Abstract
Research over the past decade has revealed close interaction between the nervous and immune systems in regulation of peripheral inflammation linking psychosocial stress with chronic somatic disease and aging. Moreover emerging data suggests that chronic inflammations lead to a pro-inflammatory status underlying premature aging called inflammaging. In this context, the spleen can be seen as a switch board monitoring peripherally derived neuroendocrine-immune mediators in the blood and keeping up a close communication with the central stress response via its mainly sympathetic innervation. The effect aims at balanced and well-timed stress axis activation and immune adaptation in acute peripheral inflammatory events. Constant adjustment to the needs generated by environmental and endogenous challenges is provided by neuroendocrine-immune plasticity. However, maladaptive plasticity induced e.g., by chronic stress-axis activation and excessive non-neuronal derived neuroendocrine mediators may be at the heart of the observed stress sensitivity promote inflammaging under chronic inflammatory conditions. We here review the role of neurotransmitters, neuropeptides and neurotrophins as stress mediators modulating the immune response in the spleen and their potential role in inflammaging.
Keywords: stress, neuroimmunology, psychodermatology, neurotrophin/neuropeptide stress axis, neuroimmune plasticity, inflammaging
Introduction
Neuroendocrine-immune interaction links stress and the immune response and allows individuals to respond to endogenous or exogenous as well as physiological or psychological stressors (Fig. 1). It can take place anywhere where nerve fibers depositing neurotransmitters or neuropeptides meet with cells of the immune system or were blood born neuroendocrine-immune mediators meet with nerve fibers. Respective interaction takes place mainly in peripheral organs at the self-environment interface or in immune-competent tissues such as the spleen.1-8
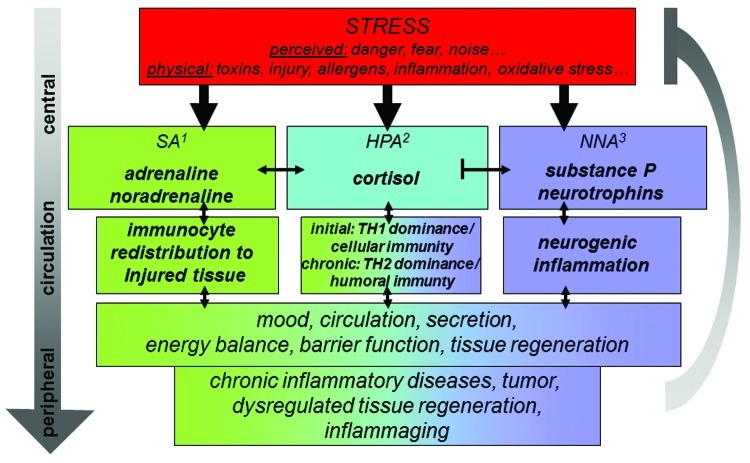
Figure 1. Schematic display of the three stress axis and their activation by a wide variety of stressors as well as selected key effects on the immune system and subsequently chronic disease and inflammaging regulated by inflammatory processes. 1sympathetic nervous system; 2hypothalamus pituitary adrenal axis; 3neurotrophin neuropeptide axis.
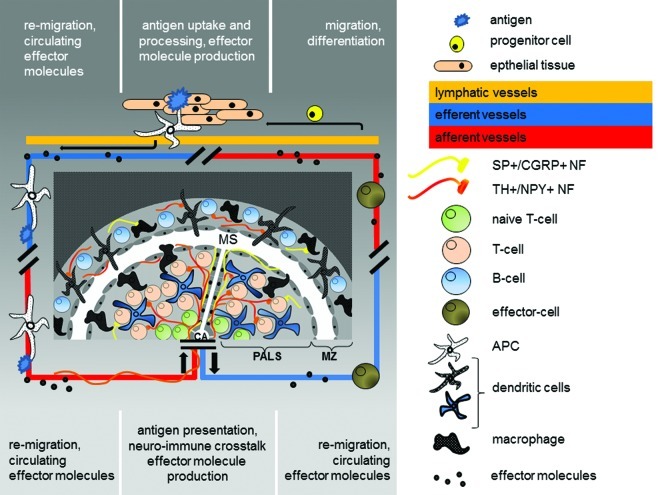
Lymphoid organs provide the space for differentiation and neuroendocrine modulation of the cells of the immune system passing through them and interacting within.5,9,10 The spleen takes a special position among the lymphoid organs since it is supplied with lymphocytes solely via the blood stream (Fig. 2).11 It is the largest lymphoid organ with the highest lymphocyte throughput of all lymphatic tissues and it is the site of cell pooling, elimination of unneeded cells and regulatory effects on a wide variety of cells of the immune system. High amounts of cytokines are produced by the splenic cells, leaving it via the blood stream and acting centrally and peripherally. The best examined cytokines are interleukin (IL)1, IL6 and Tumor Necrosis Factor α (TNFα).12 At the same time it shows prominent innervation of immune-competent areas as well as altered splenic immunity after central stress mediator blockade.13,14 It is therefore ideally suited to conduct and investigate neuroendocrine-immune communication in loco keeping systemic consequences and their role in chronic inflammation and subsequent inflammaging in mind (Fig. 2).
Figure 2. Schematic drawing of the splenic white pulp as the screening area for peripheral inflammation. In the periphery an antigen is taken up by an antigen presenting cell (APC). Cytokines are produced and reaches the circulation. The APC re-migrates into the spleen via the central artery. From here the blood enters the marginal sinus (MS) via the central arterioles (CA) and through open cavities between the endothelial cells surrounding the periarteriolar lymphoid sheaths (PALS) and marginal zone (MZ) circulating cells and effector molecules can enter the splenic white pulp (PALS+MZ). The antigen is presented to lymphocytes within a special microenvironment generated by the effector molecules and the proximity to local nerve fibers which are activated upon stress. The neuro-immune crosstalk itself governs local neurotransmitter- and neuropeptide release and thereby tunes the TH1/TH2 cytokine balance. Vice versa those effector molecules as well as local produced neurotransmitters and neuropeptides finally abandon the spleen via the splenic vein and re-circulate with potential effects on peripheral inflammation and potentially inflammaging.
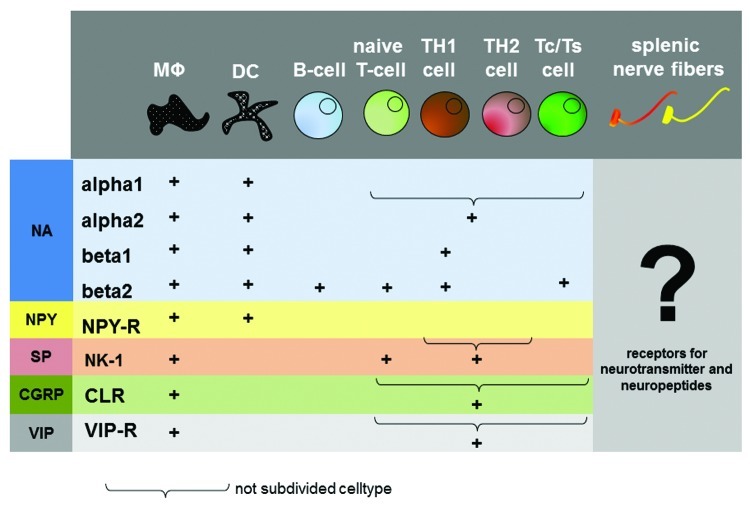
Since receptors for neurotransmitters and neuropeptides as well as neurohormones have been shown on cells of the immune system in the spleen, it is postulated that neural excitation and subsequent secretion or neuronal mediators can directly modulate the immune response in places of close contact.5,15 Vice versa, nerve fibers innervating immune competent tissues carry receptors for neuroendocrine-immune mediators derived from cells of the immune system as well as carried by the blood stream (Fig. 3). They are therefore potentially able to sense and report peripherally derived signals passing through the spleen to the central nervous system.4,5
Figure 3. Distribution of neurotransmitter and neuropeptide receptors on immune cells. The picture gives an overview about the distribution of neurotransmitter- and neuropeptide receptors on immune cells. The question mark indicates a missing link in neuro-immune crosstalk, the distribution of cytokine receptor on splenic nerve fibers. MΦ, Macrophage; DC, dendritic cell; Tc, T-cytotoxic cell; Ts, T-supressor cell; NA, noradrenalin; NPY, neuropeptide Y; SP, substance P; CGRP, calcitonine-gene related peptide; VIP, vasointestinal peptide.
To dissect the potency of this interaction, we will first introduce the term inflammaging and then discuss key neuroendocrine-immune features of the three pillars of the stress response (Fig. 1). We will than summarize what is known to date about the splenic supply with neuroendocrine mediators, discuss their impact on splenic immune cells as well as discuss the impact of mediators derived from the cells of the immune system on the central nervous system. And last but not least, we will draw some consequences for peripheral inflammatory disease on inflammaging discussing allergic inflammation as an instructive example.
Inflammaging: A Premature Aging Process Associated with Stress
To date, the process of aging and why cells lose their capacity to replicate over time is still ill understood. With respect to the immune system, less cells of the immune system are produced and on top show altered functionality such as a reduced capacity to detect neoantigen and a preserved humoral reactivity.16-19 In the present literature on unhealthy and premature aging, chronic inflammatory disease plays an important role.20 The permanent exposure to stressors—endogenous or exogenous, physiological or psychological—during the life span and the individual skill to coupe with them tends to provoke chronic inflammation which is summarized under the term inflammaging, a theory that describes how lifelong exposure to stressors meets with the organisms declining capacity to neutralize them, which finally results in the incapacity to terminate inflammatory processes.21,22 However, it has yet to be comprehensively shown, if an enhanced pro-inflammatory state is cause or result of aging associated chronic diseases.23,24 It appears that a systemic dominance of the T helper cell (TH) type 2 immune response associates with aging and thus hints at a potential role for altered splenic immune regulation.18
The Three Pillars of the Neuroendocrine-Immune Response to Stress: SA and the Fight and Flight Response
Historically, the oldest stress axis known is represented by the sympathetic axis (SA). With the discovery of noradrenaline (NA) in peripheral nerve fibers and adrenal derived adrenaline the “fight or flight response” was described by Walter Cannon Bradford.25 Only later it became evident, that activation of this axis also holds immunological implications: the mediators of the SA are for example responsible for rapid redistribution of the cells of the immune system into the targeted organs upon acute stress activation which supports the fight or flight concept also on the level of local immune defense and promotes rapid microbe or tumor cell elimination in loco.26-29 This is further promoted by activation of natural killer cells and TH1 cell differentiation (Fig. 1). However, SA-immune interaction in the spleen cannot be discussed without mention of mechanisms counteracting this acute stress response. Under complex stress-exposure (e.g., combination of LPS and restraint) or chronic inflammatory conditions splenic SA innervation and responsiveness to adrenergic mediators in splenic cells is altered and associates with immune cell apoptosis and reduced TNFα and interferon gamma (IFNγ) and thus a systemic TH2 shift as observed in the elderly.18 Due to its dense noradrenergic innervation, the spleen may be the most prominent site in the decision process promoting innate and TH1 immune responses after acute stress activation or inflammatory challenge vs. adaptive and TH2 immune responses after exaggerated or chronic challenge.5,30,31
The Three Pillars of the Neuroendocrine-Immune Response to Stress: HPA and Its Many Roles in Keeping the Immune Balance
In the fifties of the last century a second stress axis became known and the term stress in its present meaning was coined by Hans Seyle to describe the function of the hypothalamus pituitary adrenal axis (HPA).32 To date we have learned about a large number of immunological functions of the neuropeptide and endocrine mediators released after activation of this axis such as corticotrophin releasing hormone (CRH), adrenocorticotropic hormone (ACTH) and cortisol. We know now that depending on secretion mode and amount these neuroendocrine mediators act systemically as well as locally to shape an immune response that is either optimally suited to adapt to acute or to adapt to chronic inflammatory challenges (Fig. 1).33,34 However, the list of neuroendocrine-immune activities of HPA mediators continues to grow daily and the somewhat contradictory results obtained with either acute or chronic stress models continue to confuse, not unlike the diverse functions of the SA.35 The potential role for this stress axis in splenic neuroendocrine-immune regulation is less direct than the role of the SA given the lack of direct neuro-immune interaction in most investigated species.36,37 A stress-modulatory role is nonetheless feasible since cells of the immune system derived from the spleen respond to HPA mediators and are even able to generate some.5,30,34,36
The Three Pillars of the Neuroendocrine-Immune Response to Stress: NNA and Its Potential Anti-Inflammatory Function in the Spleen
Since the 1970s additional stress mediators gradually reveal their presence and role in the stress concert and the existence of a third stress axis can be postulated (Fig. 1).8,38-40 Peripheral nerve fibers including the sensory subpopulation contain neuropeptides with potent immune-modulatory activity and they are subjected to neuronal plasticity guided by neurotrophins. Corresponding to their initial discovery in peripheral nerve fibers, one of the first stress related functions described for this neurotrophin neuropeptide stress axis (NNA) was activation of neurogenic inflammation meaning release of neuropeptides such as substance P (SP) and subsequent degranulation of mast cells in organs at the self-environment border such as the skin, lung or gut.40-45 This innate immune response and the aggravation of chronic inflammatory diseases by its activation were found in virtually every chronic inflammatory disease under the respective scrutiny so far, including atopic/allergic dermatitis. With consequences for TH2 driven inflammation, mediators of the NNA also facilitate the transition to specific immune responses and are involved in TH1/TH2 balance and regulatory cell function.46-51 On first sight though, peptidergic signaling alike the HPA appears to play a minor role in splenic immune function, because of the few respective nerve fibers and contacts detectable and thus little direct neuro-immune interaction.
Neuroendocrine-Immune Interaction in the Spleen is Enabled by Its Dense Innervation
As a prerequisite for the above discussed neuroendocrine-immune interactions taking place in the spleen it is important to understand its neuro-anatomy. The number of lymphocytes and the amount of blood-born neuroendocrine-immune mediators entering the spleen is regulated through the blood flow volume via it’s sympathetic innervation.52 Noradrenerge nerve fibers represent the vast majority of all splenic nerve fibers and enter the spleen together with the blood vessels.53 Physical as well as psychoemotional stress can activate the SA and subsequent release of NA changes the tone of vascular smooth muscle of blood vessels in a receptor-dependent manner thereby redirecting the number of cells passing through the spleen to the peripheral organs.52 About 20% of the noradrenergic nerve fibers innervating the spleen reach beyond the vasculature into the splenic parenchyma.53 The densest innervation is found in the trabecular and the white pulp mostly extending from the blood vessels traveling through them.54 By contrast, the red pulp, where intense phagocytosis takes place, is characterized by basically no innervation.5 This intriguing preference of splenic innervation for the immune competent compartments already suggests neuroendocrine-immune regulatory processes taking place in the spleen.
The respective nerve fiber-immune cell contacts take place mostly in the white pulp, which is the immune-competent area of the splenic parenchyma. It is in the peri-arteriolar lymphoid sheaths (PALS) and the marginal zone (MZ) where the spatial organization of nerve fibers and cells of the immune cells brings them so closely together, that direct interaction is more than feasible.5,55,56 In the PALS nerve fibers contact T-cells and dendritic cells. In the MZ nerve fibers contact macrophages and IgM-positive B-cells. These nerve fibers appear to form direct synapse-like contacts with immune cells which fulfill the criteria for neuro-immune crosstalk.5 In addition to neuroendocrine-immune regulation through regulation of blood flow, a direct splenic neuroendocrine modulation has therefor been postulated for lymphocytes, antigen-presenting dendritic cells and macrophages (Fig. 3). This view is supported by observations such that lymphoid cell outflow is regulated by NA.27
In contrast to the well investigated SA of the spleen, little is known about a potential peptidergic innervation and its influence on neuroendocrine-immune regulation. Only a few publications so far discuss the presence of e.g., SP-containing nerve fibers.4,57-60 SP and concomitant calcitonine-gene related peptide (CGRP) containing nerve fibers reach the spleen via the central artery, travel along the trabecular system and arborize into the red pulp. In addition they can be sparsely found in the white pulp in the outer PALS in close proximity to T-lymphocytes and in the MZ adjacent to macrophages. Also, very few splenic nerve fibers contain vasointestinal peptide (VIP) and CRH.4,37
Splenic Immune Cells Respond to Neuroendocrine Mediators and Stress by Changing the Cytokine Balance: A Detailed Look
As mentioned above, NA initially promotes the TH1 response. If naïve or CD4+ T-cells are activated in the presence of NA, or T-cell receptor (TCR) activated TH1 cells are exposed to NA, they show higher rates of IFNγ production.61 Also, an acute catecholamine infusion induces proliferation of T-cytotoxic as well as NK cells.26,62 This effect is mostly mediated through α-adrenoreceptors (αAR).63,64 The most abundant receptor for NA on splenic immune cells however is the β2-adrenoceptor (β2AR).31,62,65 TH1 effector cells display a less dense expression of the β2AR within the spleen and reduction of proliferation rates after mitogen.26,62 Through this, cell mediated immune responses are suppressed while TH2 dominance is promoted.66,67 In more detail, the β2AR is expressed by naïve and effector T-lymphocytes but less on B-cells. Corresponding to chronic SA activation, IFNγ production is inhibited if NA is released before TCR is activated (Fig. 3).61
Despite the apparent lack of prominent direct peptidergic nerve fiber-immune cell interactions in the spleen, some publications discuss the proliferative or suppressive effects of SP on splenic immune cells ex vivo. However, their interest is not the understanding of neuro-immune modification occurring within the spleen but to test the general responsiveness of immune cells to the neuropeptides in question. This is a widely accepted procedure since the spleen is simple to reach and a potent source of large amounts of cells of the immune system for experimental use ex vivo.68,69
Nonetheless, these works demonstrated that SP affects the function of splenic lymphocytes and macrophages, which depends on activation of the neurokinin 1 receptor (NK1).57,70 SP promotes lymphocyte proliferation of CD4+ T-cells but not B-cells.46,71 The supported splenic cell populations appear to be either cytotoxic and acute inflammatory, or regulatory, since SP on the one hand heightens NK cell activity, LFA-1 on CD8+ cells, TNFα and IgG production, and on the other hand induces expression of CD4 together with CD25 and reduces IgE production.46,68,72-74 Another neuropeptide, CRH points in the same direction and promotes splenic TH1 cytokine production as was shown with the help of knockout mice.75
In this context, it is important to realize, that cells of the immune system themselves are capable to produce neuropeptides such as SP which can act in a paracrine fashion on cells in their close vicinity.39,70,76 Capsaicin treatment, which depletes and ultimately destroys peptidergic neurons containing e.g., SP, also reduced the amount of detectable sensory neuropeptides in the spleen indicating their in loco production.77 A notion which is further supported by the observation that CAPS, which regulate the exocytosis of neuropeptide-containing dense-core vesicles, were found in the spleen.78 Consequently, treatment with capsaicin alters spleen morphology and reduces splenic cell proliferation, NK cell activity and IL2 production as well as CD4/CD25+ T-cell number.46,79-81 With respect to TH1/TH2 balance however, both the production of TH1 and TH2 cytokines were reduced in the spleen.48
In addition to the availability of neuroendocrine mediators neuroendocrine-immune regulation is also determined by the length of time and range of diffusion allowed for neurotransmitter and neuropeptide action. This is mainly regulated through the presence and location of enzymes able to terminate their signaling. An enzyme responsible for the digestion of SP is endopeptidase 24.11 (CALLA, CD10, Neprilysin = NEP, CD10/NEP).82-84 SP is its preferred substrate but it also digests other tachykinins.85 It is highly present in histiocytes of lymphatic tissues and may therefor terminate neuropeptide action in respective areas of the spleen while blocking it results in higher numbers of splenic mature B-cells.86,87
Splenic Neuroendocrine Interaction Talks Back to the Brain
Neuroendocrine mediators and cytokines released in the spleen may also have an impact on the activation of the central stress axis, the HPA, since SP as well as pro-inflammatory mediators such as TNFα or IL1 block HPA activation at the level of the hypothalamus.4,5,88 Vice versa, decreased numbers of noradrenergic neurons in the brain relate to reduced pro-inflammatory cytokine production and cellular immunity in the spleen (Fig. 2).13 In the central response to leptin CRH acts as a suppressor of splenic immune activation.89 Also, re-challenge with TH1 inducing LPS results in reduced pro-inflammatory cytokines in the brain but increased in the spleen indicating dissociation between central stress axis activation and peripheral inflammatory response upon rechallenge.90 These observations indicate that central stress axis activation is intimately linked to splenic immune activation in both directions.
Stress Response and Pathogenesis of Chronic Inflammatory Diseases
As mentioned above, since in the late 60s and early 70s, the term stress (meaning the neuroendocrine response of the organism to any threat it encountered which required an adaption of the organisms’ bio-psycho-social functions in order to cope with it) was linked to the development and control of inflammatory disease. The initial experiments demonstrated higher susceptibility to certain viral infections first in stressed mice and then in stressed humans.91 At the same time it became evident that the immune response can be conditioned in a manner resembling the Pavlov reflex.92 This conditioning process appears to work through neuro-immune plasticity, an observation that holds important implications for the therapeutic options provided by the analysis and manipulation of the neuroendocrine system in the spleen and can be broadly summarized under the term placebo.93,94
In peripheral organs at the self-environment interface stress-induced neuroendocrine-immune plasticity has been reported a number of times.42,95,96 A social stress- and NGF-dependent change in lymphoid innervation however has been reported only in lymph nodes of primates, where higher density of nerve fibers negatively correlated with IFNγ expression and a higher vulnerability to viral infection.97 A number of disease pathologies however associate with distinct neuroendocrine-immune changes involving the spleen. Especially in aging a TH2 bias of the immune response is observed and associates with altered splenic supply of SA neurons as well as enhanced neuropeptide signaling.54,98 The age-related decline of noradrenergic nerve fibers is concomitant with reduced splenic NK-cell activity, declined IL2 and IFNγ production and decreased T- and B-cell proliferation.99 This may explain the observed susceptibility to viral infection and TH2 driven diseases such as asthma bronchiale and autoimmunity as in Lupus erythematodes. Independent of aging, other chronic inflammatory autoimmune diseases such as Crohn disease are also associated with neuroendocrine-immune plasticity that hint at pro-inflammatory effects of neuroendocrine-immune states that promote TH1 immunity in cellular dominated inflammatory diseases and those that promote TH2 immunity in humoral dominated inflammatory diseases. Specifically, NA promotes acute and attenuates chronic inflammation in Crohn disease and chemical sympathectomy changes the severity of adjuvant-induced arthritis.100-102
Much has been published recently concerning the concept of an anti-inflammatory axis acting through vagal/parasympathetic nerval activation, the counter-player of the SA. Several studies have shown that disruption of cholinergic nerves promotes overshooting inflammatory events e.g., in an acute inflammatory model such as sepsis. At the same time, pharmacological inhibition of distinct acetylcholine receptors promotes TH1 responses and sepsis while cholinergic agonists promote TH2 immunity.103,104 It is actually long standing knowledge that these cholinergic immune regulatory functions act through the spleen.103-105 They may interfere with acute SA activation of splenic immune responses albeit the vagus does not directly innervate the spleen and it remains to be determined how the two connect.15,106,107
Atopic/Allergic Dermatitis: A TH-2 Driven Model Disease for Peripheral Inflammation Tending Inflammaging
The key symptoms of atopic/allergic dermatitis—pruritus and disfiguring inflammatory skin lesions at visible predilection sites such as the face—greatly reduce the quality of life of the affected patients with psychosocial, medical and economic consequences.108,109 Moreover the lesioned skin tends to look prematurely aged. A vicious circle of itching, scratching and subsequent aggravation of the lesions is linked to stress and malfunctioning stress-adaptation as occurring after excessive, uncontrollable and unpredictable biophysical as well as psychosocial stress exposure.109,110
IL4 predominates in the initially developing atopic lesion and contributes to the characteristic TH2 bias.111 Subsequently, IgE serum levels are increased, vascular cell adhesion molecule (VCAM) is induced on blood vessel epithelia in the skin and eosinophils, mast cells and lymphocytes are recruited into the inflammation site.95,112-114 Approximately 48 h later, the cytokine profile changes and additional IFNγ, the key cytokine of TH1 immune responses, comes into play.115,116 At the same time neurotrophin dependent neuronal plasticity occurs in the skin and enhanced numbers of contacts between peptidergic nerve fibers and mast cells as well as dendritic cells alters the susceptibility to stress aggravation and stress intervention.3,8,38,43 The higher susceptibility of dendritic cells to stress resulting in a higher production of IFNγ could facilitate inflammaging of lesioned skin in allergic inflammation. Emerging data suggests its role in in inflammation-induced premature aging together with IL12, a major spleen derived TH1 cytokine.117
Numerous works by Buske-Kirschbaum and colleagues have impressively shown that individuals with chronic HPA activation and atopic individuals share a decreased reactivity of the HPA axis to acute stress exposure. This neuroendocrine situation facilitates the production of TH2 cytokines and is thus thought to contribute to the pathogenesis and stress aggravation of atopic/allergic inflammatory diseases.118-122 ACTH and glucocorticoids also directly inhibit the production of IFNγ, TNFα or IL12 and enhance the production of IL4, IL10 or IL13 even in TH1 cells.38,123 Altered cortisol levels are also associated with inflammaging and may contribute to the decreased control of allergic inflammation.124
Interestingly, the local production of SP was shown in allergic dermatitis lesions in addition to increased nerve fiber numbers immunoreactive for SP. Stress enhances PPT1 mRNA levels in lesional allergic dermatitis skin and SP-immunoreactivity is found in cells of the immune system in allergic inflammatory sites. Thus a non-neuronal source for SP is provided for at least in skin.95 Intriguingly, NGF is also induced by stress in peripheral tissues and skin derived NGF enhances SP+ cutaneous innervation and induces marginal zone hyperplasia in transgenic mice.110,125,126 It also counteracts TH1 cytokine production and enhances IL4 production in eosinophils via the low-affinity pan-neurotrophin receptor p75NTR.110,127-133
Summary
In summary, as a hub of neuro-immune communication, the spleen is well suited to investigate the effects of stress and subsequent release of neurotransmitters and neuropeptides on the modulation of the immune responses e.g., in chronic inflammatory disease and associated aging processes such as inflammaging. The potential for neuro-immune regulation through this interaction is illustrated by the high number of neuroendocrine responsive lymphocytes that daily circulate through the densely innervated and neuroendocrine active spleen. An immune-modulating effect of stressors that activate the neuroendocrine stress response in the spleen is feasible in both directions: from nerve fibers to cells of the immune system passing through the spleen and from cells of the immune system as well as the circulation to nerve fibers innervating the immune competent areas (Fig. 2). Stress effects in diseases such as atopic/allergic dermatitis, which deteriorate after induction of neurogenic inflammation and TH2 driven inflammation, seem to depend upon the communication between neurogenic and immunogenic components not only in the skin but also in the lymphatics. Mostly however, this has been investigated either on the level of the central stress responses along the HPA and SA or on the level of local neuroendocrine-immune interaction in the affected peripheral tissue and their draining lymphatics. Little attention has been paid to potential guardian role of the spleen.
With this review we hope to have drawn attention to the spleen as a potential switch board between the central and the peripheral stress as well as the immune response with a focus on the NNA. In summary, release of neuronal and/or non-neuronal NNA mediators in the spleen may have an acute pro-inflammatory effect through activation of innate immune responses while on the long run it may promote regulation of inflammation and thereby might conduct inflammaging in peripheral tissues. It therefore complements the neuroendocrine effects of the SA and HPA. By following this idea, we hope to provide insight into potential pathogenic mechanisms of stress-aggravated chronic inflammatory disease in context with inflammaging and to further promote research to develop neuro-immune-modulatory therapeutic strategies in the management of allergic skin diseases and inflammaging.23,95,120,121,134,135
Glossary
Abbreviations:
- HPA
hypothalamic-pituitary-adrenocortical axis
- SA
sympathetic axis
- NNA
neurotrophin neuropeptide stress axis
- NA
noradrenaline
- CRH
corticotrophin releasing hormone
- ACTH
adrenocorticotropic hormone
- SP
Substance P
- NK1
neurokinin-1 SP receptor
- CGRP
calcitonine-gene related peptide
- VIP
vasointestinal peptide
- TH
T-helper cell
- IL
interleukin
- TNF
tumor necrosis factor
- IFN
interferon
- MZ
marginal zone
- PALS
peri-arteriolar lymphoid sheaths
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/22023
References
- 1.Felten DL, Felten SY, Bellinger DL, Madden KS. Fundamental aspects of neural-immune signaling. Psychother Psychosom. 1993;60:46–56. doi: 10.1159/000288679. [DOI] [PubMed] [Google Scholar]
- 2.Baluk P. Neurogenic inflammation in skin and airways. J Investig Dermatol Symp Proc. 1997;2:76–81. doi: 10.1038/jidsymp.1997.15. [DOI] [PubMed] [Google Scholar]
- 3.Steinhoff M, Ständer S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–88. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- 4.Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol. 2003;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 5.Straub RH. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol Sci. 2004;25:640–6. doi: 10.1016/j.tips.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Besedovsky HO, Rey AD. Physiology of psychoneuroimmunology: a personal view. Brain Behav Immun. 2007;21:34–44. doi: 10.1016/j.bbi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Hendrix S, Picker B, Liezmann C, Peters EM. Skin and hair follicle innervation in experimental models: a guide for the exact and reproducible evaluation of neuronal plasticity. Exp Dermatol. 2008;17:214–27. doi: 10.1111/j.1600-0625.2007.00653.x. [DOI] [PubMed] [Google Scholar]
- 8.Liezmann C, Klapp B, Peters EM. Stress, atopy and allergy: A re-evaluation from a psychoneuroimmunologic persepective. Dermatoendocrinol. 2011;3:37–40. doi: 10.4161/derm.3.1.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felten SY, Felten DL, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, et al. Noradrenergic sympathetic innervation of lymphoid organs. Prog Allergy. 1988;43:14–36. [PubMed] [Google Scholar]
- 10.Romano TA, Felten SY, Felten DL, Olschowka JA. Neuropeptide-Y innervation of the rat spleen: another potential immunomodulatory neuropeptide. Brain Behav Immun. 1991;5:116–31. doi: 10.1016/0889-1591(91)90011-X. [DOI] [PubMed] [Google Scholar]
- 11.Pabst R. The spleen in lymphocyte migration. Immunol Today. 1988;9:43–5. doi: 10.1016/0167-5699(88)91258-3. [DOI] [PubMed] [Google Scholar]
- 12.Rosas-Ballina M, Tracey KJ. The neurology of the immune system: neural reflexes regulate immunity. Neuron. 2009;64:28–32. doi: 10.1016/j.neuron.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engler H, Doenlen R, Riether C, Engler A, Besedovsky HO, Del Rey A, et al. Chemical destruction of brain noradrenergic neurons affects splenic cytokine production. J Neuroimmunol. 2010;219:75–80. doi: 10.1016/j.jneuroim.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Mikoluc B, Michalkiewicz J, Motkowski R, Smolka D, Pietrucha B, Piotrowska-Jastrzebska J, et al. Neutrophil phenotypic characteristics in children with congenital asplenia and splenectomized for hereditary spherocytosis. Immunol Invest. 2012;41:61–74. doi: 10.3109/08820139.2011.581730. [DOI] [PubMed] [Google Scholar]
- 15.Karimi K, Bienenstock J, Wang L, Forsythe P. The vagus nerve modulates CD4+ T cell activity. Brain Behav Immun. 2010;24:316–23. doi: 10.1016/j.bbi.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Ginaldi L, De Martinis M, Monti D, Franceschi C. The immune system in the elderly: activation-induced and damage-induced apoptosis. Immunol Res. 2004;30:81–94. doi: 10.1385/IR:30:1:081. [DOI] [PubMed] [Google Scholar]
- 17.Berger TG, Steinhoff M. Pruritus in elderly patients--eruptions of senescence. Semin Cutan Med Surg. 2011;30:113–7. doi: 10.1016/j.sder.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Li W, Liu Z, Zhang YY, Peng Y, Feng DG, et al. Ageing-associated changes in cellular immunity based on the SENIEUR protocol. Scand J Immunol. 2012;75:641–6. doi: 10.1111/j.1365-3083.2012.02698.x. [DOI] [PubMed] [Google Scholar]
- 19.Park HR, Jo SK. Lasting effects of an impairment of Th1-like immune response in γ-irradiated mice: A resemblance between irradiated mice and aged mice. Cell Immunol. 2011;267:1–8. doi: 10.1016/j.cellimm.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Ostan R, Bucci L, Capri M, Salvioli S, Scurti M, Pini E, et al. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation. 2008;15:224–40. doi: 10.1159/000156466. [DOI] [PubMed] [Google Scholar]
- 21.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–46. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franceschi C, Bonafè M. Centenarians as a model for healthy aging. Biochem Soc Trans. 2003;31:457–61. doi: 10.1042/BST0310457. [DOI] [PubMed] [Google Scholar]
- 24.Maas DW, Westendorp RG, van der Mast RC. Immune activation and depression in the elderly. Ned Tijdschr Geneeskd. 2008;152:1413–7. [PubMed] [Google Scholar]
- 25.Cannon WB. Bodily changes in pain, hunger, fear and rage: An Account of Recent Researches Into the Function of Emotional Excitement 1927. New York, 1929. [Google Scholar]
- 26.Van Tits LJ, Michel MC, Grosse-Wilde H, Happel M, Eigler FW, Soliman A, et al. Catecholamines increase lymphocyte beta 2-adrenergic receptors via a beta 2-adrenergic, spleen-dependent process. Am J Physiol. 1990;258:E191–202. doi: 10.1152/ajpendo.1990.258.1.E191. [DOI] [PubMed] [Google Scholar]
- 27.Rogausch H, del Rey A, Oertel J, Besedovsky HO. Norepinephrine stimulates lymphoid cell mobilization from the perfused rat spleen via beta-adrenergic receptors. Am J Physiol. 1999;276:R724–30. doi: 10.1152/ajpregu.1999.276.3.R724. [DOI] [PubMed] [Google Scholar]
- 28.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 29.Dragos D, Tanasescu MD. The effect of stress on the defense systems. Journal of medicine and life 2010; 3:10-8. [PMC free article] [PubMed]
- 30.Meltzer JC, MacNeil BJ, Sanders V, Pylypas S, Jansen AH, Greenberg AH, et al. Stress-induced suppression of in vivo splenic cytokine production in the rat by neural and hormonal mechanisms. Brain Behav Immun. 2004;18:262–73. doi: 10.1016/j.bbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007) Brain Behav Immun. 2007;21:736–45. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selye H. The Physiology and Pathology of Exposure to STRESS. Montreal: ACTA. INC. Medical Publishers, 1950. [Google Scholar]
- 33.Elenkov IJ, Chrousos GP. Stress system--organization, physiology and immunoregulation. Neuroimmunomodulation. 2006;13:257–67. doi: 10.1159/000104853. [DOI] [PubMed] [Google Scholar]
- 34.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–17. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pace TW, Heim CM. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Bellinger DL, Felten DL, Lorton D, Brouxhon S. Effects of interleukin-2 on the expression of corticotropin-releasing hormone in nerves and lymphoid cells in secondary lymphoid organs from the Fischer 344 rat. J Neuroimmunol. 2001;119:37–50. doi: 10.1016/S0165-5728(01)00362-9. [DOI] [PubMed] [Google Scholar]
- 37.Bellinger DL, Brouxhon SM, Lubahn C, Tran L, Kang JI, Felten DL, et al. Strain differences in the expression of corticotropin-releasing hormone immunoreactivity in nerves that supply the spleen and thymus. Neuroimmunomodulation. 2001;9:78–87. doi: 10.1159/000049010. [DOI] [PubMed] [Google Scholar]
- 38.Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697–704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tausk F, Elenkov I, Moynihan J. Psychoneuroimmunology. Dermatol Ther. 2008;21:22–31. doi: 10.1111/j.1529-8019.2008.00166.x. [DOI] [PubMed] [Google Scholar]
- 40.Castellani ML, Galzio RJ, Felaco P, Tripodi D, Toniato E, De Lutiis MA, et al. VEGF, substance P and stress, new aspects: a revisited study. J Biol Regul Homeost Agents. 2010;24:229–37. [PubMed] [Google Scholar]
- 41.Harvima IT, Nilsson G, Naukkarinen A. Role of mast cells and sensory nerves in skin inflammation. G Ital Dermatol Venereol. 2010;145:195–204. [PubMed] [Google Scholar]
- 42.Hendrix S, Peters EM. Neuronal plasticity and neuroregeneration in the skin -- the role of inflammation. J Neuroimmunol. 2007;184:113–26. doi: 10.1016/j.jneuroim.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–53. doi: 10.1016/S0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 44.Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: A link to neurogenic skin disorders. Brain Behav Immun. 1999;13:225–39. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- 45.Arck P, Handjiski B, Hagen E, Pincus M, Bruenahl C, Bienenstock J, et al. Is there a ‘gut-brain-skin axis’? Exp Dermatol. 2010;19:401–5. doi: 10.1111/j.1600-0625.2009.01060.x. [DOI] [PubMed] [Google Scholar]
- 46.Santoni G, Perfumi MC, Spreghini E, Romagnoli S, Piccoli M. Neurokinin type-1 receptor antagonist inhibits enhancement of T cell functions by substance P in normal and neuromanipulated capsaicin-treated rats. J Neuroimmunol. 1999;93:15–25. doi: 10.1016/S0165-5728(98)00173-8. [DOI] [PubMed] [Google Scholar]
- 47.Pavlovic S, Liezmann C, Blois SM, Joachim R, Kruse J, Romani N, et al. Substance P is a key mediator of stress-induced protection from allergic sensitization via modified antigen presentation. J Immunol. 2011;186:848–55. doi: 10.4049/jimmunol.0903878. [DOI] [PubMed] [Google Scholar]
- 48.Mihara K, Kuratani K, Matsui T, Nakamura M, Yokota K. Vital role of the itch-scratch response in development of spontaneous dermatitis in NC/Nga mice. Br J Dermatol. 2004;151:335–45. doi: 10.1111/j.1365-2133.2004.06036.x. [DOI] [PubMed] [Google Scholar]
- 49.Herberth G, Daegelmann C, Weber A, Röder S, Giese T, Krämer U, et al. LISAplus Study Group Association of neuropeptides with Th1/Th2 balance and allergic sensitization in children. Clin Exp Allergy. 2006;36:1408–16. doi: 10.1111/j.1365-2222.2006.02576.x. [DOI] [PubMed] [Google Scholar]
- 50.Mathers AR, Tckacheva OA, Janelsins BM, Shufesky WJ, Morelli AE, Larregina AT. In vivo signaling through the neurokinin 1 receptor favors transgene expression by Langerhans cells and promotes the generation of Th1- and Tc1-biased immune responses. J Immunol. 2007;178:7006–17. doi: 10.4049/jimmunol.178.11.7006. [DOI] [PubMed] [Google Scholar]
- 51.Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52:40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 52.Rogausch H, Zwingmann D, Trudewind M, del Rey A, Voigt KH, Besedovsky H. Local and systemic autonomic nervous effects on cell migration to the spleen. J Appl Physiol. 2003;94:469–75. doi: 10.1152/japplphysiol.00411.2002. [DOI] [PubMed] [Google Scholar]
- 53.Felten DL. Direct innervation of lymphoid organs: substrate for neurotransmitter signaling of cells of the immune system. Neuropsychobiology. 1993;28:110–2. doi: 10.1159/000119011. [DOI] [PubMed] [Google Scholar]
- 54.Madden KS, Thyagarajan S, Felten DL. Alterations in sympathetic noradrenergic innervation in lymphoid organs with age. Ann N Y Acad Sci. 1998;840:262–8. doi: 10.1111/j.1749-6632.1998.tb09566.x. [DOI] [PubMed] [Google Scholar]
- 55.Felten DL, Ackerman KD, Wiegand SJ, Felten SY. Noradrenergic sympathetic innervation of the spleen: I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res. 1987;18:28–36, 118-21. doi: 10.1002/jnr.490180107. [DOI] [PubMed] [Google Scholar]
- 56.Bellinger DL, Felten SY, Collier TJ, Felten DL. Noradrenergic sympathetic innervation of the spleen: IV. Morphometric analysis in adult and aged F344 rats. J Neurosci Res. 1987;18:55–63, 126-9. doi: 10.1002/jnr.490180110. [DOI] [PubMed] [Google Scholar]
- 57.Lorton D, Bellinger DL, Felten SY, Felten DL. Substance P innervation of spleen in rats: nerve fibers associate with lymphocytes and macrophages in specific compartments of the spleen. Brain Behav Immun. 1991;5:29–40. doi: 10.1016/0889-1591(91)90005-U. [DOI] [PubMed] [Google Scholar]
- 58.Bellinger DL, Lorton D, Romano TD, Olschowka JA, Felten SY, Felten DL. Neuropeptide innervation of lymphoid organs. Ann N Y Acad Sci. 1990;594:17–33. doi: 10.1111/j.1749-6632.1990.tb40464.x. [DOI] [PubMed] [Google Scholar]
- 59.Stevens-Felten SY, Bellinger DL. Noradrenergic and peptidergic innervation of lymphoid organs. Chem Immunol. 1997;69:99–131. doi: 10.1159/000058655. [DOI] [PubMed] [Google Scholar]
- 60.Baig JA, Iqbal MP, Rehman R, Qureshi AA, Ahmed M. Anti-inflammatory role of methotrexate in adjuvant arthritis: effect on substance p and calcitonin gene-related Peptide in thymus and spleen. J Coll Physicians Surg Pak. 2007;17:490–4. [PubMed] [Google Scholar]
- 61.Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- 62.Laukova M, Vargovic P, Csaderova L, Chovanova L, Vlcek M, Imrich R, et al. Acute stress differently modulates β1, β2 and β3 adrenoceptors in T cells, but not in B cells, from the rat spleen. Neuroimmunomodulation. 2012;19:69–78. doi: 10.1159/000329002. [DOI] [PubMed] [Google Scholar]
- 63.Bergmann M, Sautner T. Immunomodulatory effects of vasoactive catecholamines. Wien Klin Wochenschr. 2002;114:752–61. [PubMed] [Google Scholar]
- 64.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–69. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 65.Fuchs BA, Campbell KS, Munson AE. Norepinephrine and serotonin content of the murine spleen: its relationship to lymphocyte beta-adrenergic receptor density and the humoral immune response in vivo and in vitro. Cell Immunol. 1988;117:339–51. doi: 10.1016/0008-8749(88)90123-2. [DOI] [PubMed] [Google Scholar]
- 66.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–48. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 67.Montoro J, Mullol J, Jáuregui I, Dávila I, Ferrer M, Bartra J, et al. Stress and allergy. J Investig Allergol Clin Immunol. 2009;19(Suppl 1):40–7. [PubMed] [Google Scholar]
- 68.Carucci JA, Auci DL, Herrick CA, Durkin HG. Neuropeptide-mediated regulation of hapten-specific IgE responses in mice. I. Substance P-mediated isotype-specific suppression of BPO-specific IgE antibody-forming cell responses induced in vivo and in vitro. J Leukoc Biol. 1995;57:110–5. doi: 10.1002/jlb.57.1.110. [DOI] [PubMed] [Google Scholar]
- 69.Kang HS, Trzaska KA, Corcoran K, Chang VT, Rameshwar P. Neurokinin receptors: relevance to the emerging immune system. Arch Immunol Ther Exp (Warsz) 2004;52:338–47. [PubMed] [Google Scholar]
- 70.Arsenescu R, Blum AM, Metwali A, Elliott DE, Weinstock JV. IL-12 induction of mRNA encoding substance P in murine macrophages from the spleen and sites of inflammation. J Immunol. 2005;174:3906–11. doi: 10.4049/jimmunol.174.7.3906. [DOI] [PubMed] [Google Scholar]
- 71.Grassin-Delyle S, Buenestado A, Vallat L, Naline E, Marx S, Decocq J, et al. Expression and proliferative effect of hemokinin-1 in human B-cells. Peptides. 2011;32:1027–34. doi: 10.1016/j.peptides.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 72.Dickerson C, Undem B, Bullock B, Winchurch RA. Neuropeptide regulation of proinflammatory cytokine responses. J Leukoc Biol. 1998;63:602–5. doi: 10.1002/jlb.63.5.602. [DOI] [PubMed] [Google Scholar]
- 73.Blum AM, Metwali A, Elliott DE, Weinstock JV. T cell substance P receptor governs antigen-elicited IFN-gamma production. Am J Physiol Gastrointest Liver Physiol. 2003;284:G197–204. doi: 10.1152/ajpgi.00271.2002. [DOI] [PubMed] [Google Scholar]
- 74.Kang BN, Kim HJ, Jeong KS, Park SJ, Kim SH, Kim SR, et al. Regulation of leukocyte function-associated antigen 1-mediated adhesion by somatostatin and substance P in mouse spleen cells. Neuroimmunomodulation. 2004;11:84–92. doi: 10.1159/000075317. [DOI] [PubMed] [Google Scholar]
- 75.Benou C, Wang Y, Imitola J, VanVlerken L, Chandras C, Karalis KP, et al. Corticotropin-releasing hormone contributes to the peripheral inflammatory response in experimental autoimmune encephalomyelitis. J Immunol. 2005;174:5407–13. doi: 10.4049/jimmunol.174.9.5407. [DOI] [PubMed] [Google Scholar]
- 76.Blum A, Setiawan T, Hang L, Stoyanoff K, Weinstock JV. Interleukin-12 (IL-12) and IL-23 induction of substance p synthesis in murine T cells and macrophages is subject to IL-10 and transforming growth factor beta regulation. Infect Immun. 2008;76:3651–6. doi: 10.1128/IAI.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nilsson G, Alving K, Ahlstedt S, Hökfelt T, Lundberg JM. Peptidergic innervation of rat lymphoid tissue and lung: relation to mast cells and sensitivity to capsaicin and immunization. Cell Tissue Res. 1990;262:125–33. doi: 10.1007/BF00327753. [DOI] [PubMed] [Google Scholar]
- 78.Sadakata T, Sekine Y, Oka M, Itakura M, Takahashi M, Furuichi T. Calcium-dependent activator protein for secretion 2 interacts with the class II ARF small GTPases and regulates dense-core vesicle trafficking. FEBS J. 2012;279:384–94. doi: 10.1111/j.1742-4658.2011.08431.x. [DOI] [PubMed] [Google Scholar]
- 79.Zhukova EM, Vorob’eva NF. [Morphofunctional changes in the rat spleen after capsaicin blockade of peripheral afferent neurons] Morfologiia. 1998;114:44–6. [PubMed] [Google Scholar]
- 80.Santoni G, Perfumi M, Bressan AM, Piccoli M. Capsaicin-induced inhibition of mitogen and interleukin-2-stimulated T cell proliferation: its reversal by in vivo substance P administration. J Neuroimmunol. 1996;68:131–8. doi: 10.1016/0165-5728(96)00081-1. [DOI] [PubMed] [Google Scholar]
- 81.Santoni G, Perfumi M, Birarelli P, Procaccini A, Piccoli M. In vivo capsaicin treatment inhibits rat NK cell cytotoxic functions. Immunopharmacol Immunotoxicol. 1995;17:511–28. doi: 10.3109/08923979509016384. [DOI] [PubMed] [Google Scholar]
- 82.Yamaoka J, Kawana S. Rapid changes in substance P signaling and neutral endopeptidase induced by skin-scratching stimulation in mice. J Dermatol Sci. 2007;48:123–32. doi: 10.1016/j.jdermsci.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Toyoda M, Makino T, Kagoura M, Morohashi M. Expression of neuropeptide-degrading enzymes in alopecia areata: an immunohistochemical study. Br J Dermatol. 2001;144:46–54. doi: 10.1046/j.1365-2133.2001.03951.x. [DOI] [PubMed] [Google Scholar]
- 84.Scholzen TE, Steinhoff M, Bonaccorsi P, Klein R, Amadesi S, Geppetti P, et al. Neutral endopeptidase terminates substance P-induced inflammation in allergic contact dermatitis. J Immunol. 2001;166:1285–91. doi: 10.4049/jimmunol.166.2.1285. [DOI] [PubMed] [Google Scholar]
- 85.Matsas R, Rattray M, Kenny AJ, Turner AJ. The metabolism of neuropeptides. Endopeptidase-24.11 in human synaptic membrane preparations hydrolyses substance P. Biochem J. 1985;228:487–92. doi: 10.1042/bj2280487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bowes MA, Kenny AJ. An immunohistochemical study of endopeptidase-24.11 and aminopeptidase N in lymphoid tissues. Immunology. 1987;60:247–53. [PMC free article] [PubMed] [Google Scholar]
- 87.Salles G, Rodewald HR, Chin BS, Reinherz EL, Shipp MA. Inhibition of CD10/neutral endopeptidase 24.11 promotes B-cell reconstitution and maturation in vivo. Proc Natl Acad Sci U S A. 1993;90:7618–22. doi: 10.1073/pnas.90.16.7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jessop DS, Renshaw D, Larsen PJ, Chowdrey HS, Harbuz MS. Substance P is involved in terminating the hypothalamo- pituitary-adrenal axis response to acute stress through centrally located neurokinin-1 receptors. Stress. 2000;3:209–20. doi: 10.3109/10253890009001125. [DOI] [PubMed] [Google Scholar]
- 89.Okamoto S, Irie Y, Ishikawa I, Kimura K, Masayuki Saito Central leptin suppresses splenic lymphocyte functions through activation of the corticotropin-releasing hormone-sympathetic nervous system. Brain Res. 2000;855:192–7. doi: 10.1016/S0006-8993(99)02409-9. [DOI] [PubMed] [Google Scholar]
- 90.del Rey A, Randolf A, Wildmann J, Besedovsky HO, Jessop DS. Re-exposure to endotoxin induces differential cytokine gene expression in the rat hypothalamus and spleen. Brain Behav Immun. 2009;23:776–83. doi: 10.1016/j.bbi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solomon GF. Psychoneuroimmunology: interactions between central nervous system and immune system. J Neurosci Res. 1987;18:1–9. doi: 10.1002/jnr.490180103. [DOI] [PubMed] [Google Scholar]
- 92.Ader R, Cohen N, Felten D. Psychoneuroimmunology: interactions between the nervous system and the immune system. Lancet. 1995;345:99–103. doi: 10.1016/S0140-6736(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 93.Niemi MB, Pacheco-López G, Kou W, Härting M, del Rey A, Besedovsky HO, et al. Murine taste-immune associative learning. Brain Behav Immun. 2006;20:527–31. doi: 10.1016/j.bbi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 94.Elsenbruch S, Kotsis V, Benson S, Rosenberger C, Reidick D, Schedlowski M, et al. Neural mechanisms mediating the effects of expectation in visceral placebo analgesia: an fMRI study in healthy placebo responders and nonresponders. Pain. 2012;153:382–90. doi: 10.1016/j.pain.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 95.Pavlovic S, Daniltchenko M, Tobin DJ, Hagen E, Hunt SP, Klapp BF, et al. Further exploring the brain-skin connection: stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J Invest Dermatol. 2008;128:434–46. doi: 10.1038/sj.jid.5701079. [DOI] [PubMed] [Google Scholar]
- 96.Joachim RA, Kuhlmei A, Dinh QT, Handjiski B, Fischer T, Peters EM, et al. Neuronal plasticity of the “brain-skin connection”: stress-triggered up-regulation of neuropeptides in dorsal root ganglia and skin via nerve growth factor-dependent pathways. J Mol Med (Berl) 2007;85:1369–78. doi: 10.1007/s00109-007-0236-8. [DOI] [PubMed] [Google Scholar]
- 97.Sloan EK, Capitanio JP, Cole SW. Stress-induced remodeling of lymphoid innervation. Brain Behav Immun. 2008;22:15–21. doi: 10.1016/j.bbi.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kruszewska B, Felten SY, Moynihan JA. Alterations in cytokine and antibody production following chemical sympathectomy in two strains of mice. J Immunol. 1995;155:4613–20. [PubMed] [Google Scholar]
- 99.ThyagaRajan S, Madden KS, Teruya B, Stevens SY, Felten DL, Bellinger DL. Age-associated alterations in sympathetic noradrenergic innervation of primary and secondary lymphoid organs in female Fischer 344 rats. J Neuroimmunol. 2011;233:54–64. doi: 10.1016/j.jneuroim.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Straub RH, Grum F, Strauch U, Capellino S, Bataille F, Bleich A, et al. Anti-inflammatory role of sympathetic nerves in chronic intestinal inflammation. Gut. 2008;57:911–21. doi: 10.1136/gut.2007.125401. [DOI] [PubMed] [Google Scholar]
- 101.Lorton D, Lubahn C, Sweeney S, Major A, Lindquist CA, Schaller J, et al. Differences in the injury/sprouting response of splenic noradrenergic nerves in Lewis rats with adjuvant-induced arthritis compared with rats treated with 6-hydroxydopamine. Brain Behav Immun. 2009;23:276–85. doi: 10.1016/j.bbi.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 102.Del Rey A, Wolff C, Wildmann J, Randolf A, Straub RH, Besedovsky HO. When immune-neuro-endocrine interactions are disrupted: experimentally induced arthritis as an example. Neuroimmunomodulation. 2010;17:165–8. doi: 10.1159/000258714. [DOI] [PubMed] [Google Scholar]
- 103.Xue N, Liang H, Yao H, Song XM, Li JG. The role of spleen in vagus nerve stimulation for treatment against septic shock in rats. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2011;23:263–6. [PubMed] [Google Scholar]
- 104.Vida G, Peña G, Deitch EA, Ulloa L. α7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J Immunol. 2011;186:4340–6. doi: 10.4049/jimmunol.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gushchin GV, Shkhinek EK. [Participation of cholinergic mechanisms in the regulation of immunological processes] Farmakol Toksikol. 1979;42:635–9. [PubMed] [Google Scholar]
- 106.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science (New York, NY 2011; 334:98-101. [DOI] [PMC free article] [PubMed]
- 107.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 108.Bieber T. Atopic dermatitis. Annals of dermatology 2010; 22:125-37. [DOI] [PMC free article] [PubMed]
- 109.Buske-Kirschbaum A. Cortisol responses to stress in allergic children: interaction with the immune response. Neuroimmunomodulation. 2009;16:325–32. doi: 10.1159/000216190. [DOI] [PubMed] [Google Scholar]
- 110.Peters EM, Liezmann C, Spatz K, Daniltchenko M, Joachim R, Gimenez-Rivera A, et al. Nerve growth factor partially recovers inflamed skin from stress-induced worsening in allergic inflammation. J Invest Dermatol. 2011;131:735–43. doi: 10.1038/jid.2010.317. [DOI] [PubMed] [Google Scholar]
- 111.Novak N, Bieber T. Pathophysiologie der atopischen Dermatitis. Dtsch Arztebl. 2004;101:94–102. [Google Scholar]
- 112.Worm M. Dtsch Med Wochenschr. 1946;2008(Suppl 3):S63–6. doi: 10.1055/s-2008-1067322. Allergic skin reactions. Current survey. [DOI] [PubMed] [Google Scholar]
- 113.Herz U, Bunikowski R, Renz H. Role of T cells in atopic dermatitis. New aspects on the dynamics of cytokine production and the contribution of bacterial superantigens. Int Arch Allergy Immunol. 1998;115:179–90. doi: 10.1159/000023899. [DOI] [PubMed] [Google Scholar]
- 114.Yusuf-Makagiansar H, Anderson ME, Yakovleva TV, Murray JS, Siahaan TJ. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med Res Rev. 2002;22:146–67. doi: 10.1002/med.10001. [DOI] [PubMed] [Google Scholar]
- 115.Novak N, Bieber T, Kraft S. Immunoglobulin E-bearing antigen-presenting cells in atopic dermatitis. Curr Allergy Asthma Rep. 2004;4:263–9. doi: 10.1007/s11882-004-0069-2. [DOI] [PubMed] [Google Scholar]
- 116.Raap U, Kapp A. Neuroimmunological findings in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2005;5:419–24. doi: 10.1097/01.all.0000183111.78558.4d. [DOI] [PubMed] [Google Scholar]
- 117.Alvarez-Rodríguez L, López-Hoyos M, Muñoz-Cacho P, Martínez-Taboada VM. Aging is associated with circulating cytokine dysregulation. Cell Immunol. 2012;273:124–32. doi: 10.1016/j.cellimm.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 118.Joachim RA, Quarcoo D, Arck PC, Herz U, Renz H, Klapp BF. Stress enhances airway reactivity and airway inflammation in an animal model of allergic bronchial asthma. Psychosom Med. 2003;65:811–5. doi: 10.1097/01.PSY.0000088582.50468.A3. [DOI] [PubMed] [Google Scholar]
- 119.Buske-Kirschbaum A, Jobst S, Hellhammer DH. Altered reactivity of the hypothalamus-pituitary-adrenal axis in patients with atopic dermatitis: pathologic factor or symptom? Ann N Y Acad Sci. 1998;840:747–54. doi: 10.1111/j.1749-6632.1998.tb09613.x. [DOI] [PubMed] [Google Scholar]
- 120.Buske-Kirschbaum A, Geiben A, Höllig H, Morschhäuser E, Hellhammer D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. J Clin Endocrinol Metab. 2002;87:4245–51. doi: 10.1210/jc.2001-010872. [DOI] [PubMed] [Google Scholar]
- 121.Buske-Kirschbaum A, Geiben A, Hellhammer D. Psychobiological aspects of atopic dermatitis: an overview. Psychother Psychosom. 2001;70:6–16. doi: 10.1159/000056219. [DOI] [PubMed] [Google Scholar]
- 122.Buske-Kirschbaum A, Gierens A, Höllig H, Hellhammer DH. Stress-induced immunomodulation is altered in patients with atopic dermatitis. J Neuroimmunol. 2002;129:161–7. doi: 10.1016/S0165-5728(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 123.Webster EL, Torpy DJ, Elenkov IJ, Chrousos GP. Corticotropin-releasing hormone and inflammation. Ann N Y Acad Sci. 1998;840:21–32. doi: 10.1111/j.1749-6632.1998.tb09545.x. [DOI] [PubMed] [Google Scholar]
- 124.Giunta S. Exploring the complex relations between inflammation and aging (inflamm-aging): anti-inflamm-aging remodelling of inflamm- aging, from robustness to frailty. Inflamm Res. 2008;57:558–63. doi: 10.1007/s00011-008-7243-2. [DOI] [PubMed] [Google Scholar]
- 125.Shah BR, Cowper PA, O’Brien SM, Jensen N, Patel MR, Douglas PS, et al. Association between physician billing and cardiac stress testing patterns following coronary revascularization. JAMA. 2011;306:1993–2000. doi: 10.1001/jama.2011.1604. [DOI] [PubMed] [Google Scholar]
- 126.Carlson SL, Albers KM, Beiting DJ, Parish M, Conner JM, Davis BM. NGF modulates sympathetic innervation of lymphoid tissues. J Neurosci. 1995;15:5892–9. doi: 10.1523/JNEUROSCI.15-09-05892.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann N Y Acad Sci. 2000;917:876–93. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- 128.Kimata H. Suckling reduces allergic skin responses and plasma levels of neuropeptide and neurotrophin in lactating women with atopic eczema/dermatitis syndrome. Int Arch Allergy Immunol. 2003;132:380–3. doi: 10.1159/000074906. [DOI] [PubMed] [Google Scholar]
- 129.Kimata H. Enhancement of allergic skin wheal responses and in vitro allergen-specific IgE production by computer-induced stress in patients with atopic dermatitis. Brain Behav Immun. 2003;17:134–8. doi: 10.1016/S0889-1591(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 130.Kimata H. Kissing reduces allergic skin wheal responses and plasma neurotrophin levels. Physiol Behav. 2003;80:395–8. doi: 10.1016/j.physbeh.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 131.Botchkarev VA, Yaar M, Peters EM, Raychaudhuri SP, Botchkareva NV, Marconi A, et al. Neurotrophins in skin biology and pathology. J Invest Dermatol. 2006;126:1719–27. doi: 10.1038/sj.jid.5700270. [DOI] [PubMed] [Google Scholar]
- 132.Nassenstein C, Schulte-Herbrüggen O, Renz H, Braun A. Nerve growth factor: the central hub in the development of allergic asthma? Eur J Pharmacol. 2006;533:195–206. doi: 10.1016/j.ejphar.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 133.Peters EM, Raap U, Welker P, Tanaka A, Matsuda H, Pavlovic-Masnicosa S, et al. Neurotrophins act as neuroendocrine regulators of skin homeostasis in health and disease. Hormone and metabolic research Hormon- und Stoffwechselforschung 2007; 39:110-24. [DOI] [PubMed]
- 134.Peters EM, Kuhlmei A, Tobin DJ, Müller-Röver S, Klapp BF, Arck PC. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav Immun. 2005;19:252–62. doi: 10.1016/j.bbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 135.Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65:S173–6. doi: 10.1301/nr.2007.dec.S173-S176. [DOI] [PubMed] [Google Scholar]