling, mass spectrometry, and site-directed mutagenesis (20) to identify a surface disulfide bond in β3 whose integrity is required for the homophilic binding of the β3 subunit in vivo. Furthermore, we use evolutionary tracing analysis (24, 25) to confirm that the cysteine residues of this disulfide bond are completely conserved in all known β3 and β1 subunits. Our work clarifies the relationship between structure and function in the β3-subunit Ig domain and raises new questions about the multiple roles of β subunits.
MATERIALS AND METHODS
Reagents
All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Fisher Scientific (Pittsburgh, PA, USA) unless otherwise stated. Bis-(sulfosuccinimidyl) suberate (BS3) was purchased from Pierce (Rockford, IL, USA). Tris-(2-carboxyethyl) phosphine (TCEP) was purchased from Pierce. Peptide N-glycosidase F (PNGase F) was purchased from New England Biolabs (Ipswich, MA, USA). Nupage lithium dodecylsulfate (LDS) sample buffer was from Invitrogen (Carlsbad, CA, USA). Mouse monoclonal anti-c-Myc epitope antibody 9E10 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal anti-6His antibody was purchased from AbD Serotec (Kidlington, UK). Rabbit polyclonal anti-green fluorescent protein (GFP; ab6556).was purchased from Abcam (Cambridge, UK). Purified goat anti-rabbit IgG was purchased from Jackson Laboratories (Bar Harbor, ME, USA). Horseradish peroxidase-conjugated goat anti-mouse antibody (P0447) was purchased from Dako A/S (Copenhagen, Denmark). Horseradish peroxidase-conjugated anti-rabbit antibody (170-6515) was purchased from Bio-Rad (Hercules, CA, USA). Talon metal affinity resin was purchased from Clontech (Palo Alto, CA, USA). Anti-c-Myc agarose conjugate (A7470) was from Sigma-Aldrich. Polyclonal rabbit anti-human β1 antibodies directed to the C termini of the protein were generated at GlaxoSmithKline (Stevenage, UK). Polyclonal rabbit antisera raised against the peptide RPEGGKDFLIYE of the human β3-Ig domain has been described previously (26).
β-Subunit constructs
All Nav β-subunit isoforms adopt a broadly similar shape. The extracellular domain (ECD) consists of a single Ig domain, connected via a “stalk” to a single transmembrane α helix (see Fig. 1A). The β3 cDNA constructs that were used in this work and their corresponding protein products are summarized in Fig. 1B. The C-terminal enhanced GFP (EGFP)-tagged full-length β3 wild-type subunit (β3-wt-EGFP), the C-terminal EGFP-tagged β3 subunit lacking its ECD (β3-ΔECD-EGFP), and the C-terminal EGFP-tagged β3 subunit containing the C24A point mutation (β3-[C24A]-EGFP) were cloned into the plasmid pcDNA3.1 and have been described previously (20). The cDNA encoding full-length β3 wild-type subunit containing a C-terminal Myc-tag (β3-wt-Myc), was cloned into the plasmid pFastBacMam and has been described previously (21). The EGFP-tagged constructs were generated from the sequences of rat β3, and the Myc construct was modified from human β3. However, the ECD sequences of rat and human β3 are 99.9% identical (19). We have previously established that neither the Myc tag nor the EGFP tag interferes with surface expression in any of these constructs (20, 21). To create the His-tagged free-Ig domain of β3 (β3-Ig-6His; Fig. 1B), nucleotides translating to residues T130-E191 of the mature human protein, and cloned into the plasmid pFastBacMam, were deleted, and the ECD nucleotides were fused to a C-terminal 6His tag using the QuickChange method of mutagenesis (Stratagene, Cambridge, UK). Positive clones were identified by restriction digestion of isolated plasmids and verified by sequencing. The cDNA clones encoding full-length wild-type human β1 (β1-wt) was subcloned into the plasmid pFastBacMam. Generation of the β2-subunit expression vector pCIH-β2 (encoding β2-wt), has been described previously (27).
Figure 1.
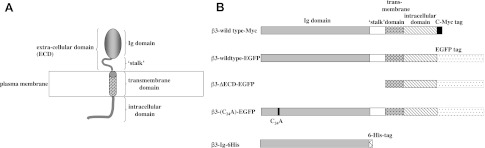
Summary of the Nav β-subunit structure and β3 constructs. A) All Nav β subunits possess a single extracellular Ig domain, a “stalk ” that links the Ig domain to a single α-helix transmembrane domain and an intracellular domain. In β3, the intracellular domain is largely disordered but has a juxtamembrane region with the potential to form a short amphipathic α helix (21). B) The epitope-tagged wild-type and mutant β3-subunit constructs that are referred to in the text.
Quantification of cell-associated and secreted β3-Ig-6His
Approximately 2 × 106 human embryonic kidney 293 (HEK293) cells were transiently transfected with a total of 10 μg of plasmid DNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The total plasmid was divided into pFastBacMam-β3-IG-6His and a 3-fold molar excess of each of the full-length subunits: pFastBacMam-β3-wt (myc-tagged), pFastBacMam-β1, pCIHβ2, or pFastBacMam-GFP (control). Transfected cultures were incubated in 10 ml of Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum (FCS) for 2 d at 37°C and 5% CO2. At the end of this period, the medium was decanted from the flasks, volumetrically measured, combined with 1 ml of cell lysis buffer [1% Triton in phosphate-buffered saline (PBS) with Complete Protease Inhibitor Cocktail; Roche, Basel, Switzerland], and filtered through a 0.4-μm syringe filter (Minisart High Flow; Sartorius AG, Göttingen, Germany). The attached cells were gently rinsed twice with PBS, drained, and lysed at 4°C in 0.5 ml of lysis buffer with occasional vortexing for 30 min. The lysate was transferred to a clean tube (1.5 ml), and the remaining cell matter was washed off the surface of the flask with a further 0.5 ml of lysis buffer, and lysates were combined. The lysate was centrifuged at 10,000 g for 10 min at 4°C. All the supernatant was carefully removed and diluted with the same volume of DMEM with 10% FCS as the recovered cell medium.
We used an enzyme-linked immunoadsorbent assay (ELISA) to detect β3-Ig-6His. The wells of a flat-bottomed 96-well ELISA plate were coated overnight with 4 μg/ml goat anti-mouse IgG antibody in 0.05 M Na2CO3 (pH 9.5). The following day, wells were aspirated and incubated with 0.05 ml of 1:1000 dilution of mouse monoclonal anti-His tag in PBS containing 1% (w/v) bovine serum albumin (BSA) for 1 h. Wells were incubated for 4 h at room temperature with 0.2 ml of either experimental samples (1 mg/ml total protein) or β3-Ig-6His standard, purified as described below, and diluted into DMEM with 10% FCS. To ensure that all the β3-Ig-6His in the assay was monomeric, samples of supernatant, cell extract, and standards were made in 0.1% sodium dodecylsulfate (SDS), heated at 70°C for 10 min, and cooled immediately prior to their addition to the ELISA plate. The wells were then washed 3 times with PBS containing 0.1% (v/v) Triton X-100 and incubated at room temperature for 3 h with a 1:100 dilution in PBS with 0.1% Triton X-100 (v/v) of a rabbit polyclonal antiserum raised against the β3-Ig domain (26).Wells were washed as above and incubated for another hour at room temperature with peroxidase-labeled sheep anti-rabbit IgG serum diluted 1:10,000 in PBS with 0.1% (w/v) BSA. The wells were again washed 3 times as above and incubated with 0.2 ml of 0.4 mg/ml o-phenylenediaminedihydrochloride in 0.05 M phosphate citrate buffer with 0.03% (w/v) sodium perborate. The plates were left for ∼15 min, and the absorbance was measured at 450 nm using an Emax ELISA plate reader (Molecular Devices, Sunnyvale, CA, USA). Samples were measured in triplicates. Samples of medium and lysate from untransfected cells were treated in an identical manner, and their absorbances were subtracted from medium and lysate samples of transfected cells as appropriate. Statistically significant differences from GFP-expressing control cells were determined by Student's t test.
Denaturing gel electrophoresis and Western blotting
Protein and cell-extract samples were heated in NuPAGE LDS denaturing sample buffer (Invitrogen)with 10 mM dithiothreitol (DTT) for 4 min at 100°C. Samples were separated using NuPAGE electrophoresis (Invitrogen) in 12% polyacrylamide gel with 4% stacking gel according to the manufacturer's instructions. Gels were dry-transferred onto nitrocellulose using the iBlot Dry Transfer system (Invitrogen).
Coimmunoprecipitation of β subunits
For coexpression studies, HEK293 cells at 70–90% confluency on a 100-mm dish were transfected with a total 5 μg of plasmids using Lipofectamine 2000 (Invitrogen) as described in Results. The attached cells were washed gently 3 times and detached with PBS. The cells were pelleted at 120 g for 7 min at 4°C on a benchtop centrifuge, and the pellet was lysed at 4°C in lysis buffer (1 ml): 50 mM Tris-HCl, 150 mM NaCl, and 1% (v/v) Triton X-100 (pH 7.4) containing Complete Protease Inhibitor Cocktail (Roche). The lysate was transferred to a 1.5-ml tube and clarified by centrifugation at 10,000 g for 14 min. The total protein concentration of the lysate was estimated using a Nanodrop 1000 (Thermo Fisher, Waltham, MA, USA). Cell lysates (15 mg of total protein) were incubated with 50 μl (50% slurry) of anti-c-Myc agarose conjugate, if c-Myc-hWT β3 was being pulled down, or Talon metal affinity resin for His-tag immunoprecipitation of β3-IG-6His at 4°C overnight with end-over-end rotation. The samples were spun at 2000 g on a benchtop centrifuge at 4°C for 2 min, and the unbound flow-through fraction was collected for analysis later. The resin was washed 5 times with ice-cold lysis buffer and spun at 2000 g between washes. For the anti-c-Myc agarose resin, 0.1 M NaOH (pH 11–12) was used to elute c-Myc tagged proteins in 3 consecutive 200-μl fractions; for the Talon beads, the first 2 fractions were eluted in 200 μl 150 mM imidazole in lysis buffer, and the final resin strip was eluted in 200 μl 150 mM EDTA (pH 8.3). The flow-through fraction, wash fractions, and eluted fractions (20 μl) were separated by denaturing polyacrylamide gel electrophoresis (PAGE) as described, and probed with anti-c-Myc, anti-6His, anti-GFP, or anti-β1 antibodies as appropriate. Negative controls were treated similarly, except that one of the binding partners was omitted in the transfection.
To demonstrate trans binding between subunits, HEK293 cells at 70–90% confluency on a 100-mm dish were separately transfected with 5 μg appropriate plasmid as described. After 2 d, cells were detached by gentle shaking with PBS, and equal numbers were reseeded together under conditions of >90%confluency for 24 h to allow cell contact. Cells were then processed for immunoprecipitation and Western blottings as above.
Structural modeling and evolutionary trace analysis
Homologs of the human β3-Ig domain were identified using FUGUE (28), and a model structure was generated using Modeler 9.8 (29). The ECD of macrophage complement receptor CRIg [Protein Data Bank (PDB) 2icc; ref. 30], and the ECD of the myelin membrane adhesion molecule P0 (PDB 1neu; ref. 31) were used as templates. The model with the lowest Modeler Objective Function was chosen for refinement to optimize stereochemistry and disulfide bond formation. Analysis of the final model using Molprobity (32) identified 97.5% of residues in favored regions of the Ramachandran plot, 0.8% as outliers, and only 0.9% as possessing bad rotamers.
Full-length β-subunit sequences (8 from β1, 11 from β2, 13 from β3, and 9 from β4; Supplemental Fig. S1) were obtained from the U.S. National Center for Biotechnology Information (NCBI) and single-nucleotide polymorphism (SNP) databases. Multiple sequence alignments were carried out using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/). Evolutionary trace analysis was carried out using TraceSuite II (ref. 25; http://mordred.bioc.cam.ac.uk/∼jiye/evoltrace/evoltrace.html). The chosen sequences were aligned, and 2 traces were run: the first tracked amino acids conserved across all four β subunits; the second identified amino acids only conserved between β1 and β3 subunits. Conserved residues were mapped onto the modeled structure of human β3.
Purification of β3-Ig-6His
A BacMam virus construct incorporating the β3-IG-6His cDNA was generated using standard methods for the Bac-to Bac system (Invitrogen; ref. 33). Approximately 2 × 107 nonadherent HEK293F cells were transduced with the β3-Ig-6His BacMam virus and grown for 2 d. The cells were pelleted at 120 g for 3 min, and the medium was filtered through a 0.2-μm filter to remove cells and cell debris. All chromatographic steps were performed using a fast protein liquid chromatography (FPLC) system (Amersham Pharmacia Biotech, Little Chalfont, UK) at 4°C. The filtered medium was applied to a Ni Sepharose column (HisTrap HP column; 17-5247-01; Amersham Biosciences) in equilibration buffer (5 mM imidazole, 20 mM Na2HPO4 buffer, pH 8.0, containing 500 mM NaCl). Nonspecific proteins were eluted with 15 ml of equilibration buffer. The β3-free IG-6His was then eluted with equilibration buffer containing a linear gradient from 5 to 300 mM imidazole. The eluted protein was concentrated to a final volume of ∼350 μl using an Amicon Ultra-15 centrifugal filter device (Millipore, Billerica, MA, USA) with a 10-kDa molecular mass cutoff. For the protein used in cross-linking studies, the β3-Ig-6His cDNA was recloned into the mammalian expression vector pTT3 using the Gateway cloning system (Invitrogen; ref. 34), and transiently transfected into HEK293F cells by following the manufacturer's instructions.
Digestion of β3-Ig-6His with PNGase F
Approximately 10 μg of purified β3-Ig-6His was incubated in 10 μl PNGase F buffer (0.05 M sodium phosphate buffer pH 7.5, 0.5% SDS, 1% Nonidet P-40, 0.05 M DTT) at 100°C for 10 min. After cooling, the sample was digested with 500 U PNGase F for 60 min at 37°C. Samples were examined by denaturing PAGE as described.
Disulfide bond identification by mass spectrometry
Edman degradation
β3-IG-6His protein (10 μg) was desalted with a ProSorb cartridge (Applied Biosystems, Foster City, CA, USA) and subjected to Edman degradation on an Applied Biosystems 494HT protein sequencer according to the manufacturer's instructions. The procedure was repeated after treating the protein in two ways: alkylation with 40 mM iodoacetamide for 1 h at 20°C, followed by quenching with 0.2 M dithiothreitol for 10 min; or reduction with 8 mM TCEP for 1 h at 20°C, followed by alkylation as in the first method.
Trypsin digestion
β3-Ig-6His was digested for 3 h at 37°C with modified porcine trypsin (14:1 w/w; Promega, Madison, WI, USA) at pH 8 in 100 mM ammonium bicarbonate. The resulting peptides were desalted (μC18ZipTip; Millipore) and examined by matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS; TofSpec 2E; Waters Corp., Milford, MA. USA) using α-cyano-4-hydroxycinnamic acid matrix (10 mg/ml in 50% acetonitrile-water containing 0.1% trifluoroacetic acid (TFA). A sample of the digest was also reduced by 5 mM TCEP for 30 min at 20°C. Internal calibration was to known β3-Ig-6His tryptic peptides.
Cleavage with 1-cyano-4-dimethylaminopyridinum tetrafluoroborate (CDAP)
β3-Ig-6His protein (0.2 μg) was reduced with 1.5 mM TCEP in 5 μl 0.1 M Tris.Cl (pH 7.5) for 30 min at 20°C under argon, then alkylated with 10 mM iodoacetamide at 37°C, for 45 min under argon. The pH of the medium was reduced to 5.5 by adding 1 M sodium citrate (pH 4.5), and the protein was digested with 0.5 μl of 1 mg/ml GluC endoproteinase for 4 h at 37°C. The solution pH was raised to 7 with 200 mM ammonium bicarbonate and quenched by incubation with 1 M 2-mercaptoethanol for 20 min at 20°C. The peptides were recovered and desalted using C18ZipTip, eluted with 50%acetonitrile/0.01%TFA, and dried under vacuum. They were resuspended in 5 μl 20 mM sodium citrate (pH 4.5) containing fresh 1.5 mM CDAP and incubated for 30 min at 20°C. Peptides were again recovered, desalted, and dried as above (using μC18ZipTip), resuspended in 3 μl 1 M NH4OH (1 h, 20°C) to effect cleavage, and dried. Finally, peptides were resuspended in 5 μl 0.2% heptafluorobutyric acid and examined by MALDI-MS and electrospray MS/MS to confirm their identities.
Cross-linking experiments
Purified samples (50 μl) of β3-Ig-6His protein (2 mg/ml in 20 mM HEPES buffer, pH 7.5, and 150 mM NaCl) were incubated with 10 mM BS (3) cross-linker for the indicated times at 37°C. At individual time points, 4-μl samples were removed and quenched with 4 μl, 0.5 M Tris HCl (pH 7.4) for 1 h at room temperature. Samples were examined by denaturing PAGE as described above. To generate the β3-Ig-6His protein with the selectively reduced C2–24 disulfide bond, the protein was incubated with 1.5 mM TCEP for 30 min at 20°C, and the reduced cysteines were trapped by alkylation with 8 mM iodoacetamide for 1 h at 20°C.
RESULTS
Secretion of the free β3-Ig domain is retarded in cells expressing β3 and β1 subunits
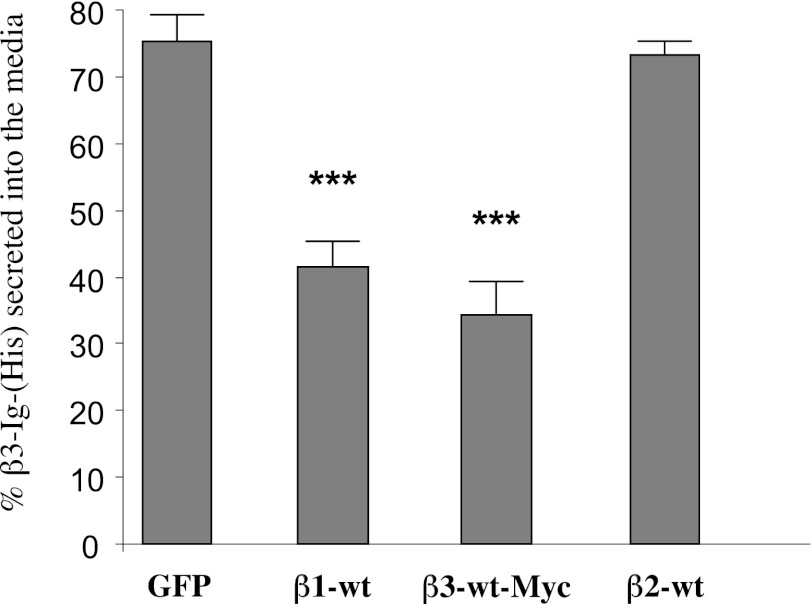
The sequence similarity between the β1 and β3 subunits compared to other members of the Nav β-subunit family (19) prompted us to examine the question of shared function, especially in the context of homophilic and heterophilic binding behavior. In our first experiment, we asked whether the secretion of the free β3-Ig domain, lacking any obvious form of membrane attachment, would be retarded if it were coexpressed in cells together with other full-length, membrane-anchored β subunits. For this purpose. we generated the construct β3-Ig-6His, which encodes only the complete β3-Ig domain (Fig. 1). We used the human cell line HEK293 for all our transfection studies. This cell line is widely employed to investigate both the cell biological and electrophysiological properties of Nav channels and has undetectable levels of endogenous Nav α- and β-subunit expression (21, 35). Cells were separately cotransfected with β3-Ig-6His cDNA together with a plasmid encoding one of the following: EGFP control, β1-wt, β2-wt, or β3-wt-Myc. The amount of β3-Ig-6His retained in the cell lysate and the amount secreted into the medium was measured by ELISA. Cells coexpressing β3-wt-Myc or β1-wt subunits retained significantly more β3-Ig-6His relative to control cells transfected with EGFP alone. By contrast, the secretion of β3-Ig-6His from cells coexpressing β2-wt showed no difference from the control (Fig. 2).
Figure 2.
Secretion of the free β3-Ig domain is reduced by coexpression with full length β3 and β1 subunits, but not β2. HEK293 cells were cotransfected with plasmids expressing β3-Ig-6His and β1-wt, β2-wt, β3-wt-Myc, and GFP control. The percentage of total β3-Ig-6His secreted into the medium is indicated. ***P < 0.01 vs. control.
β3-Ig domain binds homophilically and heterophilically to β1
The initial experiments suggest, but do not prove, that the β3-Ig-6His protein can bind the full-length β3 and β1 subunit. To demonstrate binding directly, HEK293 cells were cotransfected with β3-Ig-6His and β3-wt-EGFP, or β1-wt. The proteins from lysed cells were incubated with immobilized anti-His Talon beads, and the precipitate was examined by Western blotting using either anti-GFP or anti-β1 antibodies, as outlined in Materials and Methods. Both the full-length β3 and β1 subunits were successfully coprecipitated with the β3-Ig-6His protein (Fig. 3A, B). When the experiment was repeated using cells cotransfected with β3-Ig-6His and β3-ΔECD-EGFP, the free β3-Ig domain was no longer precipitated (Fig. 3C, D). In most experiments, the β3-Ig-6His was resolved on denaturing PAGE as a closely spaced doublet of approximate molecular mass 20–26 kDa. This is higher than expected for the predicted molecular mass of the protein (16 kDa). The higher molecular mass and the doublet pattern are due to glycosylation, since digestion of the purified β3-Ig-6His protein with PNGase F changed its mobility to a single band of molecular mass 15–16 kDa, consistent with the size expected from its amino acid sequence alone (Fig. 3E).
Figure 3.
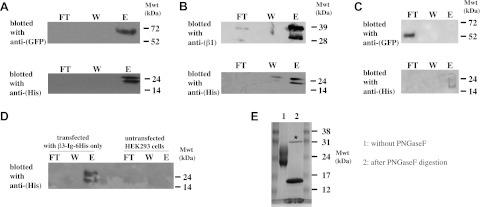
The β3 Ig domain binds β3 and β1. A–C) HEK293 cells were cotransfected with plasmids expressing β3-Ig-6His and β3-wt-EGFP(A); β1-wt (B), or β3-ΔECD-EGFP (C). Lysates were precipitated with Talon (anti-His) beads and blotted with antibodies as indicated. D) Control lanes showing cells transfected with only β3-Ig-6His together with untransfected cells. Lysates were precipitated with Talon (anti-His) beads and blotted with antibodies as indicated. FT, flow through; W, wash; E, elution fraction. E) PNGase F digestion reduces the higher-molecular-mass doublet characteristic of β3-Ig-6His protein to a single band of 15–16 kDa. Mwt, molecular weight (mass). Asterisk indicates the added PNGase F enzyme.
To establish that the β3-mediated binding can occur when both of the interacting partners were full length and tethered in membranes, we cotransfected cells with plasmids encoding β3-wt-Myc and β3-wt-EGFP constructs. Binding was detected by coimmunoprecipitation using anti-Myc resin and Western blotting for both GFP and Myc epitope. When lysates from cells cotransfected with both the β3-wt-Myc and the β3-wt-EGFP constructs were incubated with the anti-Myc resin, the EGFP-tagged β3 subunit was coprecipitated, along with β3-wt-Myc (Fig. 4A). However, when lysates from cells transfected with β3-wt-EGFP alone were incubated with the anti-Myc resin, none of the β3-wt-EGFP protein was isolated, thus confirming the specificity of the immunoprecipitation conditions (Fig. 4B). Interestingly, if extracts from cells separately transfected with β3-wt-Myc and β3-wt-EGFP were mixed together postlysis and then immunoprecipitated using anti-Myc resin, then no β3-wt-EGFP was coimmunoprecipitated (Fig. 4C). This suggests that the interaction between β3 subunits depends on the structural integrity of the transfected cells.
Figure 4.
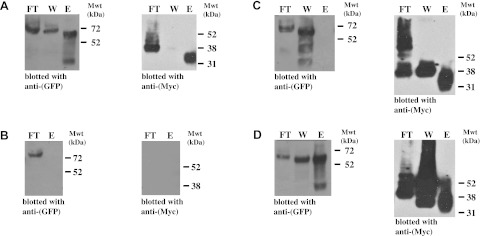
Cotransfected full-length β3 subunits bind homophilically. A–C) HEK293 cells were cotransfected with plasmids expressing β3-wt-EGFP and β3-wt-Myc (A); transfected with β3-wt-EGFP alone (B); separately transfected with plasmids expressing β3-wt-EGFP and β3-wt-Myc, then cell lysates were mixed together prior to immunoprecipitation (C); or separately transfected with plasmids expressing β3-wt-EGFP and β3-wt-Myc, then cells were cocultured for 2 d under conditions of 70–90% confluency prior to cell lysis (D). In each case, equal volumes of samples were immunoprecipitated with anti-Myc resin and blotted with antibodies as indicated. FT, flow through; W, wash; E, elution fraction; Mwt, molecular weight (mass).
We deliberately used sufficient anti-Myc resin to ensure a large molar excess of anti-Myc antibody compared to Myc-tagged protein (Materials and Methods). Nevertheless, in these experiments, a significant proportion of both Myc-tagged β3 subunits, together with associated β3-wt-EGFP, failed to bind the anti-Myc resin and remained in the unbound fraction or was removed in the buffer wash. Moreover, the mobility of the β3-wt-Myc protein in the unbound fractions was consistently slightly slower on denaturing PAGE compared to the β3-wt-Myc eluted from the anti-Myc resin (Fig. 4A, C). The reason for this behavior is not clear. It is possible that in the nonbinding fractions, the β3-wt-Myc protein has a C-terminal modification that prevents interaction of its Myc-tag with the resin. It is known for example that the C-terminal region of the β3 subunit contains a serine residue that can be phosphorylated (36).
The experiments described above cannot distinguish cis from trans interactions. To investigate whether trans homophilic binding is possible, we separately transfected HEK293 cells with the Myc-tagged β3 subunit and the EGFP-tagged β3 subunit. After 2 d, the cells were detached from their plates, and equal numbers of cells were cocultured under conditions where 70–90% of the cells were confluent and touching. Under these conditions, the EGFP-tagged β3 subunit was successfully coprecipitated from cell lysates incubated with anti-Myc resin (Fig. 4D). Hence, the β3 subunits can interact homophilically in trans when expressed in vivo.
Ig domain of the β1 and β3 subunits contains an evolutionarily conserved surface cluster of amino acids
If the β3-Ig domain can bind homophilically and heterophilically to β1, then both β-subunit Ig domains may share at least one conserved surface cluster of amino acids corresponding to a common functional site. To test this prediction, we used evolutionary trace analysis, a bioinformatic technique that can identify conserved regions within extended protein families. Evolutionary trace analysis can be a powerful way of identifying and prioritizing residues of potential functional importance (24). In evolutionary trace analysis, a phylogenetic tree is first generated from all aligned sequences. A “consensus ” sequence is then derived for each group of proteins that originate from a common node. By comparing these consensus sequences between groups, we classify each amino acid residue in one of three ways: absolutely conserved residues (rank 1); residues that are fully conserved within a group but differ in the nature of their conservation between groups (rank 2); and nonconserved residues (rank 3). This information is then mapped onto a protein structure so as to identify the location of conserved clusters, both surface and buried (25). Currently, no high-resolution structures exist for any of the Nav β-subunit Ig domains. However, the X-ray crystallographic structure of myelin P0 has been successfully used to model the Ig domains of both β3 (19, 20) and β1 (17, 37). Here we provide an improved model for the β3-subunit Ig domain, which uses both myelin P0 and macrophage complement receptor CRIg structures as templates (Fig. 5A). The model was optimized with respect to stereochemical constraints, as described in Materials and Methods.
Figure 5.
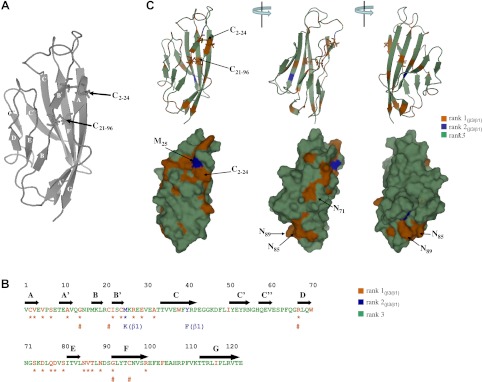
A conserved face on the β3 Ig domain revealed by evolutionary tracing. A) Ig domain of β3 showing both predicted β-sheet locations. The β3 Ig domain was modeled with the program Modeler using myelin P0 and macrophage complement receptor CRIg as templates (see Materials and Methods). The predicted β-sheet locations are indicated. The location of the C2–24 and C21–96 disulfide bonds, as described in the text, are indicated. B) Primary sequence of the Ig domain of human β3. Predicted β-sheet locations are indicated. Rank 1(β3/β1), rank 2(β3/β1), and rank 3 amino acids as determined by evolutionary tracing are color coded. Rank 1(β3/β1) and rank 2(β3/β1) residues that are predicted to be surface-localized are indicated by star. Rank 1(β3/β1) amino acids that are also perfectly conserved in all four β subunits are identified with pound symbol (#). For the two rank 2(β3/β1) residues (M25 and Y40), their equivalent β1 residues are indicated. C) Rank 1(β3/β1), rank 2(β3/β1), and rank 3 residues mapped onto the β3-Ig domain model. Ig domain is shown in both cartoon format to emphasize the predicted secondary structural elements, and in surface rendering to emphasize the surface-localized rank 1(β3/β1) and rank 2(β3/β1) residues. The three views of the Ig domain correspond to successive 120° rotations about the vertical axis. Location of the C2–24 and C21–96 disulfide bonds, and the location of specific amino acids discussed in the text, are labeled.
Phylogenetic analysis confirmed that β1 and β3 share a greater sequence similarity to each other than either does to β2 or β4 (Supplemental Fig. S1). Since we were particularly interested in the conserved features between β1 and β3, we focused our attention on the rank 1(β3/β1) classification, which corresponds to those residues fully conserved between β3 and β1 but differs from β2 and β4 (Fig. 5B, C). Included in these rank 1(β3/β1) residues were five amino acids whose conservation extended to all four β subunits across all species examined (Fig. 5B). These five included the buried disulfide bond corresponding to C21–96 in β3 (Fig. 5A). The C21–96 disulfide bond equivalent is also conserved in virtually all known Ig domains and probably functions to stabilize the two β-sheet faces of the Ig domain (38). A mutation that abolished this disulfide bond in β1 causes an inherited form of epilepsy (39). In β3, the mutation of C96 to alanine generates a nonfunctional protein (20). Almost all of the rank 1(β3/β1) sequences were surface-localized residues (Fig. 5B, C). It is also noticeable that the surface rank 1(β3/β1) residues occur almost exclusively along one face of the Ig domain and segregate into two clusters. The smaller cluster forms a self-contained patch corresponding to residues 85–87 and residue 89 in β3. The second and more extensive region of β3/β1 conservation forms two elongated tracks extending about half the length of the Ig domain. One track extends along the A-A′ β-sheet strand, the other contains sequences within the loops connecting the B to B′ and D to E β-sheet strands (Fig. 5C). Of the rank 2(β3/β1) residues (i.e., those amino acids that are fully conserved in β1 and β3, but different between the two isoforms), only one, corresponding to M25 in β3, is predicted to be surface localized. This residue lies directly adjacent to the rank 1(β3/β1) sequences within the D and E β-sheet strands (Fig. 5B, C).
Cysteine residues C2 and C24 of β3 form a surface-localized disulfide bond
A striking feature of the larger rank 1(β3/β1) surface cluster is the presence of two cysteines corresponding to C2 and C24 in β3, which lie adjacent to each other on the predicted antiparallel A and B′ β-sheet strands (Fig. 5A, C). We have previously suggested that cysteines C2 and C24 are sufficiently close in the predicted β3-Ig domain structure that they are likely to form a disulfide bond (19, 20). So far, however, direct experimental evidence has been lacking. To test our model, we purified the β3-Ig-6His protein secreted from HEK293F cells, cleaved it using specific proteases and examined the resultant peptides using mass spectrometry.
Edman degradation of the purified secreted protein identified the amino-terminal sequence as VCVEVP. This is the sequence expected if the endoplasmic reticulum targeting signal had been efficiently removed from the mature protein (19) and confirms that the secreted β3-Ig-6His protein was processed correctly within the secretory pathway. However, the amidocarboxymethylcysteine (cam) derivative at residue 2 was observed only when the protein was reduced and alkylated. Untreated and alkylated-only protein gave no signal at residue 2 (data not shown). This finding is consistent with cysteine C2 forming a disulfide bond in the intact structure.
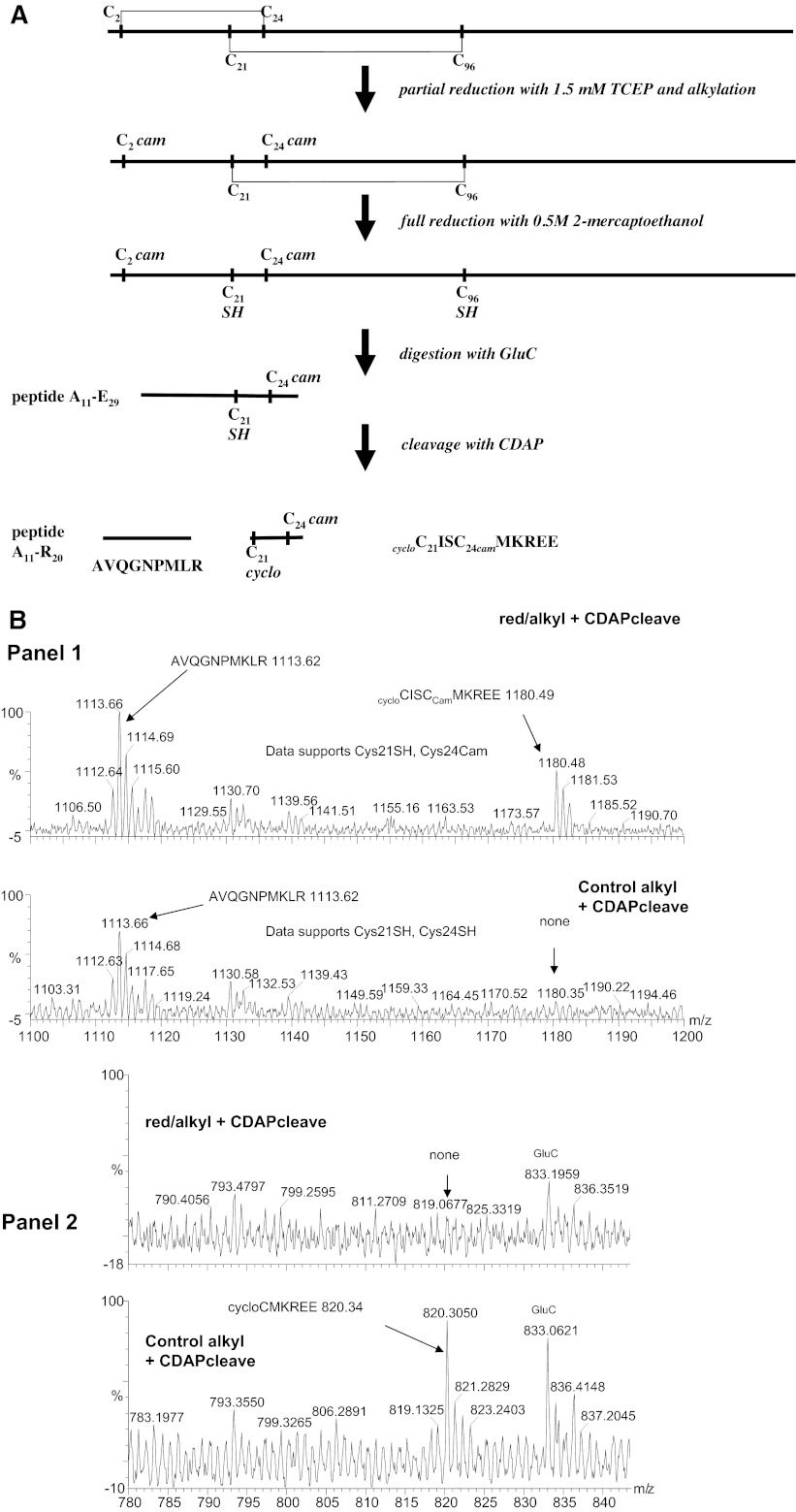
To establish disulfide connectivity, we used partial reduction by TCEP (Materials and Methods), and the protocol is outlined in Fig. 6A. Our working assumption was that the surface-location of the presumed C2–24 disulfide bond would make it more amenable to reduction compared to the buried C21–96 disulfide bond. The aim was to expose β3-Ig-6His to sufficient TCEP so that only the disulfide bond to cysteine C2 was reduced while maintaining intact the likely internal disulfide to cysteine C96. The β3-Ig-6His protein was exposed to a series of TCEP concentrations from 0 to 2 mM. Each sample was then alkylated with excess iodoacetamide to capture the reduced cysteine and excess alkylation reagent was quenched with 0.5 M 2-mercaptoethanol, which also reduced the remaining disulfide bond. Cysteines selectively reduced by the low TCEP concentration were, therefore, recognized by their alkylation signature, given by cam. Preliminary experiments established that almost full reduction of the C2–24 disulfide bond occurred between 1 and 2 mM TCEP (Supplemental Figs. S2 and S3). However, at 2 mM and above, the C21–96 disulfide became vulnerable to cleavage. Hence, 1.5 mM TCEP was chosen for the selective reduction of the C2–24 disulfide bond.
Figure 6.
Residues C2 and C24 form a disulfide bond in the β3 Ig domain. A) Outline of the protocol used to confirm the C(2–24) disulfide bond. The β3-Ig-6His was partially reduced with 1.5 mM TCEP and then alkylated to generate cam-modified sulfhydryls. The remaining disulfide bond was reduced with 2-mercaptoethanol to generate free SH groups. The protein was digested with GluC to generate peptide A11–E29 containing cam-modified C24 and unmodified C21 residues. Cleavage with CDAP generated two peptides diagnostic for the selective cam modification at C24 during the partial reduction with 1.5 mM TCEP. B) Mass analysis of peptides generated by CDAP-cleavage of a GluC digest of β3-Ig-6His (alkylated with and without prior reduction by 1.5mM TCEP). All peptide samples were fully reduced by 2-mercaptoethanol before mass analysis. Panel 1: masses corresponding to AVQGNPMKLR and cycloC21ISC24camMKREE indicating cleavage at C21; TCEP-reduction permits alkylation of C24 only, which must, therefore, be the disulfide partner of C2. The identities of these peptides were confirmed by tandem MS/MS analysis. Panel 2: alkylation of C24 should prevent formation of cycloC24MKREE, whose absence is confirmed in the sample. This peptide is readily seen in the fully reduced control; the additional expected peptide of cycloC21IS in the control was too small for analysis. Electrospray MS/MS analysis of target peptides was used to further confirm their identity (Supplemental Fig. S4).
Having reduced the β3-Ig-6His with 1.5 mM TCEP as described above, the protein was then digested with endopetidase GluC. This enzyme was chosen because it released a peptide (A11–E29) containing the two cysteines, C21 and C24 (Figs. 5B and 6A). Electrospray MS/MS of A11–E29 (1C, 1Ccam) proved unrewarding as the fragmentation pattern from MS2 and various MS3–4 attempts failed to yield fragments in the diagnostic region of the two cysteines owing to the flanking K and R residues in the sequence (AVQGNPMKLRCISCMKREE) and dominant cleavages at P. A different strategy of analyzing this peptide was needed. To identify the modified cysteine partner, the peptides produced by endopeptidase GluC digestion were desalted and incubated with CDAP, a reagent that cleaves peptides at free cysteines only (40). The cam-modified peptide (A11–E29) was cleaved by CDAP into two peptides: AVQGNPMLR and cycloC21ISC24camMKREE (Fig. 6 and Supplemental Fig. S4). Hence, C24 is the residue susceptible to limited TCEP reduction and is therefore disulfide bonded to C2 in the intact β3-Ig-6His protein.
C2–24 disulfide bond in the full-length, membrane-bound β3 is essential for homophilic binding
The existence of the C2–24 disulfide bond in β3 raised the question of whether it was required for β3 homophilic binding. We repeated the coimmunoprecipitation experiments described above using the construct β3-[C24A]-EGFP (Fig. 1B) in which the C2–24 disulfide bond was disrupted by site-directed mutagenesis. We have previously shown that the C24A mutant is expressed normally on the plasma membrane of transfected cells (20). When coexpressed in HEK293 cells together with β3-Ig-6His, the β3[C24A]-EGFP construct was not coprecipitated with anti-His Talon beads. In contrast, the β3-wt-EGFP protein was successfully coprecipitated under the same cotransfection conditions (Fig. 7A, B). Similarly, the trans homophilic interaction between the β3-wt-Myc and the β3-wt-EGFP subunits was completely abolished by the C24A mutation (Fig. 7C, D). We could detect no coimmunoprecipitation with β3-C24A-EGFP even when the Western blots were deliberately overexposed (data not shown).
Figure 7.
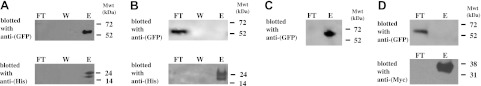
The C2-24 disulfide bond is required for β3 homophilic binding in transfected cells. A, B) HEK293 cells were cotransfected with plasmids expressing β3-wt-EGFP and β3-Ig-6His (A), or cotransfected with β3-[C24A]-EGFP and β3-Ig-6His (B). Samples were precipitated with Talon beads, and blotted as indicated. C) HEK293 cells were separately transfected with plasmids expressing β3-wt-EGFP and β3-wt-Myc. Cells were cocultured for 2 d under conditions of 70–90% confluency prior to cell lysis. Samples were immunoprecipitated with anti-Myc agarose and blotted as indicated. D) Cells were separately transfected with plasmids expressing β3-[C24A]-EGFP and β3-wt-Myc, then cells were cocultured for 2 d under conditions of 70–90% confluency prior to cell lysis and immunoprecipitation with anti-Myc agarose and blotting as indicated. FT, flow through; W, wash; E, elution fraction.
C2–24 disulfide bond is not required for dimerization of the free β3-Ig domain
The failure of the membrane-tethered β3-[C24A]-EGFP subunit to bind homophilically with wild-type β3 could be explained in two ways. The oxidized C2–24 disulfide bond could itself be a direct component of the homophilic binding site. Alternatively, the C2–24 disulfide bond may play a more indirect role, by for example ensuring the correct positioning of the binding site between individual β3 subunits in the constrained environment of the membrane. To distinguish between these two model classes, we examined the homophilic association between purified β3-Ig-6His in free solution using cross-linking and denaturing PAGE, with and without selective reduction of the C2–24 disulfide bond. Similar cross-linking experiments have been used to investigate Ig domain oligomerization for other CAMs in solution (41). Cross-linking confirmed that the purified β3-Ig-6His protein could form higher-molecular-mass complexes in solution. The predominant oligomeric band was consistent with a dimer, although a fainter band consistent with at trimer was also observed. The proportion of the putative dimerized protein reached a plateau after ∼5 min (Fig. 8A). The experiment was repeated using β3-Ig-6His protein that had been reduced by 1.5 mM TCEP, conditions previously shown to reduce the C2-24 disulfide bond selectively (Materials and Methods). The higher-molecular-mass bands of the cross-linked proteins were still detectable. Furthermore, the extent of dimerization was similar to that obtained with native β3-Ig-6His protein, although the rate of dimerization was noticeably accelerated (Fig. 8B). We therefore conclude that the oxidized C2–24 disulfide bond is unlikely to be a direct component of the dimerization site itself, but may influence the accessibility of the binding site, and/or the stability of the Ig domain. The cross-linked, selectively reduced Ig domain also showed a larger proportion of very high molecular mass protein that did not enter the polyacrylamide gel (Fig. 8B). This finding suggests that in the absence of the intact C2–24 disulfide bond, there is an increased tendency of the Ig domain to form higher order and probably nonspecific oligomers, perhaps reflecting a partial destabilization of structure.
Figure 8.
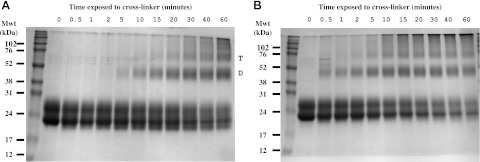
Cross-linking purified β3 Ig domain generates higher Mwt dimer and trimers. Purified native β3-Ig-6His protein (A) and purified β3-Ig-6His containing the selectively reduced and alkylated C2–24 disulfide bond (B) were cross-linked with BS3 as described in Materials and Methods for the times indicated, then examined by denaturing PAGE. Higher-molecular-mass bands corresponding to dimer (D) and trimer (T) are indicated for the case of the native β3-Ig-6His protein. Mwt, molecular weight (mass).
DISCUSSION
We have shown that separate Nav β3 subunits can bind homophilically via their Ig domains. We also show that the β3 subunit can bind heterophilically with β1. Our main aim was to identify functionally important regions of the β3-Ig domain that may influence this behavior. As a first step, we used evolutionary tracing analysis. We used a 3-dimensional prediction based on previously determined structures of proteins with similar Ig domains. We emphasize that fine details of our model are likely to require revision if and when the atomic-resolution structure of the actual β3 Ig domain is determined. Nevertheless, the deep evolutionary conservation of Ig domain structures (38), together with our mass spectrometry data discussed below, suggests that the main features of our model are likely to be correct. We have deliberately chosen the strictest criteria for our evolutionary tracing such that we only record the rank 1(β3/β1) residues that are perfectly conserved in both isoforms across the variety of vertebrate species available in the sequence data bases. This provides a clearer view of the β3/β1 conserved features, although it does risk missing information on functional regions more tolerant to amino acid changes, or on changes that only occur in specific phylogenetic lineages (25). An example of this tradeoff is shown by the N-linked glycosylation sites. There are four potential N-linked glycosylation sites in the Ig domain of mammalian β3 and β1. In β3, these four residues are N71, N85, N89, and N97 (Fig. 5B and ref. 19). Our mass spectrometry analysis indicated that all four sites may be glycosylated (data not shown). Residue N71 lies close to a region of perfect conservation between β3 and β1 (Fig. 5C). However, N71 itself failed to be included as a rank 1(β3/β1) residue. This is because the N71-equivalent residue occurs in placental mammals, marsupials and birds, but not in amphibians or fish. Hence, the N71-linked glycosylation site may be restricted to the amniotes.
Two N-linked glycosylation sites, at N85 and N89 lie within another patch of β3/β1 conserved sequences (Fig. 5C). This may have important functional implications. For example, a similarly located N-linked glycosylation site occurs in myelin P0 and is essential for trans homophilic binding. Here, the glycosylation does not occur within the regions directly responsible for homophilic binding; rather, it facilitates binding by ensuring the correct orientation of the Ig domain in the plane of the plasma membrane (42). This topological requirement may explain why β3-homophilic binding did not occur in mixed cell lysates (Fig. 4C). It may also explain the discrepancy between our results and those of McEwan et al. (23), who did not detect β3-mediated trans-homophilic binding. They employed an assay using β3-transfected Drosophila S cells and monitored cell adhesion visually. However, proteins expressed in Drosophila S cells tend to be underglycosylated, and the attached sugar residues remain in a high mannose form compared to the same protein expressed in mammalian cells (43). Similar high-mannose/underglycosylation patterns have been shown to reduce the efficiency of trans homophilic binding for other Ig domain-containing CAMs (44).
A striking feature of the evolutionary tracing analysis is a contiguous area of rank 1(β3/β1) conservation that is bounded by the cysteines C2 and C24 (Fig. 5A, C). In β3, we show that these cysteines form a disulfide bond. Given the high overall sequence similarity between β3 and β1, it is likely that the Ig domains of these two proteins fold in a very similar manner. Hence the conservation of the two cysteines, in all known β3 and β1 sequences strongly suggests that this surface disulfide bond will also exist in β1. It should be noted that an intrasubunit disulfide bond, equivalent to C2–24 cannot form in either the β2 or β4 subunits because both β2 and β4 lack a cysteine at the C2-equivalent amino acid. Nevertheless, β2 and β4 do still contain a conserved and unpaired cysteine residue in approximately the same location as C24 (45, 46). Interestingly, evidence indicates that the β2 and β4 subunits can form an intersubunit disulfide bond with the Nav α subunit (1, 46). As previously noted (46), this unpaired cysteine is likely to form the β2/β4 disulfide bond with the Nav α subunit.
Immunoprecipitation experiments indicate that the β3 intrasubunit C2–24 disulfide bond is required for homophilic binding of the membrane-localized β3 subunit in vivo. However, cross-linking experiments show that the dimerization of the purified β3-Ig domain in free solution can still occur when the C2–24 disulfide bond was selectively reduced. Taken together, these experiments suggest that the C2–24 disulfide bond is unlikely to form part of the dimerization site per se. Instead, we suggest that the C2–24 disulfide bond is required to be in the oxidized state so that the Ig domains of the in vivo membrane-bound β3 subunits can correctly align for binding. The Ig domains of cell-adhesion molecules are likely to be more topologically constrained within the full-length protein and when located on a crowded membrane surface compared to when they are examined as isolated domains in free solution (47). Further work can now be directed to the identification of the dimerization site itself, and here the evolutionary tracing analysis can again be used to guide experiments. Interestingly, an antibody raised against a peptide containing the rank 1(β3/β1) sequences of the predicted A-A′ strand has been shown to block β1-dependent homophilic trans cell adhesion (17). In our model, this region forms an extended face of the Ig domain bounded by the C2–24 disulfide bond (Fig. 5). It is therefore possible that the C2–24 disulfide bond is required to hold this face in the correct orientation for productive association of the full-length subunit in vivo. The rank 1(β3/β1) sequences within this region will be good candidates for further mutagenesis studies. Several examples of plasma membrane proteins contain surface disulfide bonds whose redox status can be functionally regulated (48). The mass spectrometry data show that under the standard tissue-culture conditions used in our experiments, the C2–24 disulfide bond is oxidized. Nevertheless, it will be interesting to see whether other physiological situations exist where the redox status of the β3 C2–24 disulfide bond can be modified in vivo. A related question is whether the C2 and the C24 residues could form an intersubunit, rather than an intrasubunit disulfide bond, and thus generate covalent β3 homodimers. However, based on mobility shift experiments in denaturing-PAGE gels lacking reducing agents, we can find no evidence for such structures in our transfected cell-lines (data not shown).
The β3-subunit Ig domain is attached to the transmembrane domain by a stalk ∼10 aa long (Fig. 1A and ref. 19). If fully extended, this would be ∼4 nm long, which is ∼75% of the longest dimension of a typical Ig domain (31). The extended stalk region should, therefore, impart rotational flexibility to the β-subunit ECD. Consequently, the β3 subunits may be capable of cis as well as trans homophilic binding. If so, this raises an interesting question about whether the β3 (and most likely β1) subunits might induce the Nav α subunits to oligomerize in electrically excitable cells. We have previously shown that the β3-[C24A]-EGFP mutant altered the voltage-dependency of inactivation for Nav1.5 (20). Hence, it was natural to assume this region defined an α-subunit binding site. However, our new data raise the possibility that the C24A mutant might interfere with α-subunit gating in more subtle and indirect ways, for example by perturbing the incorporation of Nav channels into higher order membrane clusters.
The β3 and β1 subunits have broadly distinct expression patterns, but they are also coexpressed in some tissues. For example, the hippocampus (19, 26, 49), the transverse-tubular system of cardiac myocytes (22, 50), and on the myocyte intercalated discs (data not shown). All of these examples offer the opportunity for β3 and β1 subunits to interact heterophilically. Our data showing that the β3-Ig domains can bind heterophilically to β1 differs from a previous report that failed to detect such an interaction (12). In this work, Fc fusion constructs of the free Ig domain for each β subunit were applied to cells expressing full-length β subunits. Binding was then assayed using immunofluorescence. It is possible that β3/β1 heterophilic binding has a lower affinity compared to homophilic binding such that it may be difficult to detect using assays based on the addition of free protein to cells in culture, compared to the higher concentrations possible within the lumen of the endoplasmic reticulum of cotransfected cells. In our evolutionary tracing analysis, there are only two rank 2(β3/β1) residues (i.e., fully conserved within β3 and β1 but different between the two isoforms). They are equivalent to Y40 and M25 in β3. Residue Y40 is replaced by a similarly hydrophobic phenylalanine in β1 and is predicted to be buried. However, residue M25 in β3 is replaced with a nonconservative lysine in β1. In our model, it lies directly adjacent to the C2–24 disulfide bond (Fig. 5B, C). Also several residues differ between β3 and β1 but were not recorded by our evolutionary tracing. This is because their β3- and β1-specific identities have changed over evolutionary time, although a number have remained constant within individual phylogenetic lineages. An interesting example is residue 6, which is aspartic acid in all sampled β1 sequences, and proline in all sampled β3 sequences except Xenopus laevis (Fig. 5C). Residue 6 lies within the putative A-A′ β sheet described above, and its potential importance has been noted previously (19, 23). These β3- and β1-specific differences may reduce the strength of heterophilic as compared to homophilic binding.
In summary, the use of evolutionary tracing analysis to guide our experiments has been crucial in the identification and functional analysis of an unusual structural feature in the Nav β3 subunit. We are currently applying evolutionary tracing to other aspects of the Nav β-subunit family, including those sequences conserved between all β subunits and those specific to β2 and β4. We believe that such an approach has considerable potential to generate further structure/function hypotheses concerning this versatile family of molecules.
Supplementary Material
Acknowledgments
N.R.Y. was supported by funds from Cambridge University. F.S.C. was supported by a Collaborative Awards in Science and Engineering (CASE) studentship from the U.K. Biotechnology and Biological Sciences Research Council and funds from GlaxoSmithKline. T.P.M. was supported by a Wellcome Trust Career Development Award (WT085090MA).
The authors thank John McCafferty for helping with Gateway cloning technology and Jamie Ashby for helpful comments.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BSA
- bovine serum albumin
- BS3
- bis- (sulfosuccinimidyl) suberate
- cam
- amidocarboxymethylcysteine
- CAM
- cell adhesion molecule
- CDAP
- 1-cyano-4-dimethylaminopyridinum tetrafluoroborate
- DMEM
- Dulbecco's modified Eagle's medium
- DTT
- dithiothreitol
- ECD
- extracellular domain
- EGFP
- enhanced green fluorescent protein
- ELISA
- enzyme-linked immunoadsorbent assay
- FCS
- fetal calf serum
- GFP
- green fluorescent protein
- HEK
- human embryonic kidney
- Ig
- immunoglobulin
- LDS
- lithium dodecylsulfate
- MALDI-MS
- matrix-assisted laser desorption/ionization-mass spectrometry
- Nav
- voltage-gated sodium
- PBS
- phosphate-buffered saline
- PNGase F
- peptide N-glycosidase F
- PAGE
- polyacrylamide gel electrophoresis
- SDS
- sodium dodecylsulfate
- TCEP
- tris-(2-carboxyethyl) phosphine
- TFA
- trifluoroacetic acid
REFERENCES
- 1. Catterall W. A. (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26, 13–25 [DOI] [PubMed] [Google Scholar]
- 2. Chahine M., O'Leary M. E. Regulatory role of voltage-gated Na channel beta subunits in sensory neurons. Front Pharmacol. 2, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brackenbury W. J., Isom L. L. Na channel beta subunits: overachievers of the ion channel family. Front Pharmacol.. 2, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cusdin F. S., Clare J. J., Jackson A. P. (2008) Trafficking and cellular distribution of voltage-gated sodium channels. Traffic 9, 17–26 [DOI] [PubMed] [Google Scholar]
- 5. Isom L. L., De Jongh K. S., Catterall W. A. (1994) Auxiliary subunits of voltage-gated ion channels. Neuron 12, 1183–1194 [DOI] [PubMed] [Google Scholar]
- 6. Chen C., Westenbroek R. E., Xu X., Edwards C. A., Sorenson D. R., Chen Y., McEwen D. P., O'Malley H. A., Bharucha V., Meadows L. S., Knudsen G. A., Vilaythong A., Noebels J. L., Saunders T. L., Scheuer T., Shrager P., Catterall W. A., Isom L. L. (2004) Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J. Neurosci. 24, 4030–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen C., Bharucha V., Chen Y., Westenbroek R. E., Brown A., Malhotra J. D., Jones D., Avery C., Gillespie P. J., 3rd, Kazen-Gillespie K. A., Kazarinova-Noyes K., Shrager P., Saunders T. L., Macdonald R. L., Ransom B. R., Scheuer T., Catterall W. A., Isom L. L. (2002) Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel beta 2-subunits. Proc. Natl. Acad. Sci. U. S. A. 99, 17072–17077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hakim P., Gurung I. S., Pedersen T. H., Thresher R., Brice N., Lawrence J., Grace A. A., Huang C. L. (2008) Scn3b knockout mice exhibit abnormal ventricular electrophysiological properties. Prog. Biophys. Mol. Biol. 98, 251–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Isom L. L., Catterall W. A. (1996) Na+ channel subunits and Ig domains. Nature 383, 307–308 [DOI] [PubMed] [Google Scholar]
- 10. Malhotra J. D., Kazen-Gillespie K., Hortsch M., Isom L. L. (2000) Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J. Biol. Chem. 275, 11383–11388 [DOI] [PubMed] [Google Scholar]
- 11. Ratcliffe C. F., Westenbroek R. E., Curtis R., Catterall W. A. (2001) Sodium channel beta1 and beta3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J. Cell Biol. 154, 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McEwen D. P., Isom L. L. (2004) Heterophilic interactions of sodium channel beta1 subunits with axonal and glial cell adhesion molecules. J. Biol. Chem. 279, 52744–52752 [DOI] [PubMed] [Google Scholar]
- 13. Kazarinova-Noyes K., Malhotra J. D., McEwen D. P., Mattei L. N., Berglund E. O., Ranscht B., Levinson S. R., Schachner M., Shrager P., Isom L. L., Xiao Z. C. (2001) Contactin associates with Na+ channels and increases their functional expression. J. Neurosci. 21, 7517–7525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Srinivasan J., Schachner M., Catterall W. A. (1998) Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc. Natl. Acad. Sci. U. S. A. 95, 15753–15757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xiao Z. C., Ragsdale D. S., Malhotra J. D., Mattei L. N., Braun P. E., Schachner M., Isom L. L. (1999) Tenascin-R is a functional modulator of sodium channel beta subunits. J. Biol. Chem. 274, 26511–26517 [DOI] [PubMed] [Google Scholar]
- 16. Malhotra J. D., Koopmann M. C., Kazen-Gillespie K. A., Fettman N., Hortsch M., Isom L. L. (2002) Structural requirements for interaction of sodium channel beta 1 subunits with ankyrin. J. Biol. Chem. 277, 26681–26688 [DOI] [PubMed] [Google Scholar]
- 17. Davis T. H., Chen C., Isom L. L. (2004) Sodium channel beta1 subunits promote neurite outgrowth in cerebellar granule neurons. J. Biol. Chem. 279, 51424–51432 [DOI] [PubMed] [Google Scholar]
- 18. Brackenbury W. J., Davis T. H., Chen C., Slat E. A., Detrow M. J., Dickendesher T. L., Ranscht B., Isom L. L. (2008) Voltage-gated Na+ channel beta1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo. J. Neurosci. 28, 3246–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morgan K., Stevens E. B., Shah B., Cox P. J., Dixon A. K., Lee K., Pinnock R. D., Hughes J., Richardson P. J., Mizuguchi K., Jackson A. P. (2000) beta 3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc. Natl. Acad. Sci. U. S. A. 97, 2308–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu E. J., Ko S. H., Lenkowski P. W., Pance A., Patel M. K., Jackson A. P. (2005) Distinct domains of the sodium channel beta3-subunit modulate channel-gating kinetics and subcellular location. Biochem. J. 392, 519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cusdin F. S., Nietlispach D., Maman J., Dale T. J., Powell A. J., Clare J. J., Jackson A. P. (2010) The sodium channel {beta}3-subunit induces multiphasic gating in NaV1.3 and affects fast inactivation via distinct intracellular regions. J. Biol. Chem. 285, 33404–33412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hakim P., Brice N., Thresher R., Lawrence J., Zhang Y., Jackson A. P., Grace A. A., Huang C. L. (2009) Scn3b knockout mice exhibit abnormal sino-atrial and cardiac conduction properties. Acta Physiol. (Oxf.) 198, 47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McEwen D. P., Chen C., Meadows L. S., Lopez-Santiago L., Isom L. L. (2009) The voltage-gated Na+ channel beta3 subunit does not mediate trans homophilic cell adhesion or associate with the cell adhesion molecule contactin. Neurosci. Lett. 462, 272–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lichtarge O., Bourne H. R., Cohen F. E. (1996) An evolutionary trace method defines binding surfaces common to protein families. J. Mol. Biol. 257, 342–358 [DOI] [PubMed] [Google Scholar]
- 25. Innis C. A., Shi J., Blundell T. L. (2000) Evolutionary trace analysis of TGF-beta and related growth factors: implications for site-directed mutagenesis. Protein Eng. 13, 839–847 [DOI] [PubMed] [Google Scholar]
- 26. Van Gassen K. L., de Wit M., van Kempen M., van der Hel W. S., van Rijen P. C., Jackson A. P., Lindhout D., de Graan P. N. (2009) Hippocampal Nabeta3 expression in patients with temporal lobe epilepsy. Epilepsia 50, 957–962 [DOI] [PubMed] [Google Scholar]
- 27. Meadows L. S., Chen Y. H., Powell A. J., Clare J. J., Ragsdale D. S. (2002) Functional modulation of human brain Nav1.3 sodium channels, expressed in mammalian cells, by auxiliary beta 1, beta 2 and beta 3 subunits. Neuroscience 114, 745–753 [DOI] [PubMed] [Google Scholar]
- 28. Shi J., Blundell T. L., Mizuguchi K. (2001) FUGUE: sequence-structure homology recognition using environment-specific substitution tables and structure-dependent gap penalties. J. Mol. Biol. 310, 243–257 [DOI] [PubMed] [Google Scholar]
- 29. Sali A., Blundell T. L. (1993) Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 [DOI] [PubMed] [Google Scholar]
- 30. Wiesmann C., Katschke K. J., Yin J., Helmy K. Y., Steffek M., Fairbrother W. J., McCallum S. A., Embuscado L., DeForge L., Hass P. E., van Lookeren Campagne M. (2006) Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature 444, 217–220 [DOI] [PubMed] [Google Scholar]
- 31. Shapiro L., Doyle J. P., Hensley P., Colman D. R., Hendrickson W. A. (1996) Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron 17, 435–449 [DOI] [PubMed] [Google Scholar]
- 32. Chen V. B., Arendall W. B., 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., Richardson D. C. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Condreay J. P., Witherspoon S. M., Clay W. C., Kost T. A. (1999) Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc. Natl. Acad. Sci. U. S. A. 96, 127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chapple S. D., Crofts A. M., Shadbolt S. P., McCafferty J., Dyson M. R. (2006) Multiplexed expression and screening for recombinant protein production in mammalian cells. BMC Biotechnol. 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas P., Smart T. G. (2005) HEK293 cell line: a vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods 51, 187–200 [DOI] [PubMed] [Google Scholar]
- 36. Merrick E. C., Kalmar C. L., Snyder S. L., Cusdin F. S., Yu E. J., Sando J. J., Isakson B. E., Jackson A. P., Patel M. K. (2010) The importance of serine 161 in the sodium channel beta3 subunit for modulation of Na(V)1.2 gating. Pflügers Arch. 460, 743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCormick K. A., Isom L. L., Ragsdale D., Smith D., Scheuer T., Catterall W. A. (1998) Molecular determinants of Na+ channel function in the extracellular domain of the beta1 subunit. J. Biol. Chem. 273, 3954–3962 [DOI] [PubMed] [Google Scholar]
- 38. Bork P., Holm L., Sander C. (1994) The immunoglobulin fold. Structural classification, sequence patterns and common core. J. Mol. Biol. 242, 309–320 [DOI] [PubMed] [Google Scholar]
- 39. Meadows L. S., Malhotra J., Loukas A., Thyagarajan V., Kazen-Gillespie K. A., Koopman M. C., Kriegler S., Isom L. L., Ragsdale D. S. (2002) Functional and biochemical analysis of a sodium channel beta1 subunit mutation responsible for generalized epilepsy with febrile seizures plus type 1. J. Neurosci. 22, 10699–10709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gallegos-Perez J. L., Rangel-Ordonez L., Bowman S. R., Ngowe C. O., Watson J. T. (2005) Study of primary amines for nucleophilic cleavage of cyanylated cystinyl proteins in disulfide mass mapping methodology. Anal. Biochem. 346, 311–319 [DOI] [PubMed] [Google Scholar]
- 41. Klaile E., Vorontsova O., Sigmundsson K., Muller M. M., Singer B. B., Ofverstedt L. G., Svensson S., Skoglund U., Obrink B. (2009) The CEACAM1 N-terminal Ig domain mediates cis- and trans-binding and is essential for allosteric rearrangements of CEACAM1 microclusters. J. Cell Biol. 187, 553–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Filbin M. T., Tennekoon G. I. (1993) Homophilic adhesion of the myelin P0 protein requires glycosylation of both molecules in the homophilic pair. J. Cell Biol. 122, 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gardsvoll H., Werner F., Sondergaard L., Dano K., Ploug M. (2004) Characterization of low-glycosylated forms of soluble human urokinase receptor expressed in Drosophila Schneider 2 cells after deletion of glycosylation-sites. Protein Expr. Purif. 34, 284–295 [DOI] [PubMed] [Google Scholar]
- 44. Filbin M. T., Tennekoon G. I. (1991) The role of complex carbohydrates in adhesion of the myelin protein, P0. Neuron 7, 845–855 [DOI] [PubMed] [Google Scholar]
- 45. Isom L. L., Ragsdale D. S., De Jongh K. S., Westenbroek R. E., Reber B. F., Scheuer T., Catterall W. A. (1995) Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell 83, 433–442 [DOI] [PubMed] [Google Scholar]
- 46. Yu F. H., Westenbroek R. E., Silos-Santiago I., McCormick K. A., Lawson D., Ge P., Ferriera H., Lilly J., DiStefano P. S., Catterall W. A., Scheuer T., Curtis R. (2003) Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J. Neurosci. 23, 7577–7585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van der Merwe P. A., Barclay A. N. (1994) Transient intercellular adhesion: the importance of weak protein-protein interactions. Trends Biochem. Sci. 19, 354–358 [DOI] [PubMed] [Google Scholar]
- 48. Wouters M. A., George R. A., Haworth N. L. (2007) “Forbidden” disulfides: their role as redox switches. Curr. Protein Pept. Sci. 8, 484–495 [DOI] [PubMed] [Google Scholar]
- 49. Shah B. S., Stevens E. B., Pinnock R. D., Dixon A. K., Lee K. (2001) Developmental expression of the novel voltage-gated sodium channel auxiliary subunit beta3, in rat CNS. J. Physiol. 534, 763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maier S. K., Westenbroek R. E., McCormick K. A., Curtis R., Scheuer T., Catterall W. A. (2004) Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation 109, 1421–1427 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.