Abstract
Aging is the progressive accumulation of damage to an organism over time leading to disease and death. Aging research has been very intensive in the last years aiming at characterizing the pathophysiology of aging and finding possibilities to fight age-related diseases. Various theories of aging have been proposed. In the last years advanced glycation end products (AGEs) have received particular attention in this context. AGEs are formed in high amounts in diabetes but also in the physiological organism during aging. They have been etiologically implicated in numerous diabetes- and age-related diseases. Strategies inhibiting AGE accumulation and signaling seem to possess a therapeutic potential in these pathologies. However, still little is known on the precise role of AGEs during skin aging. In this review the existing literature on AGEs and skin aging will be reviewed. In addition, existing and potential anti-AGE strategies that may be beneficial on skin aging will be discussed.
Keywords: advanced glycation end products, skin aging, photoaging, RAGE, AGEs
Introduction
Aging is defined as the progressive accumulation of damage over time, leading to disturbed function on the cellular, tissue and organ level and eventually to disease and death. Aging is a complex, multifactorial process where genetic, endogenous and environmental factors play a role.1
Skin is the largest organ of the human body and also the boundary between an organism and environment. As such, skin is subjected not only to the internal aging process but also to various external stressors, leading to distinct structural changes and affecting not only its youthful appearance, but also its various physiological functions. Aged skin shows disturbed skin permeability, angiogenesis, lipid and sweat production, immune function and vitamin D synthesis, manifesting among others as impaired wound healing, atrophy, vulnerability to external stimuli and development of several benign and malignant diseases (reviewed in Zouboulis et al.).2
Endogenously aged skin refers to changes reflecting the internal aging process of the organism and is being observed mainly in ultraviolet (UV) light-protected skin areas, such as the inner side of the arms. Macroscopically it is recognized by fine wrinkles, loss of elasticity, reduced epidermal and dermal thickness, while microscopically epidermal atrophy, decreased mitotic rate of basal keratinocytes, decreased proliferative capacity and cellular senescence, atrophy of the dermal extracellular matrix and change of the physiological properties of the connective tissue are typical characteristics.2-4 Exogenously aged skin or photoaged skin is the skin where endogenous aging processes are being aggravated by external stressors, mainly UV irradiation,2,5 but also by tobacco,6 chemicals and pollution.2,4 Apart from many similarities with endogenously aged skin, extrinsic aged skin is also characterized by a thickened epidermis and a hyperplasia of elastic tissue (solar elastosis).2,4
Until today, more than 300 theories of aging have been proposed, among them the theory of cellular senescence, decreased proliferative capacity and telomere shortening, mitochondrial DNA single mutations, the free radical theory and others, none of which can fully explain all changes observed in aging.7-11 According to the inflammatory theory of aging, a common characteristic of skin aging factors is their ability to induce or maintain proinflammatory changes and trigger a local inflammatory response which through subsequent immune responses, matrix metalloproteinase (MMP) activation and proinflammatory cytokine production contributes to the structural changes observed in aged skin.12
In the recent years, the role of advanced glycation end products (AGEs) has been increasingly discussed in skin aging, and the potential of anti-AGE strategies has received high interest from pharmaceutical companies for the development of novel anti-aging cosmeceutical compounds.
The aim of this work is to critically review the existing literature on AGEs and provide evidence that they play an important role in the pathogenesis of skin aging. Furthermore, existing and potential strategies against the deleterious effects of AGEs on skin aging will be discussed.
Biochemistry of AGEs
Glycation is the non-enzymatic reaction between reducing sugars, such as glucose, and proteins, lipids or nucleic acids.13 Glycation has to be distinguished from glycosylation, which is an enzymatic reaction. Since its first description by Maillard in 1912 and its involvement in food browning during thermal processing by Hodge 50 years later, its presence in living systems and involvement in various pathologies of the human body, including aging and diabetes, have been an intensive field of research.14,15
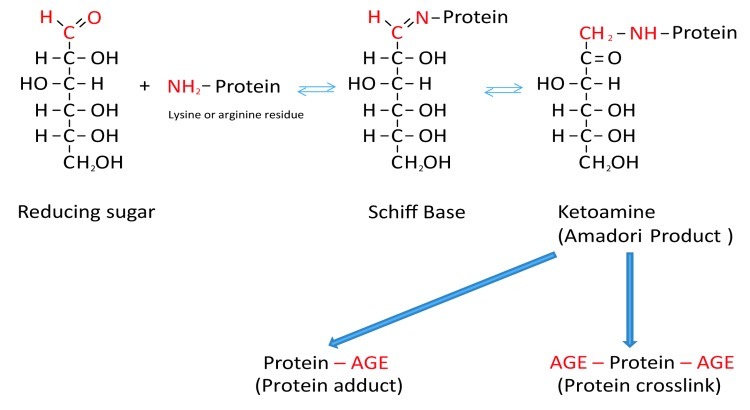
Formation of AGEs is a complicated molecular process involving simple and more complex multistep reactions. During the classical Maillard reaction electrophilic carbonyl groups of glucose or other reactive sugars react with free amino groups of amino acids (especially of basic lysine or arginine residues), forming a non-stable Schiff base.16 Further rearrangement leads to formation of a more stable ketoamine (Amadori product) (Fig. 1).13,16 Schiff bases and Amadori products are reversible reaction products. However, they can react irreversibly with amino acid residues of peptides or proteins to form protein adducts or protein crosslinks.13,16 Alternatively, they can undergo further oxidation, dehydration, polymerization and oxidative breakdown reactions to give rise to numerous other AGEs.13,17 Oxygen, reactive oxygen species (ROS) and redox active transition metals accelerate AGE formation. When an oxidative step is involved, the products are called advanced glycoxidation end products.13,17
Figure 1. Schematic presentation of the Maillard reaction. Reactive carbonyl groups of a reducing sugar react with neutrophilic free amino groups of proteins to form a reversible Schiff base. Through rearrangement a more stable Amadori product is formed. Dependent on the nature of these early glycation end products, protein adducts or protein crosslinks are formed.
AGEs are a very heterogeneous group of molecules. Since the discovery of the first glycated protein, glycated hemoglobin in diabetes, numerous other AGEs have been detected. Some of them have characteristic autofluorescent properties, which simplifies their identification in situ or in vivo.13 To date, numerous AGEs have been identified. Table 1 lists the most commonly found ones in the skin.17-28
Table 1. Detected AGEs in skin*.
| AGE | Skin compartments involved | Targets of glycation | Methods of detection |
|---|---|---|---|
| CML |
Epidermis18 Aged and diabetic dermis19-22 Photoaging-actinic elastosis20,23 |
Epidermis (SC -CK10, SS, SG)18 Collagen19-21 Vimentin22 Elastin20,23 |
LC-ESI-TOF-MS, IF, IB18 SIM/GC-MS19,21 IHC20,22,23 ELISA,23 confocal microscopy23 |
| Pentosidin |
Aged and diabetic dermis19,24,25 |
Collagen19,24,25 |
Reversed-phase HPLC,19,24 ELISA,25 IB25 |
| GO |
Aged dermis21 |
Collagen21 |
LC/MS21 |
| MGO |
Aged dermis21 |
Collagen21 |
LC/MS21 |
| Glucosepane |
Aged dermis21,26 |
Collagen21,26 |
LC/MS21,26 |
| Fructoselysine |
Aged dermis21 |
Collagen21 |
LC/MS21 |
| CEL |
Aged dermis21,27 |
Collagen21,27 |
LC/MS27 SIM/GC-MS21 |
| GOLD |
Aged dermis28 |
Collagen28 |
LC/MS28 |
| MOLD | Aged dermis28 | Collagen28 | LC/MS28 |
ELISA, enzyme-linked immunosorbent assay; GO, glyoxal; HPLC, high performance liquid chromatography; IHC, immunohistochemistry; IB, immunoblotting; IF, immunofluorescence; LC-ESI-TOF-MS, liquid chromatography–electrospray ionization time-of-flight mass spectrometry; LC/MS, liquid chromatography/mass spectrometry; MGO, methylglyoxal; SIM/GC-MS, selected ion monitoring gas chromatography-mass spectrometry; SC, stratum corneum; SG, stratum granulosum; SS, stratum spinosum; all other abbreviations are already explained in the text.
Carboxymethyl-lysine (CML) was first described by Ahmed and represents the most prevalent AGE in vivo.29,30 It is a non-fluorescent protein adduct. Mechanisms of its formation include oxidative degradation of Amadori products or direct addition of glyoxal to lysine. It seems to be the major epitope of the commonly used polyclonal anti-AGE antibodies.30
Pentosidine was first isolated and characterized by Sell and Monnier. It is composed of an arginine and a lysine residue crosslinked to a pentose.31 Pentosidine is a fluorescent glycoxidation product and forms protein-protein crosslinks.16
Dicarbonyl compounds like 3-deoxyglucosome, methylglyoxal and glyoxal derive from oxidative degradation or autooxidation of Amadori products and other pathways.13,32 These dicarbonyl compounds are very reactive molecules leading to protein crosslinks.13 Other in vivo characterized AGEs include glucosepane, carboxymethyl-hydroxy-lysine, carboxyethyl-lysine (CEL), fructose-lysine, methylglyoxal-derived hydroimidazolones and pyrraline, which form non-fluorescent protein adducts, while glyoxal-lysine dimer (GOLD) and methylglyoxal-lysine dimer (MOLD) form non-fluorescent protein crosslinks.13,17
AGEs can be exogenously ingested (through food consumption) or be endogenously produced. Endogenous AGE formation is increased in diabetes; however, AGEs are also formed at lower rates by normal metabolic processes of the organism.33 Environmental factors, such as diet and smoking influence the rate of AGE formation.34 Moreover, it seems that the level of circulating AGEs levels are genetically determined, as shown in a cohort study of healthy monozygotic and heterozygotic twins.35
The content of AGEs in the organism is not only defined by the rate of their formation but also by the rate of their removal. Many cells have developed intrinsic detoxifying pathways against accumulation of AGEs.36 The glutathione-dependent glyoxalase system, comprising of glyoxalase (Glo) I and II, has a key role in the defense against glycation.37 This system uses reduced glutathione (GSH) to catalyze the conversion of glyoxal, methylglyoxal and other α-oxoaldehydes to the less toxic D-lactate.37 Other enzymatic systems include fructosyl-amine oxidases (FAOXs) and fructosamine kinases, relatively new classes of enzymes which recognize and break Amadori products.38 However, FAOXs or “amadoriases” have been found to be expressed only in bacteria, yeast and fungi but not in mammals. They oxidatively break Amadori products but act mostly on low molecular weight compounds.39 On the contrary, fructosamine kinases are expressed in various genomes including humans.38 These intracellular enzymes phosphorylate and destabilize Amadori products leading to their spontaneous breakdown.39 Fructosamine-3-kinase (FN3K), one of the most studied enzymes in this system, is almost ubiquitary expressed in human tissues including the skin. Thus, it plays an important role in the intracellular breakdown of Amadori products.40
Receptors for AGEs
AGEs not only exert their deleterious actions due to their biological properties per se, but also through their interaction with specific receptors. Receptor for AGEs (RAGE) is a multiligand member of the immunoglobulin superfamily of cell surface receptors, encoded by a gene on chromosome 6 near the major histocompatibility complex III. It is a pattern recognition receptor binding in addition to AGEs various other molecules such as S-100/calgranulins, high motility group protein B1 (amphoterine), β-amyloid peptides and β-sheet fibrils.33,41 The binding of ligands to RAGE stimulates various signaling pathways including the mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinases (ERK) 1 and 2, phosphatidyl-inositol 3 kinase, p21Ras, stress-activated protein kinase/c-Jun-N-terminal kinase and the janus kinases.33,41 Stimulation of RAGE results in activation of the transcription factor nuclear factor kappa-B (NFκB) and subsequent transcription of many proinflammatory genes.41,42 Interestingly, RAGE-induced activation of NFκB is characterized by a sustained and self-perpetuating action, through induction of positive feedback loops and overwhelming of the autoregulatory negative feedback loops. RAGE activation leads to new synthesis of the transcriptionally active subunit p65, which overwhelms the newly synthesized inhibitor IκBα. Moreover NFκB increases further expression of RAGE, which itself further stimulates NFκB, forming a vicious cycle of self-renewing and perpetuating proinflammatory signals.41 RAGE activation can directly induce oxidative stress by activating nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase (NOX), decreasing activity of superoxide dismutase (SOD), catalase and other pathways, and indirectly by reducing cellular antioxidant defenses, like GSH and ascorbic acid.41,43,44 The reduction of GSH leads furthermore to decreased activity of Glo I, the major cellular defense system against methylglyoxal, therefore supporting further production of AGEs.37 RAGE is almost ubiquitary expressed in the organism, typically at low levels, and its expression is upregulated under various pathologic conditions.41,45 In the skin, RAGE expression was observed in both epidermis and dermis, and it was increased in sun-exposed compared with UV irradiation-protected areas. Keratinocytes, fibroblasts, dendritic cells and to a lesser extent endothelial cells and lymphocytes express RAGE.45 Not only in vivo, but also in vitro, various skin cells types have been shown to express RAGE (Table 2).43,45-51
Table 2. Expression of human RAGE in skin and skin cells*.
| Skin in situ | Methods of detection |
|---|---|
|
Young donors: High and middle epidermis Papillary dermis Old donors: Middle and basal epidermis Reticular dermis Enhanced expression in sun-exposed skin |
IHC45,46 |
|
Skin cell types in vivo Fibroblasts Dendric cells Keratinocytes Endothelial cells Mononuclear cells |
IHC45,46 |
| Cell types in vitro | |
|---|---|
|
Resident skin cells Keratinocytes Fibroblasts Melanocytes |
qRT-PCR,46 WB47 WB,43,45 qRT-PCR43,45 ? |
|
Immune cells and other cell types Mononuclear phagocytes48 Dendritic cells49 T-lymphocytes50 Vascular dermal endothelial cells |
WB,48 IF48 FC49 qRT-PCR50 qRT-PCR,51 WB51 |
FC, flow cytometry; IHC, immunohistochemistry; IF, immunofluorescence; qRT-PCR, quantitative real-time PCR; all other abbreviations are already explained in the text.
RAGE is the most studied receptor for advanced glycation end products. Another group of cell surface receptors, AGER1, AGER2 and AGER3 seem to regulate endocytosis and degradation of AGEs, thus counteracting the effects of RAGE.52 AGER1 has been further shown to counteract AGEs-induced oxidative stress via inhibition of RAGE signaling.53,54 Soluble RAGE (sRAGE) is a truncated splice variant of RAGE containing the ligand-binding domain but not the transmembrane domain and has been found in plasma. sRAGE is a soluble extracellular protein without signaling properties and it is considered as a natural decoy receptor of RAGE.55
Role of AGEs During Skin Aging
Cutaneous accumulation of AGEs is a feature of skin aging
As mentioned above, AGEs can be directly formed in the organism or be exogenously ingested. Accumulation of AGEs has been detected in various tissues during aging and diabetes, including articular collagen, skeletal and smooth vascular muscles or glomerular basement membranes.56-58 Accordingly, deposited AGEs in these tissues have been implicated in various diabetes- or age-associated pathologies such as diabetic angiopathy, age- and diabetes-associated macular degeneration and osteoarthritis.56-62
Skin, due to its easy accessibility, offers an excellent opportunity for minimal invasive or even non-invasive investigation of glycation, taking advantage of the characteristic autofluorescent properties of AGEs. Accumulation of AGEs in the skin has been therefore thoroughly studied and is detected not only in diabetes as expected but also during chronological aging.20,63,64 Glycation-associated skin autofluorescence was shown to correlate with chronological aging in a large number of healthy subjects.65
It is a general perception today that AGE accumulation is dependent on protein turnover rate; therefore long-lived proteins are thought to be mainly modified by glycation.66 Collagen types I and IV, exhibiting a slow turnover rate of about 10 y, and other dermal long-lived proteins like fibronectin mainly suffer from glycation during intrinsic chronological aging.19,20 The appearance of glycated collagen is first observed at the age of 20. It accumulates with a yearly rate of about 3,7% reaching a 30–50% increase at 80 y of age.20,67 CML was recently histochemically detected in human epidermis from healthy donors.18 The upper epidermal layers were mostly involved (stratum spinosum, granulosum and corneum) and the authors identified cytokeratin 10 (CK10) (expressed by differentiated keratinocytes) as a target protein for CML modification. The amount of CML in younger donors seemed to be weak in comparison to the older ones. The latter study had restrictions, as the size of the sample was small and heterogeneous, but indicates a potential involvement of AGEs in epidermal physiology and a possible involvement of more short-lived proteins in glycation chemistry. Moreover, in an in vitro reconstructed organ skin model, both epidermis and dermis, as well as their functions, were modified by glycation.68
AGEs also seem to highly accumulate in extrinsically aged skin. Until now, the deleterious effects of UV irradiation have been mainly attributed to proinflammatory changes, apoptosis, oxidative damage, mutagenesis and induction of MMPs.2,5 However, it has been shown that in young individuals, where typically no significant accumulation of AGEs in sun-protected skin is observed, sun-exposed areas display an increased deposition of these substances.20,69 Accumulation of AGEs was mainly found in sites of solar elastosis in sun-exposed skin, showing that UV irradiation may also precipitate the formation of AGEs in vivo.20,23 It is tempting to speculate that formation of AGEs in sun-exposed skin may be one additional mechanism mediating the various structural and functional modifications during photoaging.
Moreover, smoking, a typical aggravating factor of skin aging, accelerates formation of AGEs and increases their deposition in various tissues including skin.70,71 Another important environmental factor for aging is diet. The content of AGEs in food is highly dependent on the method of preparation, like cooking time and temperature. Fried food contains in general far higher amounts of AGEs than boiled or steamed food.72 Approximately 10–30% of ingested AGEs are absorbed in the circulation.73 Dietary AGEs directly correlate with serum levels of AGEs and inflammatory markers in healthy human subjects, respectively.73
It has been widely accepted that AGEs, once formed, can be only removed when the modified proteins degrade. However it has now become apparent that in the organism various enzymatic systems seem to be involved in the degradation or removal of AGEs. As mentioned above, Glo I is an enzyme responsible for the removal of reactive α-dicarbonyl compounds. Interestingly, decreased activity of such defense systems against AGEs has been reported during aging.44 These age-related changes may further increase the extent of deposited AGEs in a living organism over time.
Consequences of AGE deposition in skin
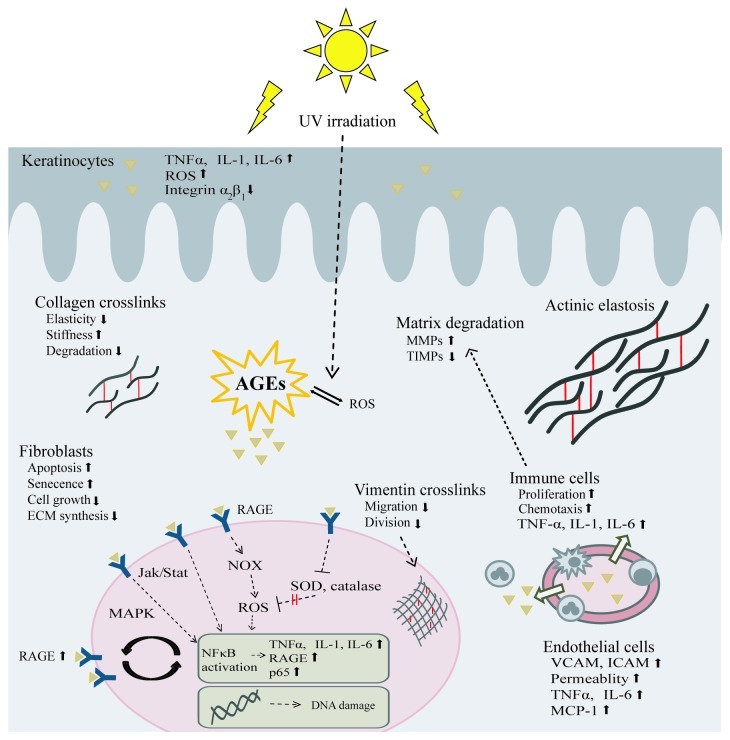
AGEs can be formed intracellularly and extracellularly. Their presence in biological molecules modifies their biomechanical and functional properties. Proteins, lipids and nucleic acids can be targets of advanced glycation, modifying enzyme-substrate interactions, protein-DNA interactions, protein-protein interactions, DNA regulation and epigenetic modulation, thus interfering with numerous physiological functions of the organism. Moreover, AGEs are themselves reactive molecules which through interaction with their receptors activate various molecular pathways in vivo, thus becoming involved in inflammation, immune response, cell proliferation and gene expression (Fig. 2).
Figure 2. Effects of AGEs on skin. AGEs are formed intracellularly and extracellularly. They can react with proteins, lipids and nucleic acids in almost all skin cells as well as on intracellular or extracellular proteins. Through alteration of the physicochemical properties of dermal proteins, decreased cell proliferation, increased apoptosis and senescence, induction of oxidative stress and proinflammatory mediators as well as other pathways, AGEs contribute to the overall picture of skin aging. Triangles represent AGEs. Abbreviations: jak/stat, januskinase/signal transducers and activators of transcription; MCP-1, monocyte chemotactic protein-1; all other abbreviations are already explained in the text.
1. Extracellular matrix proteins
Extracellular matrix (ECM) proteins have been regarded as one of the major target structures for glycation. The most abundant collagen type in the skin is type I, whereas collagen IV is being found in the basal membrane. Collagen is one of the strongest proteins. In the skin, it is not only used as a supportive framework for mechanical support for cells and tissues, but represents an active component being able to interact with cells and affect various cellular functions such as migration, differentiation and proliferation.
Collagen glycation impairs its function in various ways. Intermolecular crosslinks of adjacent collagen fibers change its biomechanical properties leading to stiffness and decreased flexibility, thus increasing its susceptibility to mechanical stimuli.74 The change of its charge and the formation of AGEs on side chains of collagen affect its contact sites with cells and other matrix proteins and inhibit its ability to react with them.75 The precise aggregation of monomers into the triple helix may be affected as well as the association of collagen IV with laminin in the basal membrane.16 Modified collagen resists degradation by MMPs, thus inhibiting its removal and replacement by newly synthesized and functional one.62 Accordingly, tissue permeability and turnover is impaired.16,76
Other extracellular matrix proteins suffering from advanced glycation are elastin and fibronectin, contributing further to dermal dysfunction.19,20,23 Of note, CML-modified elastin has been found almost exclusively in sites of actinic elastosis and not in sun-protected skin, underlining its potential role in photoaging. Indeed, UV irradiation stimulates glycation of elastin in the presence of sugars. Moreover, CML-modified elastin assembled in large and irregular structures, has decreased elasticity and is resistant to proteolytic degradation.77
It has been shown that in vitro glycated skin samples have impaired biomechanical properties.78 In vivo, decreased skin elasticity characterizes diabetic subjects in comparison to healthy controls.79
2. Intracellular proteins
Intermediate filaments such as vimentin in fibroblasts and CK10 in keratinocytes have been found to be modified by AGEs.18,22 Cytoskeletal proteins are important in providing stability of the cytoskeleton and are crucially involved in numerous cellular functions such as migration and cellular division. Various other intracellular proteins including enzymes and growth factors may be targets of non-enzymatic modification by sugars. Glycated basic fibroblast growth factor (bFGF) displays impaired mitogenic activity in endothelial cells.80 Glycation of enzymes of the ubiquitin-proteasome system and of the lysosomal proteolytic system has been shown to inhibit their action.81 Antioxidant and other protective enzymes such as Cu-Zn-SOD can be inactivated.82 Other intracellular components, such as DNA and lipids can be glycated with detrimental effects on their function.13,83
3. Receptors for AGEs: RAGE
AGEs do not only act by altering the physicochemical properties of glycated proteins. Interestingly, AGEs may bind to their cell surface receptor, RAGE, initiating a cascade of signals influencing cell cycle and proliferation, gene expression, inflammation and extracellular matrix synthesis (reviewed in Bierhaus et al.).41 As mentioned above, RAGE is broadly expressed in human skin and in epidermal keratinocytes, dermal fibroblasts and endothelial cells in vitro. It is highly found in sites of solar elastosis, and its expression is induced by advanced glycation end products and proinflammatory cytokines like TNFα.45 In skin cells RAGE has been shown to decrease cell proliferation, induce apoptosis and increase MMPs production.47 Many of these effects involve NFκB signaling.47
4. Effects of AGEs on resident skin cells
AGEs have been shown to affect various functions of skin cells in vitro (Table 3). They decrease proliferation and enhance apoptosis of human dermal fibroblasts, an effect which is at least partly RAGE-dependent and correlates with the activation of NFκB and caspases.87 In keratinocytes, AGEs decrease cell viability and migration and induce the expression of proinflammatory mediators.84 Moreover, AGEs are able to induce premature senescence in human dermal fibroblasts and in normal human keratinocytes in vitro.86,89,90 Collagen and ECM protein synthesis have been also found to be decreased, while the expression of MMPs is induced.47 Dicarbonyls such as glyoxal and methylglyoxal impair the signaling of epidermal growth factor receptor (EGFR), a receptor controlling various cellular functions such as proliferation, differentiation, motility and survival, by formation of EGFR crosslinks, blocking of phosphorylation and impaired activation of ERKs and phospholipase C.92 Various other growth factors or proteins significant for cellular functions, like bFGF, may be glycated inhibiting their functions.80 In the context of extrinsic aging, AGEs seem to render cells more sensitive to external stimuli, as UVA irradiated fibroblasts and keratinocytes exhibit decreased viability after exposure to AGEs.85,93
Table 3. Effects of AGEs/RAGE on skin morphology and physiology during aging*.
| Keratinocytes |
Proliferation ↓84 Apoptosis ↑47 ROS ↑85 MMP-9 ↑, TIPM ↓84 Senescence ↑86 NFκB, proinflammatory mediators ↑81 α2β1-integrin ↓84 |
Cell renewal ↓ Epidermal homeostasis ↓ |
| Fibroblasts |
Proliferation ↓87 Apoptosis ↑87 ECM synthesis ↓88 MMP ↑88 Senescence ↑89,90 NFκB ↑87 ROS ↑43,85,90 Contractile properties ↓22 NOX ↑43 |
Cell renewal ↓ Dermal homeostasis ↓ Skin contractile function ↓ |
| Melanocytes |
? |
? |
| Immune cells |
Proliferation ↑50 Haptotaxis, chemotaxis ↑48 NFκB, TNFα, IL-1, IL-6 ↑42,49 |
Induction and propagation of inflammation |
| Extracellular matrix proteins (collagen, fibronectin, elastin) |
Crosslinking16,19,20,23,76 Resistance to MMP degradation62,76 Impaired assembly of macromolecules to normal 3D structures16,76-78 Defect cross-talking to cells75,76 |
Elasticity ↓ Stiffness ↑ Resistance to repair mechanisms Tissue permeability ↓ |
| Vascular endothelial cells | VCAM, ICAM, E-selectin ↑91 Permeability ↑91 TNFα, IL-6 ↑91 MCP-1 ↑91 |
Induction of proinflammatory mediators and recruitment of immune cells |
ICAM, intercellular adhesion molecule; MCP-1, monocyte chemotactic protein-1; TIPM, tissue inhibitor of MMP; VCAM, vascular cell adhesion molecule; all other abbreviations are already explained in the text.
5. The role of oxidative stress
Oxidative stress has been widely accepted to mediate the deleterious effects of solar radiation in the skin during photoaging. Interestingly, in vitro exposure of AGEs to UVA irradiation leads to formation of ROS, such as superoxide anion, hydrogen peroxide and hydroxyl radicals.93 AGEs can lead to ROS formation in cells by various ways. They can stimulate NOX to induce production of superoxide anion or they can compromise cellular antioxidant defense systems, e.g. inactivation of Cu-Zn-SOD by cross-linking and site-specific fragmentation of this molecule.82 Moreover, AGEs are themselves very reactive molecules. As early as during their crosslinking reactions they can act as electron donors leading to formation of superoxide anions.94 Glycation of proteins creates active enzyme-like centers (cation-radical sites of crosslinked proteins) able to catalyze one-electron oxidation-reduction reactions leading to ROS generation with or without presence of oxygen or transition metals such as iron and copper.94-,96
Finally, autofluorescent AGEs, such as pentosidine, can act as endogenous photosensitizers leading to increased ROS formation after UVA irradiation of human skin.97 UV irradiation of human keratinocytes and fibroblasts in the presence of AGEs led to increased ROS formation and decreased proliferation in vitro.85
6. Skin AGEs as biomarkers of aging
As AGEs have been etiologically implicated in aging and aging-related pathologies, the idea of using them as biomarkers is appealing. AGEs in the skin have been initially measured by western blots (WB) with polyclonal antibodies or by autofluorescence measurements of skin biopsies, thus restricting the wide use of these measurements. An AGE-Reader (DiagnOptics B.V., Groningen, The Netherlands) has been introduced some years ago as a new, non-invasive method to measure in vivo the skin content of AGEs based on their characteristic autofluorescence.98-100
Until now it has been shown that skin autofluorescence positively correlates with various diabetes- and age-related complications such as micro- and macrovascular complications, renal disease, cardiovascular events, overall mortality, age-related macular degeneration and chronic renal disease.99,101,102 Skin glycation has been proposed as a prognostic factor for the development of diabetic complications.103 Lately it was shown that skin autofluorescence increases with chronological aging and correlates with skin deposition of AGEs, making this method a potential tool in investigating the effect of various anti-aging products of the cosmetic industry.104
Anti-AGE Strategies: Current Knowledge and Future Perspectives
Since the emergence of AGEs as an important pathogenetic factor in diabetes and aging the development of strategies against AGEs has been in the center of scientific interest. Substances able to prevent or inhibit formation of AGEs, as well as agents able to break already formed AGEs or those antagonizing their signaling have been identified. Some of them are already being tested in clinical trials.105,106
1. Substances preventing or inhibiting AGE formation
Aminoguanidine was one of the first substances identified limiting the formation of AGEs.107 Aminoguanidine is a nucleophilic hydrazine and its anti-AGE properties result from trapping of early glycation products such as carbonyl intermediate compounds. It has no effects on more advanced stages of glycation. Despite its potential effects in attenuating various diabetes- and age-related complications in animal models, its use in clinical practice is limited due to adverse effects in clinical trials with diabetic patients.108 In an in vitro skin aging model it could attenuate collagen glycation, however its effects against AGE-induced collagen modification in vivo have been contradictory.109-111 Studies on topical application of aminoguanidine in the skin are lacking.
Pyridoxamine, a naturally occurring vitamin B6 isoform, seems to be another tool in the fight against AGEs. Pyridoxamine traps reactive carbonyl intermediates, scavenges ROS and in addition inhibits post-Amadori stages of AGEs formation.112 It has shown promising results in a phase II clinical trial against diabetic nephropathy.113 Oral intake of pyridoxamine resulted in potent inhibition of skin collagen CML formation in diabetic rats.111 However, its potential against skin aging remains to be shown.
2. “AGE breakers”
Chemical substances and enzymes able to recognize and break the Maillard reaction crosslinks have been identified. Such chemical AGE breakers are dimethyl-3-phenayl-thiazolium chloride (ALT-711), N-phenacylthiazolium and N-phenacyl-4,5-dimethylthiazolium.113 They have been developed to chemically break the prototypical Maillard reaction crosslink via a thiazolium structure.113 Promising results against cardiovascular complications in diabetes and aging have been reported, although their actual ability to cleave existing protein crosslinks in tissues has been questioned.114-117 In the rat ALT-711 showed some promising results on skin hydration.113
Interference with intrinsic AGE-detoxifying enzymes like FAOXs, FN3K and the enzymatic system of Glo is another interesting strategy to remove AGEs, as enzymes recognize specific substrates and may be associated with fewer side effects.37,38,118 There are a lot of data supporting the significance of these enzyme systems in aging. As noted above decreased Glo I activity and increased accumulation of AGEs with age have been shown in many tissues and animals.37 Overexpression of Glo I significantly inhibits hyperglycemia-induced intracellular formation of AGEs in bovine aortic endothelial cells and in mouse mesangial cells by reduction of intracellular oxidative stress and apoptosis.119,120 A potential in vivo beneficial effect of Glo I against AGEs could be also shown in transgenic rats.121 Interestingly, it has been recently shown that Glo I is transcriptionally controlled by Nrf2, and that pharmacological Nrf2 activators increase Glo I mRNA and protein levels as well as its activity.122 The pharmacological induction of such enzymes could represent a novel future strategy against AGEs. Fructosamine phosphokinases are relatively new enzymes and currently under investigation, and until now no inductors or activators of their expression have been found.40 FAOXs on the other hand are not expressed in mammals, and their potential use in humans by enzymatic engineering remains to be discovered.39
3. Nutriceuticals
Since oxidation steps are crucially involved in formation of many AGEs, substances with antioxidative or metal chelating properties, may also have antiglycating activities.123 Thus, a lot of interest has been directed to nutrients and vitamins, so called “nutriceuticals,” as natural tools against AGEs.106,124
Accordingly, an increasing list of natural antioxidants and chelating agents such as ascorbic acid, α-tocopherol, niacinamide, pyridoxal, sodium selenite, selenium yeast, trolox, rivoflavin, zink and manganese has been shown to inhibit glycation of albumin in vitro.125 Alpha-lipoic acid was able to reverse tail tendon collagen glycation in fructose-fed rats, an effect which was attributed to its endogenous antioxidant action, its ability to recycle ascorbic acid, α-tocopherol and GSH as well as to its positive influence on glucose uptake and glycaemia.126 Green tea, vitamins C and E and a combination of N-acetylcystein with taurine and oxerutin could inhibit skin collagen glycation in mice.124,127 Another compound, the green tea-derived polyphenol and flavonoid epigallocatechin-3-gallate revealed also promising in vitro effects by antagonizing AGE-induced proinflammatory changes.128 In healthy human subjects, supplementation of vitamin C significantly decreased serum protein glycation.129
Many spices and herbs were shown to inhibit glycation of albumin in vitro, among them ginger, cinnamon, cloves, marjoram, rosemary and tarragon.130 Their protective effects correlated with their phenolic content. Recently, in vivo beneficial effects of some of these compounds were shown in zebrafish.131
Other promising compounds include blueberry extract and naturally occurring flavonoids, such as luteolin, quercetin and rutin, which can inhibit various stages of AGE formation.132,133 Recently, blueberry extract, an AGE-inhibitor and C-xyloside, a glycosaminoglycan synthesis stimulator, were tested for 12 weeks in female diabetic subjects. This treatment resulted in significant improvement of skin firmness, wrinkles and hydration although it failed to show a significant decrease in the cutaneous content of AGEs.132
4. Caloric restriction and dietary measures
As nutrition is an important factor in skin aging, dietary caloric restriction may be effective in preventing accumulation of AGEs in the human body. In mice restriction of caloric intake increases lifespan and delays many age-related dysfunctions by altering stress response and influencing the expression of various metabolic and biosynthetic genes.134 Dietary restriction could significantly decrease the levels of AGEs in rat and mice skin collagen.135,136 Skin collagen glycation and glycoxidation inversely correlated with lifespan whereas caloric restriction led to decreased accumulation of AGEs and increased lifespan.137 Dietary restriction may not be a pragmatic option in humans; however a restriction in intake of dietary “glycotoxins” may be more feasible. As outlined above these dietary glycotoxins derive from nutrition. In humans dietary glycotoxins significantly increase concentrations of systemic inflammatory mediators like TNFα, interleukin (IL)-6 and C-reactive protein and are thus considered as diabetogenic, nephrotoxic and proatherogenic.59,138,139 Dietary intake of AGEs correlates with serum AGEs and can induce systemic oxidative stress, increase RAGE expression, decrease antioxidant levels and shorten lifespan in mice.54 A diet with a low content in AGEs could reduce circulating AGEs and inflammatory biomarkers in patients with diabetes and renal failure thus seeming to be an important supportive therapy in diabetes.140,141 In mice low dietary AGEs had beneficial effects in wound healing and other diabetes mellitus-associated pathologies.142 There are no studies investigating the effects of AGE-poor diets on skin aging in humans. However, it has been shown that skin collagen glycation positively correlates with blood glucose levels in diabetes and that intensive treatment can reduce the levels of skin glycation, implicating that a diet low in AGEs may have a beneficial effect on skin glycation.143,144
5. Targeting RAGE
Another potential strategy against excessive accumulation of AGEs could be the antagonism of RAGE.145 Possible approaches include gene knock-down of RAGE by siRNA or anti-sense and antagonism of RAGE with putative small molecular inhibitors against RAGE-induced signaling.50,145 Promising effects in various systems have been shown in vitro and in vivo with neutralizing anti-RAGE antibodies.41 Since serum concentrations of sRAGE negatively correlate with AGE-induced pathologies, neutralization of AGEs by these decoy receptors of RAGE may be considered as another potential anti-AGE strategy. Potential protective effects of sRAGE have been shown in various diabetes and inflammatory models.41,44,45,146 Interestingly, sRAGE could also attenuate impaired wound healing in diabetic mice. Therefore, studies will be needed to investigate an analogous effect on skin aging.147
6. Others
Molecular chaperones like carnosine have lately shown promise in improving skin appearance in various studies at least in part by reducing the amounts of skin AGEs.148-150
Conclusion
There is ample evidence that AGEs play an important role in skin aging. There are also numerous studies investigating potential substances against excessive accumulation of AGEs in tissues. Some of these studies have already shown protective effects against diabetic complications. As controlled human studies investigating the effects of these anti-AGE strategies against skin aging are largely missing, this is a hot field for future research.
Glossary
Abbreviations:
- AGE
advanced glycation end product
- ALT-711
dimethyl-3-phenayl-thiazolium chloride
- bFGF
basic fibroblast growth factor
- CEL
carboxyethyl-lysine
- CML
carboxymethyl-lysine
- CK10
cytokeratin 10
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- FAOX
fructosyl-amine oxidases
- FN3K
fructosamine-3 kinase
- Glo
glyoxalase
- GOLD
glyoxal-lysine dimer
- GSH
glutathione
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- MOLD
methylglyoxal-lysine dimer
- NADPH
nicotinamide adenine dinucleotide phosphate
- NFκB
nuclear factor kappa-B
- NOX
NADPH-oxidase
- RAGE
receptor of AGE
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- sRAGE
soluble RAGE
- TNF
tumor necrosis factor
- UV
ultraviolet
- WB
western blot
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/22028
References
- 1.Viña J, Borrás C, Miquel J. Theories of ageing. IUBMB Life. 2007;59:249–54. doi: 10.1080/15216540601178067. [DOI] [PubMed] [Google Scholar]
- 2.Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3–14. doi: 10.1016/j.clindermatol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Makrantonaki E, Zouboulis CC. William J. Cunliffe Scientific Awards. Characteristics and pathomechanisms of endogenously aged skin. Dermatology. 2007;214:352–60. doi: 10.1159/000100890. [DOI] [PubMed] [Google Scholar]
- 4.Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci. 2007;1119:40–50. doi: 10.1196/annals.1404.027. [DOI] [PubMed] [Google Scholar]
- 5.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–70. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 6.Bernhard D, Moser C, Backovic A, Wick G. Cigarette smoke--an aging accelerator? Exp Gerontol. 2007;42:160–5. doi: 10.1016/j.exger.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Medvedev ZA. An attempt at a rational classification of theories of ageing. Biol Rev Camb Philos Soc. 1990;65:375–98. doi: 10.1111/j.1469-185X.1990.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 8.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89:10114–8. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michikawa Y, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286:774–9. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- 11.Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–61. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 12.Giacomoni PU, Rein G. Factors of skin ageing share common mechanisms. Biogerontology. 2001;2:219–29. doi: 10.1023/A:1013222629919. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Maillard LC. Action des acides amines sur les sucres: formation des melanoidines par voie methodique. C R Acad Sci (Paris) 1912;154:66–8. [Google Scholar]
- 15.Hodge JE. Dehydrated foods, chemistry of browning reactions in model systems. J Agric Food Chem. 1953;1:928–43. doi: 10.1021/jf60015a004. [DOI] [Google Scholar]
- 16.Paul RG, Bailey AJ. Glycation of collagen: the basis of its central role in the late complications of ageing and diabetes. Int J Biochem Cell Biol. 1996;28:1297–310. doi: 10.1016/S1357-2725(96)00079-9. [DOI] [PubMed] [Google Scholar]
- 17.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids. 2003;25:275–81. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 18.Kawabata K, Yoshikawa H, Saruwatari K, Akazawa Y, Inoue T, Kuze T, et al. The presence of N(ε)-(Carboxymethyl) lysine in the human epidermis. Biochim Biophys Acta. 2011;1814:1246–52. doi: 10.1016/j.bbapap.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, et al. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–9. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeanmaire C, Danoux L, Pauly G. Glycation during human dermal intrinsic and actinic ageing: an in vivo and in vitro model study. Br J Dermatol. 2001;145:10–8. doi: 10.1046/j.1365-2133.2001.04275.x. [DOI] [PubMed] [Google Scholar]
- 21.Fan X, Sell DR, Zhang J, Nemet I, Theves M, Lu J, et al. Anaerobic vs aerobic pathways of carbonyl and oxidant stress in human lens and skin during aging and in diabetes: A comparative analysis. Free Radic Biol Med. 2010;49:847–56. doi: 10.1016/j.freeradbiomed.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kueper T, Grune T, Prahl S, Lenz H, Welge V, Biernoth T, et al. Vimentin is the specific target in skin glycation. Structural prerequisites, functional consequences, and role in skin aging. J Biol Chem. 2007;282:23427–36. doi: 10.1074/jbc.M701586200. [DOI] [PubMed] [Google Scholar]
- 23.Mizutari K, Ono T, Ikeda K, Kayashima K, Horiuchi S. Photo-enhanced modification of human skin elastin in actinic elastosis by N(epsilon)-(carboxymethyl)lysine, one of the glycoxidation products of the Maillard reaction. J Invest Dermatol. 1997;108:797–802. doi: 10.1111/1523-1747.ep12292244. [DOI] [PubMed] [Google Scholar]
- 24.Yu Y, Thorpe SR, Jenkins AJ, Shaw JN, Sochaski MA, McGee D, et al. DCCT/EDIC Research Group Advanced glycation end-products and methionine sulphoxide in skin collagen of patients with type 1 diabetes. Diabetologia. 2006;49:2488–98. doi: 10.1007/s00125-006-0355-8. [DOI] [PubMed] [Google Scholar]
- 25.Taneda S, Monnier VM. ELISA of pentosidine, an advanced glycation end product, in biological specimens. Clin Chem. 1994;40:1766–73. [PubMed] [Google Scholar]
- 26.Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM. Glucosepane is a major protein cross-link of the senescent human extracellular matrix. Relationship with diabetes. J Biol Chem. 2005;280:12310–5. doi: 10.1074/jbc.M500733200. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 1997;324:565–70. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frye EB, Degenhardt TP, Thorpe SR, Baynes JW. Role of the Maillard reaction in aging of tissue proteins. Advanced glycation end product-dependent increase in imidazolium cross-links in human lens proteins. J Biol Chem. 1998;273:18714–9. doi: 10.1074/jbc.273.30.18714. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed MU, Thorpe SR, Baynes JW. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem. 1986;261:4889–94. [PubMed] [Google Scholar]
- 30.Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34:10872–8. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- 31.Sell DR, Monnier VM. Isolation, purification and partial characterization of novel fluorophores from aging human insoluble collagen-rich tissue. Connect Tissue Res. 1989;19:77–92. doi: 10.3109/03008208909016816. [DOI] [PubMed] [Google Scholar]
- 32.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344:109–16. doi: 10.1042/0264-6021:3440109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleming TH, Humpert PM, Nawroth PP, Bierhaus A. Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process: a mini-review. Gerontology. 2011;57:435–43. doi: 10.1159/000322087. [DOI] [PubMed] [Google Scholar]
- 34.Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, et al. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A. 1997;94:13915–20. doi: 10.1073/pnas.94.25.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leslie RD, Beyan H, Sawtell P, Boehm BO, Spector TD, Snieder H. Level of an advanced glycated end product is genetically determined: a study of normal twins. Diabetes. 2003;52:2441–4. doi: 10.2337/diabetes.52.9.2441. [DOI] [PubMed] [Google Scholar]
- 36.Thornalley PJ. The enzymatic defence against glycation in health, disease and therapeutics: a symposium to examine the concept. Biochem Soc Trans. 2003;31:1341–2. doi: 10.1042/BST0311341. [DOI] [PubMed] [Google Scholar]
- 37.Xue M, Rabbani N, Thornalley PJ. Glyoxalase in ageing. Semin Cell Dev Biol. 2011;22:293–301. doi: 10.1016/j.semcdb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, Monnier VM. Enzymatic deglycation of proteins. Arch Biochem Biophys. 2003;419:16–24. doi: 10.1016/j.abb.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Van Schaftingen E, Collard F, Wiame E, Veiga-da-Cunha M. Enzymatic repair of Amadori products. Amino Acids. 2012;42:1143–50. doi: 10.1007/s00726-010-0780-3. [DOI] [PubMed] [Google Scholar]
- 40.Conner JR, Beisswenger PJ, Szwergold BS. Some clues as to the regulation, expression, function, and distribution of fructosamine-3-kinase and fructosamine-3-kinase-related protein. Ann N Y Acad Sci. 2005;1043:824–36. doi: 10.1196/annals.1333.095. [DOI] [PubMed] [Google Scholar]
- 41.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83:876–86. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 42.Miyata T, Inagi R, Iida Y, Sato M, Yamada N, Oda O, et al. Involvement of beta 2-microglobulin modified with advanced glycation end products in the pathogenesis of hemodialysis-associated amyloidosis. Induction of human monocyte chemotaxis and macrophage secretion of tumor necrosis factor-alpha and interleukin-1. J Clin Invest. 1994;93:521–8. doi: 10.1172/JCI117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loughlin DT, Artlett CM. Precursor of advanced glycation end products mediates ER-stress-induced caspase-3 activation of human dermal fibroblasts through NAD(P)H oxidase 4. PLoS One. 2010;5:e11093. doi: 10.1371/journal.pone.0011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- 45.Lohwasser C, Neureiter D, Weigle B, Kirchner T, Schuppan D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J Invest Dermatol. 2006;126:291–9. doi: 10.1038/sj.jid.5700070. [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto E, Kobayashi T, Fujimoto N, Akiyama M, Tajima S, Nagai R. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130:405–14. doi: 10.1038/jid.2009.269. [DOI] [PubMed] [Google Scholar]
- 47.Zhu P, Ren M, Yang C, Hu YX, Ran JM, Yan L. Involvement of RAGE, MAPK and NFκB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp Dermatol. 2012;21:123–9. doi: 10.1111/j.1600-0625.2011.01408.x. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt AM, Yan SD, Brett J, Mora R, Nowygrod R, Stern D. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products. J Clin Invest. 1993;91:2155–68. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hilmenyuk T, Bellinghausen I, Heydenreich B, Ilchmann A, Toda M, Grabbe S, et al. Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells. Immunology. 2010;129:437–45. doi: 10.1111/j.1365-2567.2009.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Akirav EM, Chen W, Henegariu O, Moser B, Desai D, et al. RAGE ligation affects T cell activation and controls T cell differentiation. J Immunol. 2008;181:4272–8. doi: 10.4049/jimmunol.181.6.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem. 2000;275:25781–90. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- 52.Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev. 2001;17:436–43. doi: 10.1002/dmrr.233. [DOI] [PubMed] [Google Scholar]
- 53.Lu C, He JC, Cai W, Liu H, Zhu L, Vlassara H. Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc Natl Acad Sci U S A. 2004;101:11767–72. doi: 10.1073/pnas.0401588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai W, He JC, Zhu L, Chen X, Zheng F, Striker GE, et al. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol. 2008;173:327–36. doi: 10.2353/ajpath.2008.080152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramasamy R, Yan SF, Schmidt AM. RAGE: therapeutic target and biomarker of the inflammatory response--the evidence mounts. J Leukoc Biol. 2009;86:505–12. doi: 10.1189/jlb.0409230. [DOI] [PubMed] [Google Scholar]
- 56.Verzijl N, DeGroot J, Oldehinkel E, Bank RA, Thorpe SR, Baynes JW, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000;350:381–7. doi: 10.1042/0264-6021:3500381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol. 2007;103:2068–76. doi: 10.1152/japplphysiol.00670.2007. [DOI] [PubMed] [Google Scholar]
- 58.Sell DR, Carlson EC, Monnier VM. Differential effects of type 2 (non-insulin-dependent) diabetes mellitus on pentosidine formation in skin and glomerular basement membrane. Diabetologia. 1993;36:936–41. doi: 10.1007/BF02374476. [DOI] [PubMed] [Google Scholar]
- 59.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A. 2002;99:15596–601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glenn JV, Beattie JR, Barrett L, Frizzell N, Thorpe SR, Boulton ME, et al. Confocal Raman microscopy can quantify advanced glycation end product (AGE) modifications in Bruch’s membrane leading to accurate, nondestructive prediction of ocular aging. FASEB J. 2007;21:3542–52. doi: 10.1096/fj.06-7896com. [DOI] [PubMed] [Google Scholar]
- 61.Stitt AW. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol. 2001;85:746–53. doi: 10.1136/bjo.85.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeGroot J, Verzijl N, Wenting-Van Wijk MJ, Bank RA, Lafeber FP, Bijlsma JW, et al. Age-related decrease in susceptibility of human articular cartilage to matrix metalloproteinase-mediated degradation: the role of advanced glycation end products. Arthritis Rheum. 2001;44:2562–71. doi: 10.1002/1529-0131(200111)44:11<2562::AID-ART437>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 63.Sell DR, Lane MA, Johnson WA, Masoro EJ, Mock OB, Reiser KM, et al. Longevity and the genetic determination of collagen glycoxidation kinetics in mammalian senescence. Proc Natl Acad Sci U S A. 1996;93:485–90. doi: 10.1073/pnas.93.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997;99:457–68. doi: 10.1172/JCI119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corstjens H, Dicanio D, Muizzuddin N, Neven A, Sparacio R, Declercq L, et al. Glycation associated skin autofluorescence and skin elasticity are related to chronological age and body mass index of healthy subjects. Exp Gerontol. 2008;43:663–7. doi: 10.1016/j.exger.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 66.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–31. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 67.Dunn JA, McCance DR, Thorpe SR, Lyons TJ, Baynes JW. Age-dependent accumulation of N epsilon-(carboxymethyl)lysine and N epsilon-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry. 1991;30:1205–10. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- 68.Pageon H. Reaction of glycation and human skin: the effects on the skin and its components, reconstructed skin as a model. Pathol Biol (Paris) 2010;58:226–31. doi: 10.1016/j.patbio.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 69.Yamauchi M, Prisayanh P, Haque Z, Woodley DT. Collagen cross-linking in sun-exposed and unexposed sites of aged human skin. J Invest Dermatol. 1991;97:938–41. doi: 10.1111/1523-1747.ep12491727. [DOI] [PubMed] [Google Scholar]
- 70.Nicholl ID, Stitt AW, Moore JE, Ritchie AJ, Archer DB, Bucala R. Increased levels of advanced glycation endproducts in the lenses and blood vessels of cigarette smokers. Mol Med. 1998;4:594–601. [PMC free article] [PubMed] [Google Scholar]
- 71.Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, et al. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care. 2006;29:2654–9. doi: 10.2337/dc05-2173. [DOI] [PubMed] [Google Scholar]
- 72.Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, et al. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287–91. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 73.Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, et al. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–33. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Avery NC, Bailey AJ. The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathol Biol (Paris) 2006;54:387–95. doi: 10.1016/j.patbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Haitoglou CS, Tsilibary EC, Brownlee M, Charonis AS. Altered cellular interactions between endothelial cells and nonenzymatically glucosylated laminin/type IV collagen. J Biol Chem. 1992;267:12404–7. [PubMed] [Google Scholar]
- 76.DeGroot J. The AGE of the matrix: chemistry, consequence and cure. Curr Opin Pharmacol. 2004;4:301–5. doi: 10.1016/j.coph.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 77.Yoshinaga E, Kawada A, Ono K, Fujimoto E, Wachi H, Harumiya S, et al. N(ɛ)-(carboxymethyl)lysine modification of elastin alters its biological properties: implications for the accumulation of abnormal elastic fibers in actinic elastosis. J Invest Dermatol. 2012;132:315–23. doi: 10.1038/jid.2011.298. [DOI] [PubMed] [Google Scholar]
- 78.Reihsner R, Melling M, Pfeiler W, Menzel EJ. Alterations of biochemical and two-dimensional biomechanical properties of human skin in diabetes mellitus as compared to effects of in vitro non-enzymatic glycation. Clin Biomech (Bristol, Avon) 2000;15:379–86. doi: 10.1016/S0268-0033(99)00085-6. [DOI] [PubMed] [Google Scholar]
- 79.Yoon HS, Baik SH, Oh CH. Quantitative measurement of desquamation and skin elasticity in diabetic patients. Skin Res Technol. 2002;8:250–4. doi: 10.1034/j.1600-0846.2002.00332.x. [DOI] [PubMed] [Google Scholar]
- 80.Giardino I, Edelstein D, Brownlee M. Nonenzymatic glycosylation in vitro and in bovine endothelial cells alters basic fibroblast growth factor activity. A model for intracellular glycosylation in diabetes. J Clin Invest. 1994;94:110–7. doi: 10.1172/JCI117296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak D, et al. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in nondiabetics) Aging Cell. 2012;11:1–13. doi: 10.1111/j.1474-9726.2011.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ukeda H, Hasegawa Y, Ishi T, Sawamura M. Inactivation of Cu,Zn-superoxide dismutase by intermediates of Maillard reaction and glycolytic pathway and some sugars. Biosci Biotechnol Biochem. 1997;61:2039–42. doi: 10.1271/bbb.61.2039. [DOI] [PubMed] [Google Scholar]
- 83.Baynes JW. The Maillard hypothesis on aging: time to focus on DNA. Ann N Y Acad Sci. 2002;959:360–7. doi: 10.1111/j.1749-6632.2002.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 84.Zhu P, Yang C, Chen LH, Ren M, Lao GJ, Yan L. Impairment of human keratinocyte mobility and proliferation by advanced glycation end products-modified BSA. Arch Dermatol Res. 2011;303:339–50. doi: 10.1007/s00403-010-1102-z. [DOI] [PubMed] [Google Scholar]
- 85.Wondrak GT, Roberts MJ, Jacobson MK, Jacobson EL. Photosensitized growth inhibition of cultured human skin cells: mechanism and suppression of oxidative stress from solar irradiation of glycated proteins. J Invest Dermatol. 2002;119:489–98. doi: 10.1046/j.1523-1747.2002.01788.x. [DOI] [PubMed] [Google Scholar]
- 86.Berge U, Behrens J, Rattan SI. Sugar-induced premature aging and altered differentiation in human epidermal keratinocytes. Ann N Y Acad Sci. 2007;1100:524–9. doi: 10.1196/annals.1395.058. [DOI] [PubMed] [Google Scholar]
- 87.Alikhani Z, Alikhani M, Boyd CM, Nagao K, Trackman PC, Graves DT. Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J Biol Chem. 2005;280:12087–95. doi: 10.1074/jbc.M406313200. [DOI] [PubMed] [Google Scholar]
- 88.Molinari J, Ruszova E, Velebny V, Robert L. Effect of advanced glycation endproducts on gene expression profiles of human dermal fibroblasts. Biogerontology. 2008;9:177–82. doi: 10.1007/s10522-008-9129-7. [DOI] [PubMed] [Google Scholar]
- 89.Ravelojaona V, Robert AM, Robert L. Expression of senescence-associated beta-galactosidase (SA-beta-Gal) by human skin fibroblasts, effect of advanced glycation end-products and fucose or rhamnose-rich polysaccharides. Arch Gerontol Geriatr. 2009;48:151–4. doi: 10.1016/j.archger.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 90.Sejersen H, Rattan SI. Dicarbonyl-induced accelerated aging in vitro in human skin fibroblasts. Biogerontology. 2009;10:203–11. doi: 10.1007/s10522-008-9172-4. [DOI] [PubMed] [Google Scholar]
- 91.Schmidt AM, Hori O, Chen J, Li JF, Crandall J, Zhang J, et al. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1): a potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Portero-Otín M, Pamplona R, Bellmunt MJ, Ruiz MC, Prat J, Salvayre R, et al. Advanced glycation end product precursors impair epidermal growth factor receptor signaling. Diabetes. 2002;51:1535–42. doi: 10.2337/diabetes.51.5.1535. [DOI] [PubMed] [Google Scholar]
- 93.Masaki H, Okano Y, Sakurai H. Generation of active oxygen species from advanced glycation end-products (AGE) under ultraviolet light A (UVA) irradiation. Biochem Biophys Res Commun. 1997;235:306–10. doi: 10.1006/bbrc.1997.6780. [DOI] [PubMed] [Google Scholar]
- 94.Yim MB, Yim HS, Lee C, Kang SO, Chock PB. Protein glycation: creation of catalytic sites for free radical generation. Ann N Y Acad Sci. 2001;928:48–53. doi: 10.1111/j.1749-6632.2001.tb05634.x. [DOI] [PubMed] [Google Scholar]
- 95.Lee C, Yim MB, Chock PB, Yim HS, Kang SO. Oxidation-reduction properties of methylglyoxal-modified protein in relation to free radical generation. J Biol Chem. 1998;273:25272–8. doi: 10.1074/jbc.273.39.25272. [DOI] [PubMed] [Google Scholar]
- 96.Qian M, Liu M, Eaton JW. Transition metals bind to glycated proteins forming redox active “glycochelates”: implications for the pathogenesis of certain diabetic complications. Biochem Biophys Res Commun. 1998;250:385–9. doi: 10.1006/bbrc.1998.9326. [DOI] [PubMed] [Google Scholar]
- 97.Wondrak GT. Let the sun shine in: mechanisms and potential for therapeutics in skin photodamage. Curr Opin Investig Drugs. 2007;8:390–400. [PubMed] [Google Scholar]
- 98.Meerwaldt R, Links T, Graaff R, Thorpe SR, Baynes JW, Hartog J, et al. Simple noninvasive measurement of skin autofluorescence. Ann N Y Acad Sci. 2005;1043:290–8. doi: 10.1196/annals.1333.036. [DOI] [PubMed] [Google Scholar]
- 99.Mulder DJ, Water TV, Lutgers HL, Graaff R, Gans RO, Zijlstra F, et al. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: an overview of current clinical studies, evidence, and limitations. Diabetes Technol Ther. 2006;8:523–35. doi: 10.1089/dia.2006.8.523. [DOI] [PubMed] [Google Scholar]
- 100.Tseng JY, Ghazaryan AA, Lo W, Chen YF, Hovhannisyan V, Chen SJ, et al. Multiphoton spectral microscopy for imaging and quantification of tissue glycation. Biomed Opt Express. 2010;2:218–30. doi: 10.1364/BOE.2.000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bos DC, de Ranitz-Greven WL, de Valk HW. Advanced glycation end products, measured as skin autofluorescence and diabetes complications: a systematic review. Diabetes Technol Ther. 2011;13:773–9. doi: 10.1089/dia.2011.0034. [DOI] [PubMed] [Google Scholar]
- 102.Smit AJ, Gerrits EG. Skin autofluorescence as a measure of advanced glycation endproduct deposition: a novel risk marker in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:527–33. doi: 10.1097/MNH.0b013e32833e9259. [DOI] [PubMed] [Google Scholar]
- 103.Genuth S, Sun W, Cleary P, Sell DR, Dahms W, Malone J, et al. DCCT Skin Collagen Ancillary Study Group Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes. 2005;54:3103–11. doi: 10.2337/diabetes.54.11.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beisswenger PJ, Howell S, Mackenzie T, Corstjens H, Muizzuddin N, Matsui MS. Two fluorescent wavelengths, 440(ex)/520(em) nm and 370(ex)/440(em) nm, reflect advanced glycation and oxidation end products in human skin without diabetes. Diabetes Technol Ther. 2012;14:285–92. doi: 10.1089/dia.2011.0108. [DOI] [PubMed] [Google Scholar]
- 105.Farris PK. Innovative cosmeceuticals: sirtuin activators and anti-glycation compounds. Semin Cutan Med Surg. 2011;30:163–6. doi: 10.1016/j.sder.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 106.Elosta A, Ghous T, Ahmed N. Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications. Curr Diabetes Rev. 2012;8:92–108. doi: 10.2174/157339912799424528. [DOI] [PubMed] [Google Scholar]
- 107.Edelstein D, Brownlee M. Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine. Diabetes. 1992;41:26–9. doi: 10.2337/diabetes.41.1.26. [DOI] [PubMed] [Google Scholar]
- 108.Reddy VP, Beyaz A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov Today. 2006;11:646–54. doi: 10.1016/j.drudis.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 109.Pageon H, Técher MP, Asselineau D. Reconstructed skin modified by glycation of the dermal equivalent as a model for skin aging and its potential use to evaluate anti-glycation molecules. Exp Gerontol. 2008;43:584–8. doi: 10.1016/j.exger.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 110.Sell DR, Nelson JF, Monnier VM. Effect of chronic aminoguanidine treatment on age-related glycation, glycoxidation, and collagen cross-linking in the Fischer 344 rat. J Gerontol A Biol Sci Med Sci. 2001;56:B405–11. doi: 10.1093/gerona/56.9.B405. [DOI] [PubMed] [Google Scholar]
- 111.Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, et al. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002;61:939–50. doi: 10.1046/j.1523-1755.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- 112.Voziyan PA, Hudson BG. Pyridoxamine: the many virtues of a maillard reaction inhibitor. Ann N Y Acad Sci. 2005;1043:807–16. doi: 10.1196/annals.1333.093. [DOI] [PubMed] [Google Scholar]
- 113.Vasan S, Foiles P, Founds H. Therapeutic potential of breakers of advanced glycation end product-protein crosslinks. Arch Biochem Biophys. 2003;419:89–96. doi: 10.1016/j.abb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 114.Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, et al. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res. 2003;92:785–92. doi: 10.1161/01.RES.0000065620.39919.20. [DOI] [PubMed] [Google Scholar]
- 115.Bakris GL, Bank AJ, Kass DA, Neutel JM, Preston RA, Oparil S. Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. Am J Hypertens. 2004;17:23S–30S. doi: 10.1016/j.amjhyper.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 116.Yang S, Litchfield JE, Baynes JW. AGE-breakers cleave model compounds, but do not break Maillard crosslinks in skin and tail collagen from diabetic rats. Arch Biochem Biophys. 2003;412:42–6. doi: 10.1016/S0003-9861(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 117.Monnier VM, Sell DR. Prevention and repair of protein damage by the Maillard reaction in vivo. Rejuvenation Res. 2006;9:264–73. doi: 10.1089/rej.2006.9.264. [DOI] [PubMed] [Google Scholar]
- 118.Monnier VM, Wu X. Enzymatic deglycation with amadoriase enzymes from Aspergillus sp. as a potential strategy against the complications of diabetes and aging. Biochem Soc Trans. 2003;31:1349–53. doi: 10.1042/BST0311349. [DOI] [PubMed] [Google Scholar]
- 119.Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, et al. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101:1142–7. doi: 10.1172/JCI119885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim KM, Kim YS, Jung DH, Lee J, Kim JS. Increased glyoxalase I levels inhibit accumulation of oxidative stress and an advanced glycation end product in mouse mesangial cells cultured in high glucose. Exp Cell Res. 2012;318:152–9. doi: 10.1016/j.yexcr.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 121.Brouwers O, Niessen PM, Ferreira I, Miyata T, Scheffer PG, Teerlink T, et al. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem. 2011;286:1374–80. doi: 10.1074/jbc.M110.144097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xue M, Rabbani N, Momiji H, Imbasi P, Anwar MM, Kitteringham N, et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem J. 2012;443:213–22. doi: 10.1042/BJ20111648. [DOI] [PubMed] [Google Scholar]
- 123.Price DL, Rhett PM, Thorpe SR, Baynes JW. Chelating activity of advanced glycation end-product inhibitors. J Biol Chem. 2001;276:48967–72. doi: 10.1074/jbc.M108196200. [DOI] [PubMed] [Google Scholar]
- 124.Rutter K, Sell DR, Fraser N, Obrenovich M, Zito M, Starke-Reed P, et al. Green tea extract suppresses the age-related increase in collagen crosslinking and fluorescent products in C57BL/6 mice. Int J Vitam Nutr Res. 2003;73:453–60. doi: 10.1024/0300-9831.73.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tarwadi KV, Agte VV. Effect of micronutrients on methylglyoxal-mediated in vitro glycation of albumin. Biol Trace Elem Res. 2011;143:717–25. doi: 10.1007/s12011-010-8915-7. [DOI] [PubMed] [Google Scholar]
- 126.Thirunavukkarasu V, Nandhini AT, Anuradha CV. Lipoic acid prevents collagen abnormalities in tail tendon of high-fructose-fed rats. Diabetes Obes Metab. 2005;7:294–7. doi: 10.1111/j.1463-1326.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- 127.Odetti P, Pesce C, Traverso N, Menini S, Maineri EP, Cosso L, et al. Comparative trial of N-acetyl-cysteine, taurine, and oxerutin on skin and kidney damage in long-term experimental diabetes. Diabetes. 2003;52:499–505. doi: 10.2337/diabetes.52.2.499. [DOI] [PubMed] [Google Scholar]
- 128.Rasheed Z, Anbazhagan AN, Akhtar N, Ramamurthy S, Voss FR, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res Ther. 2009;11:R71. doi: 10.1186/ar2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vinson JA, Howard HB. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J Nutr Biochem. 1996;7:659–63. doi: 10.1016/S0955-2863(96)00128-3. [DOI] [Google Scholar]
- 130.Dearlove RP, Greenspan P, Hartle DK, Swanson RB, Hargrove JL. Inhibition of protein glycation by extracts of culinary herbs and spices. J Med Food. 2008;11:275–81. doi: 10.1089/jmf.2007.536. [DOI] [PubMed] [Google Scholar]
- 131.Jin S, Cho KH. Water extracts of cinnamon and clove exhibits potent inhibition of protein glycation and anti-atherosclerotic activity in vitro and in vivo hypolipidemic activity in zebrafish. Food Chem Toxicol. 2011;49:1521–9. doi: 10.1016/j.fct.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 132.Draelos ZD, Yatskayer M, Raab S, Oresajo C. An evaluation of the effect of a topical product containing C-xyloside and blueberry extract on the appearance of type II diabetic skin. J Cosmet Dermatol. 2009;8:147–51. doi: 10.1111/j.1473-2165.2009.00428.x. [DOI] [PubMed] [Google Scholar]
- 133.Wu CH, Yen GC. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J Agric Food Chem. 2005;53:3167–73. doi: 10.1021/jf048550u. [DOI] [PubMed] [Google Scholar]
- 134.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–3. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 135.Cefalu WT, Bell-Farrow AD, Wang ZQ, Sonntag WE, Fu MX, Baynes JW, et al. Caloric restriction decreases age-dependent accumulation of the glycoxidation products, N epsilon-(carboxymethyl)lysine and pentosidine, in rat skin collagen. J Gerontol A Biol Sci Med Sci. 1995;50:B337–41. doi: 10.1093/gerona/50A.6.B337. [DOI] [PubMed] [Google Scholar]
- 136.Reiser KM. Influence of age and long-term dietary restriction on enzymatically mediated crosslinks and nonenzymatic glycation of collagen in mice. J Gerontol. 1994;49:B71–9. doi: 10.1093/geronj/49.2.b71. [DOI] [PubMed] [Google Scholar]
- 137.Sell DR, Kleinman NR, Monnier VM. Longitudinal determination of skin collagen glycation and glycoxidation rates predicts early death in C57BL/6NNIA mice. FASEB J. 2000;14:145–56. doi: 10.1096/fasebj.14.1.145. [DOI] [PubMed] [Google Scholar]
- 138.Vlassara H, Uribarri J, Ferrucci L, Cai W, Torreggiani M, Post JB, et al. Identifying advanced glycation end products as a major source of oxidants in aging: implications for the management and/or prevention of reduced renal function in elderly persons. Semin Nephrol. 2009;29:594–603. doi: 10.1016/j.semnephrol.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sebeková K, Somoza V. Dietary advanced glycation endproducts (AGEs) and their health effects--PRO. Mol Nutr Food Res. 2007;51:1079–84. doi: 10.1002/mnfr.200700035. [DOI] [PubMed] [Google Scholar]
- 140.Yamagishi S, Ueda S, Okuda S. Food-derived advanced glycation end products (AGEs): a novel therapeutic target for various disorders. Curr Pharm Des. 2007;13:2832–6. doi: 10.2174/138161207781757051. [DOI] [PubMed] [Google Scholar]
- 141.Vlassara H, Striker GE. AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol. 2011;7:526–39. doi: 10.1038/nrendo.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peppa M, Brem H, Ehrlich P, Zhang JG, Cai W, Li Z, et al. Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes. 2003;52:2805–13. doi: 10.2337/diabetes.52.11.2805. [DOI] [PubMed] [Google Scholar]
- 143.Lyons TJ, Bailie KE, Dyer DG, Dunn JA, Baynes JW. Decrease in skin collagen glycation with improved glycemic control in patients with insulin-dependent diabetes mellitus. J Clin Invest. 1991;87:1910–5. doi: 10.1172/JCI115216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Monnier VM, Bautista O, Kenny D, Sell DR, Fogarty J, Dahms W, et al. DCCT Skin Collagen Ancillary Study Group Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes. 1999;48:870–80. doi: 10.2337/diabetes.48.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hudson BI, Bucciarelli LG, Wendt T, Sakaguchi T, Lalla E, Qu W, et al. Blockade of receptor for advanced glycation endproducts: a new target for therapeutic intervention in diabetic complications and inflammatory disorders. Arch Biochem Biophys. 2003;419:80–8. doi: 10.1016/j.abb.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 146.Yan SF, Ramasamy R, Schmidt AM. Soluble RAGE: therapy and biomarker in unraveling the RAGE axis in chronic disease and aging. Biochem Pharmacol. 2010;79:1379–86. doi: 10.1016/j.bcp.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, et al. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159:513–25. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Babizhayev MA, Nikolayev GM, Nikolayeva JG, Yegorov YE. Biologic activities of molecular chaperones and pharmacologic chaperone imidazole-containing dipeptide-based compounds: natural skin care help and the ultimate challenge: implication for adaptive responses in the skin. Am J Ther. 2012;19:e69–89. doi: 10.1097/MJT.0b013e3181e71fb7. [DOI] [PubMed] [Google Scholar]
- 149.Babizhayev MA, Yegorov YE. Therapeutic uses of drug-carrier systems for imidazole-containing dipeptide compounds that act as pharmacological chaperones and have significant impact on the treatment of chronic diseases associated with increased oxidative stress and the formation of advanced glycation end products. Crit Rev Ther Drug Carrier Syst. 2010;27:85–154. doi: 10.1615/CritRevTherDrugCarrierSyst.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- 150.Babizhayev MA, Deyev AI, Savel’yeva EL, Lankin VZ, Yegorov YE. Skin beautification with oral non-hydrolized versions of carnosine and carcinine: Effective therapeutic management and cosmetic skincare solutions against oxidative glycation and free-radical production as a causal mechanism of diabetic complications and skin aging. J Dermatolog Treat. 2011 doi: 10.3109/09546634.2010.521812. In press. [DOI] [PubMed] [Google Scholar]