Abstract
It is a prevalent concept that, in line with the Wobble Hypothesis, those tRNAs having an adenosine in the first position of the anticodon become modified to an inosine at this position. Sequencing the cDNA derived from the gene coding for cytoplasmic tRNAArgACG from several higher plants as well as mass spectrometric analysis of the isoacceptor has revealed that for this kingdom an unmodified A in the wobble position of the anticodon is the rule rather than the exception. In vitro translation shows that in the plant system the absence of inosine in the wobble position of tRNAArg does not prevent decoding. This isoacceptor belongs to the class of tRNA that is imported from the cytoplasm into the mitochondria of higher plants. Previous studies on the mitochondrial tRNA pool have demonstrated the existence of tRNAArgICG in this organelle. In moss the mitochondrial encoded distinct tRNAArgACG isoacceptor possesses the I34 modification. The implication is that for mitochondrial protein biosynthesis A-to-I editing is necessary and occurs by a mitochondrion-specific deaminase after import of the unmodified nuclear encoded tRNAArgACG.
Keywords: wobble hypothesis, inosine, tRNAArg, deaminase, decoding, plant, mitochondrial tRNA import
Introduction
Codon-anticodon recognition between triplets of an mRNA and a specific tRNA is the key element in the translation of the genetic code. In general, the precision of this process is dominated by a strict Watson-Crick base-pairing scheme. However, the degeneracy of the genetic code led Crick to propose the Wobble Hypothesis,1 permitting a less restraining interaction with the third base of the codon and involving the participation of inosine for decoding C-ending codons. In formulating this concept, Crick predicted that, “…. inosine will be formed enzymically from an adenine in the nascent sRNA. This may mean that A in this position will be rare or absent, depending upon the exact specificity of the enzyme(s) involved.”1 The validity of this insight was subsequently confirmed, as evident from the collection of primary structures of numerous tRNAs2 and elucidated in detail in a review3 which pointed out a mere four exceptions to the rule.4-7
Recently, while characterizing an A-to-I editing system in Arabidopsis chloroplasts using the standard reverse transcriptase assay,8 “surprising” data, which were not commented further, were presented showing that the mature cytoplasmic tRNAArgACG counterpart showed no signs of an A-to-I conversion. This independent observation was in agreement with results that have emerged from our work on the plant arginine system9 and confirmed our doubts on the accepted nature of the wobble base in this tRNA.
The RNA sequence of a wheat tRNAArg isoacceptor whose gene encodes an ACG anticodon was reported in 1986.10 Among other modified bases that were found, particular emphasis was placed on the presence of inosine at the wobble position of the anticodon. While its presence in a plant tRNA had not been reported previously, an inosine at this position of the anticodon was in line with the Wobble hypothesis and not inappropriate in view of its established localization in murine11 and bacterial12 tRNAArg, as well as subsequently in the chloroplast isoacceptor.13 Glover et al.,14 analyzed the group of tRNAs that are nuclear encoded and subsequently imported into wheat mitochondria. Here, using chemical sequencing, reverse transcriptase sequencing and 2D TLC, the presence of I34 in tRNAArg2 confirmed the similar finding in imported potato mitochondrial tRNAArg.15 Thus, plant tRNAArgICG has been propagated in the literature16-20 and has become an accepted member of the tRNA family. In view of the discrepancy between the accepted status quo and the published8 data, we have performed a number of analyses from several plant species (including wheat) while using the corresponding isoacceptor from E.coli as a positive control. Whereas inosine is readily detected in the bacterial tRNAArg, we find no evidence for the presence of this modification in plant cytoplasmic tRNAArg.
Results
Reverse transcriptase analysis of plant tRNAArgACG
The in vivo gene product of the E.coli tDNAArgACG is a classic example of a tRNA whose anticodon A34 is deaminated by tadA to inosine.21 To confirm that the RT/PCR protocol22 was able to detect authentic inosine in tRNA, partially purified, overexpressed E. coli tRNAArgICG23 was subjected to this analysis. Accordingly, Figure 1B reveals a G at this position as a result of I34 pairing with C during reverse transcription. In contrast, the unmodified genomic sequence tRNA (Fig. 1A) retains the A34 sequence. tRNAs extracted from three plants, including examples of both monocots and dicots, when examined by this method for the presence of inosine in tRNAArg are characterized by an unequivocal A34 sequence (Fig. 1C‒E). As in the case of Arabidopsis,8 we interpret this as evidence for the lack of deamination at this position.
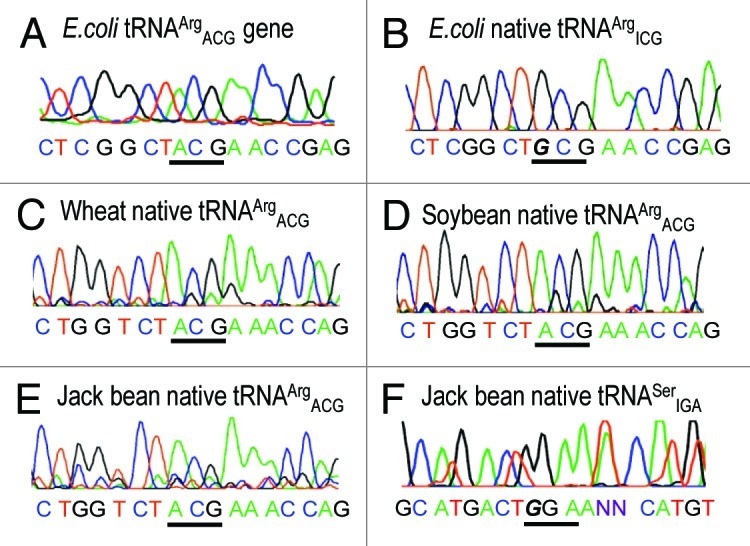
Figure 1. DNA sequence analysis of PCR products generated from genomic DNA (A) and reverse transcribed tRNAs (B‒F). Anticodon stem/loop sequences are shown for the tRNAs indicated. For the purposes of this communication, native tRNA refers to tRNAs extracted directly from the organism, as opposed to transcript tRNA prepared by in vitro transcription. In each case, the anticodon is indicated by a bar and the A-to-I deaminated base 34 (read as G by the reverse transcriptase) in italics.
Unlike prokaryote and chloroplast-encoded tRNAs, in eukaryotes tRNAArg is not the only isoacceptor in which inosine plays a role in codon-anticodon recognition.24 As a representative of the other seven potential I34-bearing tRNAs, we analyzed cytoplasmic tRNASerIGA which has been sequenced from two different plant species and by two independent groups (tobacco25; spinach26) and whose genomic sequence reveals an A34 (see e.g., NCBI trace archive gnl|ti|1697792733, for the Arabidopsis equivalent). Using primers derived from the soybean genomic sequence, the presence of inosine in the related jack bean tRNASerIGA could be confirmed (Fig. 1F).
Mass spectrometry
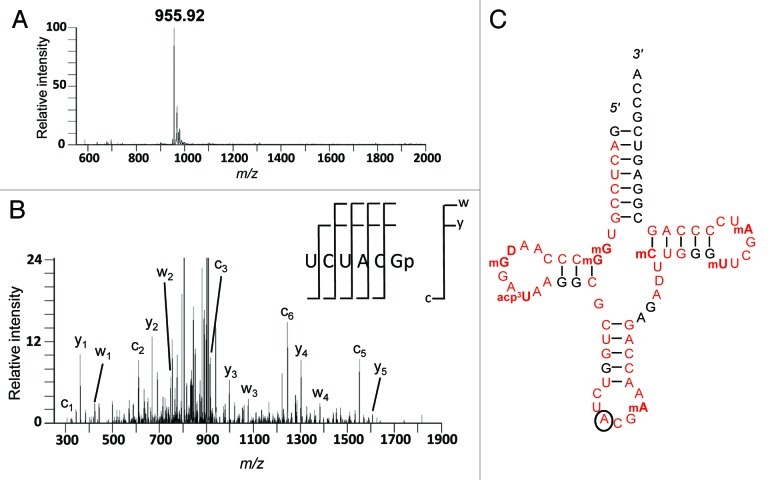
The tRNAArgACG isoacceptor was isolated from wheat leaves by oligonucleotide capture,27 subjected to RNase T1 digestion, and analyzed by LC-MS/MS.28,29 Digestion products of the tRNA containing inosine were not detected (Fig. S1); however, an unmodified version of the anticodon portion of the tRNA containing A34 was found. This RNase T1 digestion product was detected as a doubly charged species (m/z 955.9) (Fig. 2A). The doubly charged ion was selected for sequencing by tandem mass spectrometry (MS/MS), and the fragment ions (c-, y-, and w-ions) are consistent with an RNase T1 digestion product sequence of UCUACGp (Fig. 2B). This sequence is found in only one location of this tRNA, from position 31 to 37 (Fig. 2C). Further sequencing of additional RNase T1 digestion products (Figs. S2–9) were used to obtain a tRNA sequence that was in full agreement with the gene sequence and a modification pattern comparable to published data.10,14
Figure 2. Mass spectrum (A) depicting a doubly charged ion (m/z 955.92) and fragmentation pattern after collision induced dissociation (B) of the RNase T1-derived oligonucleotide covering the anticodon region of tRNAArgACG from wheat with the sequence UCUACG. All c- and y-type fragment ions and 4 of 5 possible w-type ions were detected and used to confirm the sequence. The position of A34 in the compiled sequence of the entire tRNA is circled (C). RNase T1 digestion products whose fragmentation pattern (Figs. S1–9) was used to establish the sequence are red. Modified bases are shown in bold.
In vitro translation
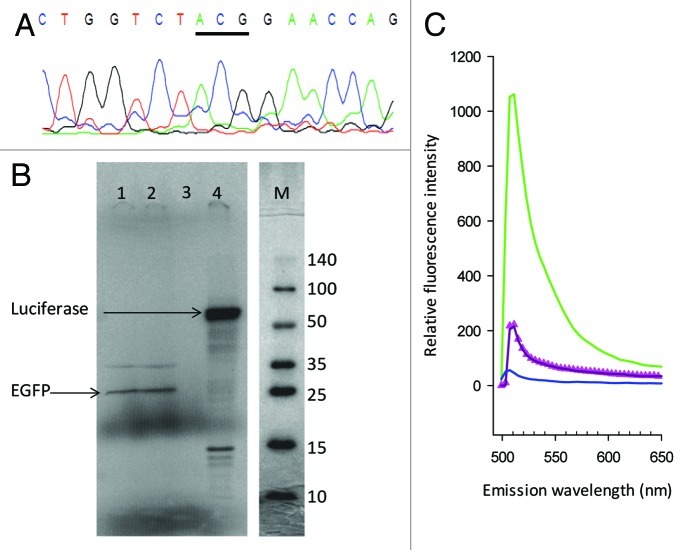
Cell free wheat germ extracts have been used for decades30 for the efficient translation of mRNA in vitro. Total tRNA from a commercial wheat germ extract was isolated and the tRNAArgACG isoacceptor sequenced by the RT/PCR procedure. As in the case of tRNA isolated from wheat leaves, this sequence was characterized by an unmodified A34 (Fig. 3A). Although the success of this extract in translating a host of different mRNAs over the years favors the conclusion that the arginine CGC codon (assumed to be read by ICG) does not hinder translation despite the lack of inosine, we tested its efficacy using a GFP gene in which all 6 arginine codons had been converted to CGC (pEGFP-N3; Accession number U57609). Figure 3B shows that a translation product of the correct size is obtained. Fluorescence measurements with the GFP translation product gave an emission spectrum typical (Emax 507 nm) for this protein (Fig. 3C) compared with the negative control (translation vector without GFP insert or luciferase gene insert). Similarly, the manufacturer’s control luciferase gene product whose arginines at positions 437 and 513 are coded by CGC, is efficiently translated to a product of the expected size. As only full-length luciferase is active (Promega, Technical Notes), the positive luminescence measurements obtained with this product (data not shown) confirmed the translation of the two CGC codons.
Figure 3. In vitro translation of CGC encoded arginine by a wheat germ extract. (A) Sequence of RT/PCR product from tRNAArgACG extracted from commercial wheat germ cell free translation extract. The anticodon is underlined. (B) SDS gel analysis of in vitro translation using wheat germ extract. Product formation was monitored by phosphorimager analysis of the incorporation of [35S]-Met into EGFP in whose gene all arginine codons had been replaced by CGC. Lanes 1 and 2, EGFP protein (predicted, 26.9 kDa); 3, Flexi ® Vector without an integrated gene sequence; 4, Luciferase positive control (predicted, 61 kDa); M, Protein Ladder. Samples and marker were run on the same gel. The unlabelled protein ladder was visualized optically and the separate images scaled to the identical size. The red molecular weight bands of 40, 70 and 260 kDa of the marker were not visible under the conditions of the image capture. (C) Fluorescence emission spectrum of the translation products EGFP (green), translation vector only (pink triangles), luciferase (purple) and no translation template (blue). Excitation was at 470 nm.
Mitochondrial decoding requires A-to-I editing
In contrast to angiosperms, which rely on the import of cytoplasmic tRNAArgACG into the mitochondria,31 the mitochondrial genome (AB251495) of the bryophyte P. patens encodes a distinct tRNAArgACG isoacceptor.32 Ligation of an RNA tail to the 3'end of moss total RNA followed by a tail specific reverse transcription provides a cDNA population from which the mitochondrial tDNAArgACG may be specifically amplified using the corresponding 5' targeted primer. Sequence analysis of this amplicon clearly shows the effect of ACG to ICG conversion with the tell-tale evidence of GCG in the DNA sequence trace (Fig. 4).
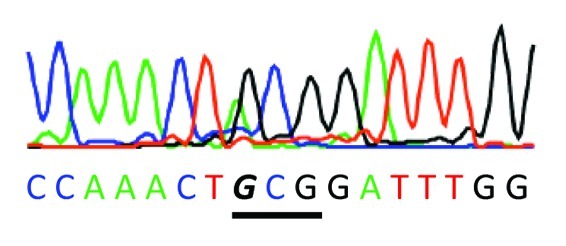
Figure 4. DNA sequence of the RT/PCR product derived from moss mitochondrial tRNAArgACG. Anticodon stem/loop sequence is shown. The anticodon is indicated by a bar and the A-to-I deaminated base 34 (read as G by the reverse transcriptase) is in italics
Discussion
The concept that A34 of tRNAArgACG in all eukaryotes, eubacteria and plant chloroplasts is converted to I34 is firmly anchored in the literature. Auxilien, et al.,33 pointed out that in archaea, in the mitochondria of single-cell organisms and in animal mitochondria no tRNA has been found to contain inosine 34. Following the revelation that the Arabidopsis cytoplasmic tRNAArgACG showed a complete lack of A-to-I conversion,8 we have performed a series of experiments that substantiate our claim that higher plant cytoplasmic tRNAArgACG does not harbor an inosine in its anticodon.
RT PCR of tRNAs is the method of choice for detecting certain modified bases, including inosine.22 To avoid potential trace contamination with genomic DNA, we chose to ligate an RNA oligonucleotide to the 3' end of the tRNA of interest, to which the reverse transcription primer is targeted. For amplification, specificity was dictated by the 5' tRNA-specific primer. Total tRNA from the leaves of wheat (T. aestivum), soybean (G. max) and jack bean (C. ensiformis) was isolated and the nominal tRNAArgICG isoacceptor analyzed. In none of the cytoplasmic tRNAArgACG of the three plants could inosine be detected (Fig. 1C‒E), in contrast to native tRNAArgICG from E. coli (Fig. 1B).
In order to confirm the lack of deamination of tRNAArgACG, the specific isoacceptor was enriched by oligonucleotide capture27 and subjected to mass spectrometric analysis which confirmed conclusively the absence of inosine. Discrepancies with respect to the identity of other modified bases documented by Barciszewska, et al.10 were observed as unmodified m22G26 and unmethylated C32 (Figs. S1 and 2). The RNase T1 fragment containing D47 and mC49 was also observed in its unmodified form (Fig. S9), which is consistent with an unmodified C49 as reported by Glover et al.14 Confirmation of Cm3214,15 in the mitochondrial tRNAArgACG pool may point to a modification which is characteristic for the imported species (see below).
In angiosperms, the mitochondrial genome codes for a certain number of tRNAs34 and represent a subclass of tRNAs none of which have an A34 and hence do not require A-to-I editing to fulfill their decoding function. Cytoplasmic tRNAArgACG is imported into the mitochondria of angiosperms31 but is encoded by the mitochondrial genome of, e.g., mosses and some algae.32,35 The fraction of cytoplasmic tRNA that is imported into the mitochondria varies greatly36 but represents less than 0.15% for tRNAArgACG in Chlamydomonas. Imported tRNAs have been characterized in wheat14 and in potato.15 In both these plants, the presence of I34 in tRNAArgICG was conclusively and independently shown.
Should an inosine in the wobble position of tRNAArgACG be essential for mitochondrial protein synthesis, one would expect to find evidence for this modification in the mitochondrial cDNA of plants that do not rely on its import. Of the non-vascular plants that encode tRNAArgACG in their mitochondrial genome,32 we have examined this isoacceptor in the moss P. patens. Using primers specific for the tRNAArgACG encoded by the mitochondrial DNA an RT-PCR product was generated whose sequence is in accordance with the gene sequence (Acc. No. AB251495, position 54682–54609) with the exception of an A34I conversion (Fig. 4) whose efficiency resembles that documented for the chloroplast encoded tRNAArgACG editing in Arabidopsis.8
Evidently, inosine in the first position of the anticodon is required by the plant mitochondrial decoding system with the example of moss disproving the long-held supposition that inosine does not occur in mitochondrial tRNAs.37,38 In moss the three genetic compartments encode individual tRNAArgACG isoacceptors of which the chloroplast and the mitochondrial transcripts are deaminated by organelle-specific deaminases. The cytoplasmic translation machinery of plants has evolved to dispense with this requirement yet the mitochondria of angiosperms rely on the post-import modification of the nuclear encoded tRNAArgACG to provide a functional decoding ability. Post-import alterations in the structure of tRNAs have been recorded for plant isoacceptors of Leu (G18 to Gm18),39 for trypanosome Leu, Lys and Tyr (C32 to Cm3240), Glu (U34 dethiolation41), Trp (C34 to U34 and U33 thiolation42) and for Leishmania Glu, Gln, Lys (U34 to Um34 and dethiolation43). The mitochondrion-specific A-to-I conversion reported here is the first example of an adjustment of the decoding properties within the plant organelle’s genetic system. The originally published sequence of tRNAArgICG,10 which has been the single source for globally specifying the presence of I34 in plant cytoplasmic tRNAArg, makes no explicit claim as to its subcellular origin but has been assumed to be cytoplasmic.2 On the basis of its inosine content, it is now apparent that it should be re-assigned as originating from the mitochondrial RNA pool.
In eukaryotes 7–8 cytoplasmic tRNAs contain I at the first position of the anticodons.24 If, as is proposed for the A34I conversion in chloroplasts,44 the editing of tRNAArgACG in mitochondria is a remnant from the prokaryotic origins of the organelles, those cytoplasmic tRNAs which are targets for other deaminases should still reflect their inosine content following RT/PCR. In accordance with this, inosine could be found in tRNASerIGA (which is not imported into mitochondria) in total jack bean tRNA (Fig. 1F), in line with the published sequences of these spinach and tobacco cytoplasmic tRNAs.25,26 tRNAValIAC, which has been characterized as a component of the imported mitochondrial tRNA pool,14 exists in its IAC-anticodon form in the plant cytoplasm45
Characterization of the deaminase in yeast17,33 provided evidence for the presence of a single enzyme for all A34-containing tRNAs. Since we confirmed A-to-I editing in the jack bean tRNASerIGA, and the imported tRNAValIAC is performed in the cytoplasm,45 it would appear that in plants the cytoplasmic deaminase specifically avoids tRNAArgACG modification. The yeast TAD2 activity is dependent on the presence of a purine at the central position of the anticodons but, exceptionally, also edits the ACG anticodon.33 This exception appears to have been lost by the corresponding deaminase in plants. The only A-to-I deaminase found in plants to date, TADA, has been shown to be targeted exclusively to the chloroplasts8,38 and is responsible for I34 formation in the plastid-encoded tRNAArgACG transcript. An additional, mitochondrial targeted, tadA-like deaminase would have to be responsible for the post-import A34I conversion in the mitochondrial isoacceptor.
The absence of inosine in cytoplasmic tRNAArgACG raises the question of how CGC codons in the nuclear genome are translated. CGC codons are widespread in the plant genome, yet no gene for a functional tRNA with a GCG anticodon exists.24 The decoding functionality of an unmodified A34 was examined in an in vitro translation system. If inosine is required to read a C in the wobble position, as demanded by the Wobble Hypothesis, the presence of CGC codons might be expected to prevent or reduce the efficiency of translation or induce mistranslation. A gene for GFP in which all arginine codons had been replaced by CGC was, nevertheless, efficiently translated by a commercial wheat germ extract in which an unmodified wobble base in tRNAArg had been established (Fig. 3). Furthermore, in GFP the presence of native Arg96 or mutant Lys96 has been shown to be critical for fluorophore formation.46 The fact that the product of in vitro translation expressed the optical properties of GFP suggests that mistranslation of the codon has not occurred. Although we cannot claim that tRNAArgACG rather than another arginine isoacceptor is responsible for the translation of the CGC codon, it is evident that its decoding is independent of the presence of inosine. Other examples in which decoding occurs by an unmodified A34 are nematode6 and yeast mitochondrial tRNAArgACG7 although their genomes use the CGC codon (http://www.kazusa.or.jp/codon/). Alternative codon reading mechanisms, such as two-out-of-three,47 provide a potential solution, especially since two G:C base pairs are available. The plausible existence of an A:C base pair which would be formed by the unmodified A34 has been emphasized48 and such structures are known from other regions of the tRNA49 enabling the stabilization of the two-out-of-three mechanism. This would also be consistent with the situation in the Salmonella mutant in which the tRNAProGGG had been replaced by a tRNAProAGG but the CCC codon reading ability was not impaired by the A:C mismatch.4 With the tRNAThrAGU of mycoplasma, the only reported cytoplasmic tRNA with an unmodified A34, to date,5 the translation of all threonine codons except ACA was demonstrated in vitro.50 The functionality of a partially unmodified cytoplasmic trypanosome tRNAThrAGU has been proposed.51 In contrast, Cantara, et al.,52 recently showed by an in vitro assay that in the context of an E.coli ribosome, a tRNAArg is not bound by a CGC codon unless A34 is modified to I34.
In generalizing our observation, it is now apparent that the few exceptions to an inosine in the first position of the tRNAArg anticodon that had been documented to date were merely indicative of a much more widespread phenomenon encompassing the plant kingdom.
Materials and Methods
Oligonucleotides (Table 1) were synthesized by Sigma-Aldrich and the polymerase chain reaction system was purchased from Genaxxon BioScience. All chemicals used were commercially available pro analysis quality unless stated otherwise. DNA sequence analysis was performed using BigDye version 1.1 chemicals (Applied Biosystems/Life Technologies) in combination with an ABI Prism 310 Genetic Analyzer. Concentration of nucleic acids was determined by measuring the absorbance at 260 nm using a NanoDrop 1000 (Thermo Fisher Scientific). E. coli tRNA, enriched in tRNAArgACG to an arginine acceptance of 760 pmol/A260, was obtained from an expression construct described by Wu, et al.23
Table 1. Primers used for reverse transcription, PCR and sequencing.
| tRNA | Reverse transcription | PCR | Sequencing |
|---|---|---|---|
| Soybean tRNAArgACG |
1 |
1+3 |
7 |
| Jack bean tRNAArgACG |
1 |
1+3 |
7 |
| Wheat tRNAArgACG |
1 |
1+3 |
7 |
|
E. coli tRNAArgICG |
1 |
1+4 |
1 |
| Jack bean tRNASerIGA |
1 |
1+5 |
8 |
| Moss mitochondrial tRNAArgICG |
1 |
2+6 |
7 |
|
E.coli tRNAArgACG gene |
|
4+9 |
4 |
|
Primer |
Sequence |
||
| 1 |
M13 universal primer |
||
| 2 |
ACGGCCAGTGCAGTGAGTGGCATG |
||
| 3 |
TTTAATACGACTCACTATAGACTCCATGGCCCAATGGAT |
||
| 4 |
TTTAATACGACTCACTATAGCATCCGTAGCTCAGCTGGA |
||
| 5 |
TTTAATACGACTCACTATAGTGGACGTGCCGGAGTGGTTA |
||
| 6 |
TTTAATACGACTCACTATAGTGCTTGTAGCTCAATCGGATA |
||
| 7 |
TACAGGAAACAGCTATGACCCATCGTGATCCTTAGACTTCATACACTTACTTTAATACGACTCACTATA |
||
| 8 |
ACGGCCAGTGCAGTGAGTGG |
||
| 9 | CCCTGCAGTGGTGCATCCGGGAGGATTCGA | ||
The following combination of primers was used to characterize the tRNAs described, after ligation of an RNA tail to the 3' end.
Isolation and enrichment of native plant tRNAArgACG isoacceptor
Plants for total RNA extraction were grown in the greenhouse (jack bean – C. ensiformis) or in the field (wheat—T. aestivum- and soybean—G. max) under standard growth conditions (Dachswanger Mühle). Total tRNA from plants was isolated as described53 with some minor modifications and the wheat tRNAArgACG isoacceptor purified according to Spears, et al.27 A DEAE cellulose column (DE52 – Whatman) was prepared by suspending 5 g DEAE cellulose in 150 ml 0.1 M NaOH and carefully stirring it for 1h at 65°C. The cellulose was washed three times with H2O, resuspended and stirred in 150 ml 0.2 M acetic acid for 15 min and washed again five times with H2O. After washing it was taken up in 150 ml 0.14 M sodium acetate, pH 4.8 and equilibrated at 4°C overnight. For purification, total tRNA was bound to the DEAE cellulose column, washed once with 150 ml 0.14 M sodium acetate, pH 4.8 and a second time with 200 ml 0.14 M sodium acetate /0.25 M NaCl until A260 < 0.1, tRNA was then eluted with 0.14 M sodium acetate, pH 4.8/1 M NaCl. The isoacceptor corresponding to tRNAArgACG was isolated from total plant tRNA using biotinylated oligonucleotides covering the anticodons loop/stem region [(Btn)AGAATCTCTGGTTTCGTAGACCAGCGCCTT] on a streptavidin-Sepharose column (GE Healthcare). Purification to homogeneity was achieved by preparative denaturing PAGE on a 10% polyacrylamide gel containing 7 M urea. After ethanol precipitation the concentration of the tRNAArgACG isoacceptor was determined, yielding 3–6 µg (species-dependent) of the isoacceptor from 200–300 g leaves.
Ligation, reverse transcription, PCR and sequencing
In order to sequence tRNA and to avoid trace DNA contamination, an RNA primer was ligated to the 3' end of the isolated native tRNA. About 1 µg of total RNA was ligated to 20 pmol of a 5'-phosphorylated, 3'-periodate-oxidized RNA oligonucleotide [5'(Phos)CUCACUGCACUGGCCGUCGUUUUACCUox]. The oligonucleotide was designed as described9 to enable the use of the universal M13 primer for reverse transcription. Ligation was performed in ligation buffer using 10 units of T4 RNA ligase (Affymetrix-USB) and 40 U RNasin (Promega) in a total volume of 50 µl. The reaction was incubated for 90 min at RT. The total volume of the ligation was vacuum concentrated to 11 µl for reverse transcription and the ligation product was annealed to 20 pmol of universal M13 primer for 10 min at 65°C. Reverse transcription was performed under standard conditions using 10 U transcriptor reverse transcriptase (Roche) as described in the company’s manual. Incubation was for 30 min at 55°C and the reaction was terminated by heating to 85°C for 5 min. PCR was performed with 2 µl of the cDNA using a combination of 3' tail and 5' specific primers for wheat, jack bean, soybean and E.coli tRNAArgACG (Table 1). The amplicons were analyzed on 2% agarose gel, purified with the QIAquick PCR purification Kit (Qiagen) and sequenced using primers from either end (Table 1).
RNase T1 digestion of tRNA and LC-MS/MS
Purified tRNAArgACG from wheat was digested with RNase T1 by mixing 1 µg of the sample with 50 U of RNase T1 in 20 mM ammonium acetate and incubating for 2 h at 37°C. Digestion products were separated using a Thermo Surveyor HPLC system with an Xbridge C18 1.0 × 150 mm column (Waters) at room temperature with a flow rate of 30 µL/min. Before each run the column was equilibrated for 15 min at 95% Buffer A (200 mM HFIP, 8.15 mM TEA pH 7.0) and Buffer B (200 mM HFIP, 8.15 mM TEA:methanol 50:50 v:v pH 7.0). The gradient used was 5% B for 5 min., 30% B at 7 min. and 95% B at 50 min and held at 95% B for 5 min. The eluent was directed into a LTQ-XL (Thermo Scientific) for mass spectral analysis with a capillary temperature of 275°C, spray voltage of 4.5 kV, sheath, auxiliary and sweep gases were set to 25, 14 and 10 arbitrary units, respectively. Collision induced dissociation tandem mass spectrometry set to 35% with a Q value of 0.35 for 30 msec was used to obtain sequence information of the digestion products in data dependent mode. The data dependent settings will select each individual ion for CID for 15 scans or 30 sec before placing it on an exclusion list for 30 sec.
In vitro translation
In vitro translation was conducted with the TNT® Coupled Wheat Germ Extract Systems from Promega. As DNA template for in vitro translation the Flexi® Vector system from Promega was used. The EGFP coding sequence was amplified from pEGFP-N3 and was verified by sequencing. The restriction sites within the pF3A WG (BYDV) Flexi® Vector (Promega) as well as those appended to the PCR product were digested with SfaI (isoschizomer of SgfI) and MssI (isoschizomer of PmeI) from Fermentas using the Fermentas digestion protocol with buffer B for double digestions. Approximately 100 ng digested PCR product was ligated with 200 ng Flexi® Vector in a 20 µl reaction containing 400 U T4 DNA Ligase (New England BioLabs) for 4h at room temperature. Transformation of competent E.coli cells and sequence verification was accomplished according to the Flexi® Vector systems protocol from Promega. Plasmids containing the correct sequence were used for in vitro translation. In vitro translation was performed as described in the TNT® Coupled Wheat Germ Extract Systems users’ protocol with [35S]-methionine for protein detection. Translation products were analyzed on a 12% SDS (w/v) polyacrylamide gel and compared with a protein molecular mass marker (Spectra™ Multicolor Broad Range Protein Ladder, Fermentas). Newly translated proteins were detected by exposure of the dried gel to phosphorimager plates followed by visualization using the molecular imager PharosFX system from BioRad. The fluorescence of EGFP was measured with a NanoDrop 3300 Fluorospectrometer (Thermo Scientific).
Supplementary Material
Acknowledgments
We are grateful to M. Tomek for providing moss RNA. We thank R. and L. Schneider of the Dachswanger Mühle for a generous supply of wheat and soybean leaves.
Glossary
Abbreviations:
- GFP
green fluorescent protein
- TEA
triethylammonium acetate
- HFIP
hexafluoroisopropanol
- PAGE
polyacrylamide gel electrophoresis
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the German Research Foundation (DFG) (Ig9–6) to G.L.I. and by the National Science Foundation (CHE0910751) to P.A.L.
Supplemental Material
Supplemental material may be found here: www.landesbioscience.com/journals/rnabiology/article/21839
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/21839
References
- 1.Crick FH. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966;19:548–55. doi: 10.1016/S0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- 2.Jühling F, Mörl M, Hartmann RK, Sprinzl M, Stadler PF, Pütz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37(Database issue):D159–62. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agris PF, Vendeix FA, Graham WD. tRNA’s wobble decoding of the genome: 40 years of modification. J Mol Biol. 2007;366:1–13. doi: 10.1016/j.jmb.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Chen P, Qian Q, Zhang S, Isaksson LA, Björk GR. A cytosolic tRNA with an unmodified adenosine in the wobble position reads a codon ending with the non-complementary nucleoside cytidine. J Mol Biol. 2002;317:481–92. doi: 10.1006/jmbi.2002.5435. [DOI] [PubMed] [Google Scholar]
- 5.Andachi Y, Yamao F, Iwami M, Muto A, Osawa S. Occurrence of unmodified adenine and uracil at the first position of anticodon in threonine tRNAs in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1987;84:7398–402. doi: 10.1073/pnas.84.21.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe Y, Tsurui H, Ueda T, Furusihima-Shimogawara R, Takamiya S, Kita K, et al. Primary sequence of mitochondrial tRNA(Arg) of a nematode Ascaris suum: occurrence of unmodified adenosine at the first position of the anticodon. Biochim Biophys Acta. 1997;1350:119–22. doi: 10.1016/S0167-4781(96)00211-4. [DOI] [PubMed] [Google Scholar]
- 7.Sibler AP, Dirheimer G, Martin RP. Codon reading patterns in Saccharomyces cerevisiae mitochondria based on sequences of mitochondrial tRNAs. FEBS Lett. 1986;194:131–8. doi: 10.1016/0014-5793(86)80064-3. [DOI] [PubMed] [Google Scholar]
- 8.Delannoy E, Le Ret M, Faivre-Nitschke E, Estavillo GM, Bergdoll M, Taylor NL, et al. Arabidopsis tRNA adenosine deaminase arginine edits the wobble nucleotide of chloroplast tRNAArg(ACG) and is essential for efficient chloroplast translation. Plant Cell. 2009;21:2058–71. doi: 10.1105/tpc.109.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igloi GL, Schiefermayr E. Amino acid discrimination by arginyl-tRNA synthetases as revealed by an examination of natural specificity variants. FEBS J. 2009;276:1307–18. doi: 10.1111/j.1742-4658.2009.06866.x. [DOI] [PubMed] [Google Scholar]
- 10.Barciszewska MZ, Keith G, Kubli E, Barciszewski J. The primary structure of wheat germ tRNAArg--the substrate for arginyl-tRNAArg:protein transferase. Biochimie. 1986;68:319–23. doi: 10.1016/S0300-9084(86)80029-3. [DOI] [PubMed] [Google Scholar]
- 11.Harada F, Nishimura S. Transfer RNAs containing the G-Psi-Psi-C sequence. 1. 2 Arginine transfer-RNAs of mouse leukemia cells. Biochem Int. 1980;1:539–46. [Google Scholar]
- 12.Chakraburtty K. Effect of sodium bisulfite modification on the arginine acceptance of E. coli tRNA Arg. Nucleic Acids Res. 1975;2:1793–804. doi: 10.1093/nar/2.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfitzinger H, Weil JH, Pillay DT, Guillemaut P. Codon recognition mechanisms in plant chloroplasts. Plant Mol Biol. 1990;14:805–14. doi: 10.1007/BF00016513. [DOI] [PubMed] [Google Scholar]
- 14.Glover KE, Spencer DF, Gray MW. Identification and structural characterization of nucleus-encoded transfer RNAs imported into wheat mitochondria. J Biol Chem. 2001;276:639–48. doi: 10.1074/jbc.M007708200. [DOI] [PubMed] [Google Scholar]
- 15.Maréchal-Drouard L, Guillemaut P, Cosset A, Arbogast M, Weber F, Weil JH, et al. Transfer RNAs of potato (Solanum tuberosum) mitochondria have different genetic origins. Nucleic Acids Res. 1990;18:3689–96. doi: 10.1093/nar/18.13.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curran JF. Decoding with the A:I wobble pair is inefficient. Nucleic Acids Res. 1995;23:683–8. doi: 10.1093/nar/23.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber AP, Keller W. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science. 1999;286:1146–9. doi: 10.1126/science.286.5442.1146. [DOI] [PubMed] [Google Scholar]
- 18.Percudani R. Restricted wobble rules for eukaryotic genomes. Trends Genet. 2001;17:133–5. doi: 10.1016/S0168-9525(00)02208-3. [DOI] [PubMed] [Google Scholar]
- 19.Murphy FV, 4th, Ramakrishnan V. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat Struct Mol Biol. 2004;11:1251–2. doi: 10.1038/nsmb866. [DOI] [PubMed] [Google Scholar]
- 20.Agris PF. Decoding the genome: a modified view. Nucleic Acids Res. 2004;32:223–38. doi: 10.1093/nar/gkh185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf J, Gerber AP, Keller W. tadA, an essential tRNA-specific adenosine deaminase from Escherichia coli. EMBO J. 2002;21:3841–51. doi: 10.1093/emboj/cdf362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motorin Y, Muller S, Behm-Ansmant I, Branlant C. Identification of modified residues in RNAs by reverse transcription-based methods. Methods Enzymol. 2007;425:21–53. doi: 10.1016/S0076-6879(07)25002-5. [DOI] [PubMed] [Google Scholar]
- 23.Wu J-F, Wang E-D, Wang Y-L, Gilbert E, Jean G. Gene Cloning, Overproduction and Purification of Escherichia coli tRNA(Arg)(2) Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 1999;31:226–32. [PubMed] [Google Scholar]
- 24.Paris Z, Fleming IMC, Alfonzo JD. Determinants of tRNA editing and modification: Avoiding conundrums, affecting function. Semin Cell Dev Biol. 2012;23:269–74. doi: 10.1016/j.semcdb.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francis MA, Dudock BS. Nucleotide sequence of spinach cytoplasmic serine (IGA) tRNA. Nucleic Acids Res. 1989;17:7996. doi: 10.1093/nar/17.19.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teichmann T, Urban C, Beier H. The tRNA(Ser)-isoacceptors and their genes in Nicotiana rustica: genome organization, expression in vitro and sequence analyses. Plant Mol Biol. 1994;24:889–901. doi: 10.1007/BF00014443. [DOI] [PubMed] [Google Scholar]
- 27.Spears JL, Gaston KW, Alfonzo JD. Analysis of tRNA editing in native and synthetic substrates. Methods Mol Biol. 2011;718:209–26. doi: 10.1007/978-1-61779-018-8_13. [DOI] [PubMed] [Google Scholar]
- 28.Kowalak JA, Pomerantz SC, Crain PF, McCloskey JA. A novel method for the determination of post-transcriptional modification in RNA by mass spectrometry. Nucleic Acids Res. 1993;21:4577–85. doi: 10.1093/nar/21.19.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandal D, Köhrer C, Su D, Russell SP, Krivos K, Castleberry CM, et al. Agmatidine, a modified cytidine in the anticodon of archaeal tRNA(Ile), base pairs with adenosine but not with guanosine. Proc Natl Acad Sci U S A. 2010;107:2872–7. doi: 10.1073/pnas.0914869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts BE, Paterson BM. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973;70:2330–4. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michaud M, Cognat V, Duchêne A-M, Maréchal-Drouard L. A global picture of tRNA genes in plant genomes. Plant J. 2011;66:80–93. doi: 10.1111/j.1365-313X.2011.04490.x. [DOI] [PubMed] [Google Scholar]
- 32.Terasawa K, Odahara M, Kabeya Y, Kikugawa T, Sekine Y, Fujiwara M, et al. The mitochondrial genome of the moss Physcomitrella patens sheds new light on mitochondrial evolution in land plants. Mol Biol Evol. 2007;24:699–709. doi: 10.1093/molbev/msl198. [DOI] [PubMed] [Google Scholar]
- 33.Auxilien S, Crain PF, Trewyn RW, Grosjean H. Mechanism, specificity and general properties of the yeast enzyme catalysing the formation of inosine 34 in the anticodon of transfer RNA. J Mol Biol. 1996;262:437–58. doi: 10.1006/jmbi.1996.0527. [DOI] [PubMed] [Google Scholar]
- 34.Small I, Akashi K, Dietrich A, Duchene A-M, Lancelin D, Maréchal-Drouard L, et al. The strange evolutionary history of plant mitochondrial tRNAs and their aminoacyl-tRNA synthetases. J Hered. 1999;90:333–7. doi: 10.1093/jhered/90.3.333. [DOI] [Google Scholar]
- 35.Turmel M, Otis C, Lemieux C. The complete mitochondrial DNA sequence of Mesostigma viride identifies this green alga as the earliest green plant divergence and predicts a highly compact mitochondrial genome in the ancestor of all green plants. Mol Biol Evol. 2002;19:24–38. doi: 10.1093/oxfordjournals.molbev.a003979. [DOI] [PubMed] [Google Scholar]
- 36.Vinogradova E, Salinas T, Cognat V, Remacle C, Maréchal-Drouard L. Steady-state levels of imported tRNAs in Chlamydomonas mitochondria are correlated with both cytosolic and mitochondrial codon usages. Nucleic Acids Res. 2009;37:1521–8. doi: 10.1093/nar/gkn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaub M, Keller W. RNA editing by adenosine deaminases generates RNA and protein diversity. Biochimie. 2002;84:791–803. doi: 10.1016/S0300-9084(02)01446-3. [DOI] [PubMed] [Google Scholar]
- 38.Karcher D, Bock R. Identification of the chloroplast adenosine-to-inosine tRNA editing enzyme. RNA. 2009;15:1251–7. doi: 10.1261/rna.1600609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maréchal-Drouard L, Weil JH, Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988;16:4777–88. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider A, McNally KP, Agabian N. Nuclear-encoded mitochondrial tRNAs of Trypanosoma brucei have a modified cytidine in the anticodon loop. Nucleic Acids Res. 1994;22:3699–705. doi: 10.1093/nar/22.18.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruske EI, Sendfeld F, Schneider A. Thiolated tRNAs of Trypanosoma brucei are imported into mitochondria and dethiolated after import. J Biol Chem. 2009;284:36491–9. doi: 10.1074/jbc.M109.064527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wohlgamuth-Benedum JM, Rubio MAT, Paris Z, Long S, Poliak P, Lukes J, et al. Thiolation controls cytoplasmic tRNA stability and acts as a negative determinant for tRNA editing in mitochondria. J Biol Chem. 2009;284:23947–53. doi: 10.1074/jbc.M109.029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaneko T, Suzuki T, Kapushoc ST, Rubio MA, Ghazvini J, Watanabe K, et al. Wobble modification differences and subcellular localization of tRNAs in Leishmania tarentolae: implication for tRNA sorting mechanism. EMBO J. 2003;22:657–67. doi: 10.1093/emboj/cdg066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chateigner-Boutin A-L, Small I. Organellar RNA editing. Wiley Interdiscip Rev RNA. 2011;2:493–506. doi: 10.1002/wrna.72. [DOI] [PubMed] [Google Scholar]
- 45.Barciszewska M, Jones DS. The nucleotide sequence of a valine accepting tRNA from Lupinus luteus (lupin) seeds. Nucleic Acids Res. 1987;15:1333. doi: 10.1093/nar/15.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood TI, Barondeau DP, Hitomi C, Kassmann CJ, Tainer JA, Getzoff ED. Defining the role of arginine 96 in green fluorescent protein fluorophore biosynthesis. Biochemistry. 2005;44:16211–20. doi: 10.1021/bi051388j. [DOI] [PubMed] [Google Scholar]
- 47.Lagerkvist U. “Two out of three”: an alternative method for codon reading. Proc Natl Acad Sci U S A. 1978;75:1759–62. doi: 10.1073/pnas.75.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikelsaar R. C-A base pairs in transfer ribonucleic acids and codon-anticodon complexes. J Theor Biol. 1981;92:163–80. doi: 10.1016/0022-5193(81)90390-8. [DOI] [PubMed] [Google Scholar]
- 49.Auffinger P, Westhof E. Singly and bifurcated hydrogen-bonded base-pairs in tRNA anticodon hairpins and ribozymes. J Mol Biol. 1999;292:467–83. doi: 10.1006/jmbi.1999.3080. [DOI] [PubMed] [Google Scholar]
- 50.Inagaki Y, Kojima A, Bessho Y, Hori H, Ohama T, Osawa S. Translation of synonymous codons in family boxes by Mycoplasma capricolum tRNAs with unmodified uridine or adenosine at the first anticodon position. J Mol Biol. 1995;251:486–92. doi: 10.1006/jmbi.1995.0450. [DOI] [PubMed] [Google Scholar]
- 51.Rubio MAT, Ragone FL, Gaston KW, Ibba M, Alfonzo JD. C to U editing stimulates A to I editing in the anticodon loop of a cytoplasmic threonyl tRNA in Trypanosoma brucei. J Biol Chem. 2006;281:115–20. doi: 10.1074/jbc.M510136200. [DOI] [PubMed] [Google Scholar]
- 52.Cantara WA, Bilbille Y, Kim J, Kaiser R, Leszczyńska G, Malkiewicz A, et al. Modifications modulate anticodon loop dynamics and codon recognition of E. coli tRNA(Arg1,2) J Mol Biol. 2012;416:579–97. doi: 10.1016/j.jmb.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 53.Steinmetz A, Weil JH. Isolation and characterization of chloroplast and cytoplasmic transfer RNAs. Methods Enzymol. 1986;118:212–31. doi: 10.1016/0076-6879(86)18075-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.