Abstract
Skin aging is a complex process and underlies multiple influences with the probable involvement of heritable and various environmental factors. Several theories have been conducted regarding the pathomechanisms of aged skin, however fundamental mechanisms still remain poorly understood. This article addresses the influence of genetics on skin aging and in particular deals with the differences observed in ethnic populations and between both genders. Recent studies indicate that male and female aged skin differs as far as the type, the consistency and the sensitivity to external factors is concerned. The same has been also documented between elderly people of different origin. Consequently, the aging process taking place in both genders and in diverse ethnic groups should be examined separately and products specialized to each population should be developed in order to satisfy the special needs.
Keywords: skin, aging, genetics, gender, biomarkers, ethnic differences
Introduction
As our society is growing older, the consequences of aging have begun to gain particular attention. Improvement of quality of life at old age and prevention of age-associated diseases have become the main focus of the aging research. In particular, skin aging has gained increasing interest, not only because it is the most obvious sign of the aging process but mostly because it represents a window for human health, being likely to predict systemic diseases and their outcome, such as chronic metabolic diseases including obesity,1 osteoporosis,2 the outcome of cardiac surgery3 or the occurrence of neurodegenerative diseases.4
Skin has long been recognized to protect the organisms from deleterious environmental (physical, chemical, microbiological) agents, and is crucial for the maintenance of temperature, electrolyte and fluid balance. In addition, skin is a sensory organ, a biofactory for the synthesis, processing and/or metabolism of a wide range of structural proteins, glycans, lipids and it fulfils the requirements of a classic endocrine organ.5 With accelerating age skin loses its structural and morphological characteristics and as a consequence all its functions deteriorate.6 This deterioration is enhanced cumulatively by various environmental physical, chemical and mechanical insults. Exposed areas of the body e.g., face and neck suffer the most by the influence of extrinsic factors such as UV irradiation and overexposure of these regions may result in premature skin aging and skin diseases such as non-healing ulcerations and benign (e.g., actinic keratosis) and malignant skin tumors (e.g., basal cell carcinoma, spinal cell carcinoma and malignant melanoma). These processes are augmented by mitochondrial DNA mutations, protein oxidation, disturbed defense against protein macromolecular damage and apoptosis induction. On the other hand, in non-exposed areas e.g., inner side of the upper arm aging is mainly attributed to intrinsic factors, e.g., genetic predisposition and changes in the endocrine environment and reflects degradation processes of the entire organism.6,7 Hormone replacement therapy and the use of antioxidants have been suggested to be a promising strategy to retard the skin aging process.
Genes and Mutations
In current years, microarray technology has become a valuable tool for screening genetic material of several model organisms in order to map genes and pathways which are involved in the mechanisms of aging and extension of lifespan.8,9 This information has also led to the development of new strategies to enable better skin repair and anti-aging benefits.10
Gene expression patterns were examined in sun-protected (buttocks) and sun-exposed skin (extensor forearm) from 10 young (age 19 to 20 y) and 10 aged women (age 63 to 67 y) in order to examine gene expression profiles associated with chronological skin aging and photoageing. Both chronologic and photoageing were associated with downregulation of the biological process of lipid synthesis. In particular, genes were downregulated involved in the cholesterol and fatty acid synthesis. In addition, genes associated with epidermal differentiation, including keratin filaments and cornified envelop components were downregulated. An upregulation of the biological processes of inflammatory response and wound healing, the molecular functions of cytokine activity and protease activity and the cellular component theme of extracellular matrix was also observed in both skin aging types. The elastin gene expression was upregulated with aging only in the photodamaged arm and remained unchanged in the sun-protected buttock. This finding corresponds to the histopathological findings which show typical elastotic changes the so called ‘solar elastosis’ in photoaged skin.11
Further studies conducted to investigate changes in gene expression during skin aging have been performed on naturally aged human foreskin obtained from children and elderly males. Some of the mechanisms proposed to be involved in the induction of aging comprise disturbed lipid metabolism, altered insulin and STAT3 signaling, upregulation of apoptotic genes partly due to the deregulation of FOXO1, downregulation of members of the jun and fos family, differential expression of cytoskeletal proteins (e.g., keratin 2A, 6A, and 16A), extracellular matrix components (e.g., PI3, S100A2, A7, A9, SPRR2B), and proteins involved in cell-cycle control (e.g., CDKs, GOS2).9 Similar results have been presented by a study related to aging of skeletal muscle.12
In a previous study, we proposed that one of the factors which may play a significant role in the initiation of aging may be the physiological decline of hormones occurring with age. Human SZ95 sebocytes in vitro treated with hormone levels that can be found in 60-year-old women produce less lipids than sebocytes treated with a hormone mixture representing that found in serum of 20-year-old women.13 A differential gene expression between SZ95 sebocytes under the 20- and 60-year-old hormone mixture detected differentially expressed genes, which are involved in biological processes such as DNA repair and stability, mitochondrial function, oxidative stress, cell cycle and apoptosis, ubiquitin-induced proteolysis and transcriptional regulation corresponding to the results by Lener et al.9 and Robinson et al.11 A comparison of these results with data obtained from the aged kidney14 identified key genes which may be of great importance for global aging. The most significantly altered signaling pathway was that of TGFβ. A disturbed function of this cascade has been also associated with tumorigenesis, i.e., in pancreatic, prostate, intestine, breast, and uterine cancer. Interestingly, genes expressed in signaling pathways operative in age-associated diseases such as Huntington’s disease, dentatorubral-pallidoluysian atrophy, and amyotrophic lateral sclerosis were also identified. These data demonstrate that hormones interact in a complex fashion, and sebocytes may be affected to a large extent by the changes in their circulating blood levels with age.13
In recent studies researchers have been focusing on gene mutations accompanying known progeroid syndromes e.g., Hutchinson-Gilford progeria, Werner’s syndrome (WS), Rothmund-Thomson syndrome, Cockayne syndrome, Ataxia teleangiectasia and Down syndrome.15 The most common skin disorders of these syndromes which are characterized by an acceleration of the aging phenotype are alopecia, skin atrophy and sclerosis, teleangiectasia, poikiloderma, thinning and graying of hair and several malignancies. Most of these syndromes are inherited in an autosomal recessive way and mostly display defects in DNA replication, recombination, repair, and transcription. Expression gene patterns of skin cells derived from Werner patients,16 old and young donors show that 91% of the analyzed genes have similar expression changes in WS and in normal aging implying transcription alterations common to WS and normal aging represent general events in the aging process.
An assessment of mRNA levels in fibroblasts isolated from young, middle-aged, and elderly patients with progeria has sown that chromosomal pathologies may lead to misregulation of key structural, signaling, and metabolic genes associated with the aging phenotype.17 In addition, in humans evidence is presented that isolated growth-hormone (GH) deficiency (IGHD), multiple pituitary hormone deficiency (MPHD) including GH, as well as primary insulin-like growth factor I (IGF-I) deficiency (GH resistance, Laron syndrome) present signs of early skin aging such as dry, thin and wrinkled skin, obesity, hyperglucaemia, reduced body lean mass, osteopenia, lowered venous access, rise in serum cholesterol, tendency for cardiovascular diseases and subsequent premature mortality.18-20 These studies illustrate the importance of the hormone environment for deterioration of the human organism and the aging process.
Gender-Specific Human Skin Aging
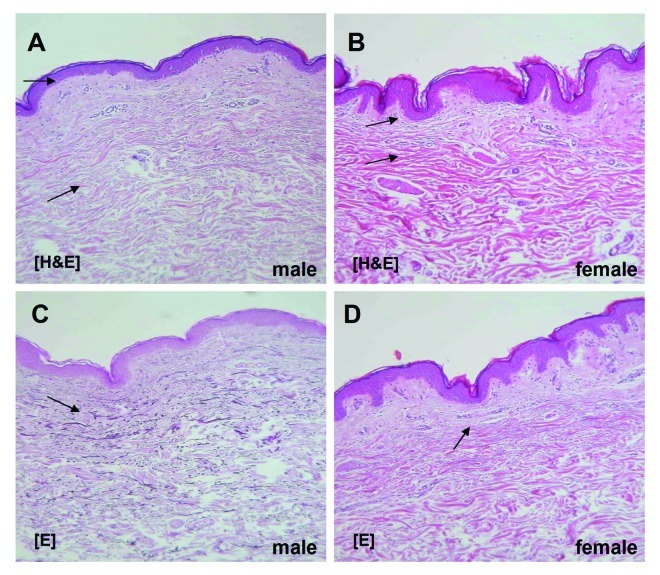
Androgens may play a substantial role in skin morphology. This fact has been described in several animal and human studies, which have documented gender-specific characteristics of the skin structure. A comparison between male and female mouse dorsal skin revealed that the dermis in the male is much thicker than in the female (+190%, p < 0.01). In contrast, epidermis and subcutaneous tissue are thicker in the female (+40%, p < 0.01 and 11-fold, p < 0.01, accordingly), thus resulting in total skin that is 40% thicker in the male. In addition, significant larger sebaceous glands were observed in males (+45%, p < 0.01).21 In females, gonadectomy resulted in a decrease of epidermal thickness (-40%, p < 0.01), an increase of the dermal thickness (+22%, p < 0.05) and of the thickness of the hair shaft. Our own data32 correspond to previous studies22 showing that in humans, male skin is thicker than female skin, while females have thicker subcutaneous tissue and provide evidence that androgens and their decline with age may play a significant role in the regulation of the dermis and the hair shaft thickness. A comparison between male and female sun-protected skin derived from the inner side of the upper arm revealed that the male dermis is much thicker than the female one (1.8-fold, p < 0.05). In contrast, epidermis and subcutaneous tissue is thicker in the female (3.5-fold, p < 0.05 and 10-fold, respectively) (Fig. 1).
Figure 1. A comparison between male (A and C) and female (B and D) endogenous aged skin. Staining via hematoxylin eosin (A and B) and elastica staining (C and D), respectively, revealed that the dermis in the male is significantly thicker than in the female. In contrast, epidermis and subcutaneous tissue are significantly thicker in the female.
In our own further experiments32 whole genome gene profiling was employed in sun-protected skin obtained from 24 European Caucasian young and elderly donors. In total, only 39 genes were common in the target lists of significant regulated genes in males and females. Furthermore, analysis of regulations in biological processes, cellular compartments, molecular functions and pathways showed that these were only in some cases overlapping or belonging to a similar cluster. These results provide biomarkers of endogenous human skin aging in both genders and indicate that the process of male aging may significantly differ from female aging.
This observation has been also verified by others. Increased lipid oxidation with age has been described in non-exposed pelvic skin only in males.23 This finding suggests an increase in oxidative potential in males which according to the authors it may be linked to either greater muscle mass and therefore, increased oxygen respiration in males and/or differences in ‘hormone related’ antioxidant capacity. Furthermore, in order to investigate a possible association between increased oxidative stress, DNA damage and poly-ADP-ribosylation, the activity of the NAD-dependent DNA repair enzyme PARP has been measured across the range of aging human skin and has been shown to correlate significantly with age only in males aged between 0–77 y (p < 0.0001; r = 0.768).23 This has been also accompanied by a significant decline in NAD+ levels in males which could lead to cell death through reduced ATP production and activation of apoptosis.23 Overall, the data show that aging may be caused by different pathways and mechanisms in males and females.
Population-Specific Human Skin Aging
Among Europeans, Asians, Americans, even between countries and cities of one continent, exist different characteristics of skin and skin aging. Several clinical manifestations in scaling, uneven distribution of melanin pigment, wrinkling, sagging, sebaceous secretion have another prevalence, intensity and physiology. People of skin of color comprise the majority of the world's population and Asian subjects comprise more than half of the total population of the earth.
Caucasians have an earlier onset and greater skin wrinkling and sagging signs than other skin types.24 In a study, Chinese women had significantly more severe wrinkles in the area around the eyes compared with Japanese women, while Thai women had significantly more severe wrinkles in the lower halves of their faces compared with Chinese women.25 In particular, Caucasian females showed marked age-related wrinkle formation in the lower areas of the face, probably due to sagging in the subzygomatic area, which suggests a higher susceptibility to sagging in the subzygomatic area of Caucasian females.25 For each facial skin area, wrinkle onset is delayed by about 10 y in Chinese women as compared with French women and is most between age 40 and 50.
In general increased pigmentary problems are seen in skin of color although one large study reported that East Asians living in the USA had the least pigment spots.24 Pigmented spot intensity is a much more important aging sign in Chinese women (severe for 30% of women over 40) than in French women (severe for less than 8% of women, irrespective of age).26 Induction of a hyperpigmentary response is thought to be through signaling by the protease-activated receptor-2 which together with its activating protease is increased in the epidermis of subjects with skin of color.24 Changes in skin biophysical properties with age demonstrate that the more darkly pigmented subjects retaining younger skin properties compared with the more lightly pigmented groups.24 Melanosomes have found to be more numerous in American and African Blacks and Australian aborigines. Melanosomes in these ethnic groups are also larger and individually dispersed compared with those found in other racial groups.24 Variations in melanosome arrangement, together with the quantity and type of melanin present, are responsible for the differences in skin pigmentation. Variability in the structure of melanosomes in diverse ethnic skin types may also contribute to differences in tyrosinase activity.24 The number of melanocytes decreases with aging.
Scaling has been studied in a wide range of ages in different ethnicities. Caucasian subjects have a higher prevalence of desquamating skin, which increases with age.27 The range of values was larger in the dorsal forearm than the upper inner arm and was greater in Caucasian subjects than African-American subjects. Another study has revealed that the hydration of the skin is influenced by ethnicity, suggesting anatomical or physiological property differences in ethnic skin. With age, the dryness of the skin is higher for African American and Caucasian women, with a higher percentage increase in Caucasian women.28
Investigations on the role of sebaceous secretion in aging and in the variability observed between different ethnicities are very limited. The dimension of facial pores can be interrelated with the activity of the sebaceous gland.29 Facial pore size and the architecture of the interfollicular epidermis differ between ethnic groups. Many endogenous and exogenous factors are known to cause enlarged pilosebaceous pores. Such factors include sex, genetic predisposition, aging, chronic UV light exposure, comedogenic xenobiotics, acne and seborrhea.29 Asian women show smaller pore areas compared with other racial groups.30 African Americans showed substantially more severe impairment of architecture around facial pores than any other racial group.30
The incidence of acne is similar across diverse racial groups but acne responses appear to show differences between the different racial groups. In response to coal tar, Caucasians develop inflammatory lesions whereas subjects with Black skin open comedones develop. Thus, in subjects with white skin rupture of the follicles occurs but in Negroid subjects hyperproliferation and retention of horny cells occur.24 Interestingly, serum levels of insulin-like growth factor-1 (IGF-1) correlate with facial pore size and impairment of the perifollicular epidermal architecture where IGF-1 positive cells were more abundant.31
Skin changes influenced by age and the ethnic difference in the skin features at young and old age can have their basis in different organization of skin surface lipids. This has been partly investigated clinically.24,27 One has to remember that barrier function relates to the total architecture of the stratum corneum (SC) and not just its lipid levels. Asian skin is reported to possess a similar basal transepidermal water loss (TEWL) to Caucasian skin and similar ceramide levels but upon mechanical challenge it has the weakest barrier function SC.24 Nevertheless, several studies indicate that Asian skin maybe more sensitive to exogenous chemicals probably due to a thinner SC and higher eccrine gland density.24
Concluding Remarks
Research that can lead to novel intervention to extend the health span and improve quality of life at older age has the potential for enormous impact in an ever-aging society. Understanding the impact of genetics and elucidating the mechanisms of skin aging in both genders and in different populations is the first step toward the development of accurate diagnostic kits and effective strategies of prevention and treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/22372
References
- 1.Monnier VM, Sell DR. Prevention and repair of protein damage by the Maillard reaction in vivo. Rejuvenation Res. 2006;9:264–73. doi: 10.1089/rej.2006.9.264. [DOI] [PubMed] [Google Scholar]
- 2.Castelo-Branco C, Pons F, Gratacós E, Fortuny A, Vanrell JA, González-Merlo J. Relationship between skin collagen and bone changes during aging. Maturitas. 1994;18:199–206. doi: 10.1016/0378-5122(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 3.Simm A, Wagner J, Gursinsky T, Nass N, Friedrich I, Schinzel R, et al. Advanced glycation endproducts: a biomarker for age as an outcome predictor after cardiac surgery? Exp Gerontol. 2007;42:668–75. doi: 10.1016/j.exger.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Makrantonaki E, Schönknecht P, Hossini AM, Kaiser E, Katsouli MM, Adjaye J, et al. Skin and brain age together: The role of hormones in the ageing process. Exp Gerontol. 2010;45:801–13. doi: 10.1016/j.exger.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Makrantonaki E, Zouboulis CC, German National Genome Research Network 2 The skin as a mirror of the aging process in the human organism--state of the art and results of the aging research in the German National Genome Research Network 2 (NGFN-2) Exp Gerontol. 2007;42:879–86. doi: 10.1016/j.exger.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3–14. doi: 10.1016/j.clindermatol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Zouboulis CC, Makrantonaki E. Hormonal therapy of intrinsic aging. Rejuvenation Res. 2012;15:302–12. doi: 10.1089/rej.2011.1249. [DOI] [PubMed] [Google Scholar]
- 8.Zahn JM, Kim SK. Systems biology of aging in four species. Curr Opin Biotechnol. 2007;18:355–9. doi: 10.1016/j.copbio.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lener T, Moll PR, Rinnerthaler M, Bauer J, Aberger F, Richter K. Expression profiling of aging in the human skin. Exp Gerontol. 2006;41:387–97. doi: 10.1016/j.exger.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Kaczvinsky JR, Jr., Grimes PE. Practical applications of genomics research for treatment of aging skin. J Drugs Dermatol. 2009;8(Suppl):s15–8. [PubMed] [Google Scholar]
- 11.Robinson MK, Binder RL, Griffiths CE. Genomic-driven insights into changes in aging skin. J Drugs Dermatol. 2009;8(Suppl):s8–11. [PubMed] [Google Scholar]
- 12.Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol Genomics. 2003;14:149–59. doi: 10.1152/physiolgenomics.00049.2003. [DOI] [PubMed] [Google Scholar]
- 13.Makrantonaki E, Adjaye J, Herwig R, Brink TC, Groth D, Hultschig C, et al. Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Aging Cell. 2006;5:331–44. doi: 10.1111/j.1474-9726.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 14.Rodwell GE, Sonu R, Zahn JM, Lund J, Wilhelmy J, Wang L, et al. A transcriptional profile of aging in the human kidney. PLoS Biol. 2004;2:e427. doi: 10.1371/journal.pbio.0020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–9. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- 16.Kyng KJ, May A, Kølvraa S, Bohr VA. Gene expression profiling in Werner syndrome closely resembles that of normal aging. Proc Natl Acad Sci U S A. 2003;100:12259–64. doi: 10.1073/pnas.2130723100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;287:2486–92. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- 18.Carroll PV, Christ ER, Bengtsson BA, Carlsson L, Christiansen JS, Clemmons D, et al. Growth Hormone Research Society Scientific Committee Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. J Clin Endocrinol Metab. 1998;83:382–95. doi: 10.1210/jc.83.2.382. [DOI] [PubMed] [Google Scholar]
- 19.Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, et al. West Midlands Prospective Hypopituitary Study Group Association between premature mortality and hypopituitarism. Lancet. 2001;357:425–31. doi: 10.1016/S0140-6736(00)04006-X. [DOI] [PubMed] [Google Scholar]
- 20.Laron Z. Do deficiencies in growth hormone and insulin-like growth factor-1 (IGF-1) shorten or prolong longevity? Mech Ageing Dev. 2005;126:305–7. doi: 10.1016/j.mad.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Azzi L, El-Alfy M, Martel C, Labrie F. Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. J Invest Dermatol. 2005;124:22–7. doi: 10.1111/j.0022-202X.2004.23545.x. [DOI] [PubMed] [Google Scholar]
- 22.Seidenari S, Pagnoni A, Di Nardo A, Giannetti A. Echographic evaluation with image analysis of normal skin: variations according to age and sex. Skin Pharmacol. 1994;7:201–9. doi: 10.1159/000211295. [DOI] [PubMed] [Google Scholar]
- 23.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006;28:79–93. doi: 10.1111/j.1467-2494.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsukahara K, Fujimura T, Yoshida Y, Kitahara T, Hotta M, Moriwaki S, et al. Comparison of age-related changes in wrinkling and sagging of the skin in Caucasian females and in Japanese females. J Cosmet Sci. 2004;55:351–71. [PubMed] [Google Scholar]
- 26.Nouveau-Richard S, Yang Z, Mac-Mary S, Li L, Bastien P, Tardy I, et al. Skin ageing: a comparison between Chinese and European populations. A pilot study. J Dermatol Sci. 2005;40:187–93. doi: 10.1016/j.jdermsci.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Chu M, Kollias N. Documentation of normal stratum corneum scaling in an average population: features of differences among age, ethnicity and body site. Br J Dermatol. 2011;164:497–507. doi: 10.1111/j.1365-2133.2010.10120.x. [DOI] [PubMed] [Google Scholar]
- 28.Diridollou S, de Rigal J, Querleux B, Leroy F, Holloway Barbosa V. Comparative study of the hydration of the stratum corneum between four ethnic groups: influence of age. Int J Dermatol. 2007;46(Suppl 1):11–4. doi: 10.1111/j.1365-4632.2007.03455.x. [DOI] [PubMed] [Google Scholar]
- 29.Roh M, Han M, Kim D, Chung K. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol. 2006;155:890–4. doi: 10.1111/j.1365-2133.2006.07465.x. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama-Nakagiri Y, Sugata K, Hachiya A, Osanai O, Ohuchi A, Kitahara T. Ethnic differences in the structural properties of facial skin. J Dermatol Sci. 2009;53:135–9. doi: 10.1016/j.jdermsci.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Sugiyama-Nakagiri Y, Ohuchi A, Hachiya A, Kitahara T. Involvement of IGF-1/IGFBP-3 signaling on the conspicuousness of facial pores. Arch Dermatol Res. 2010;302:661–7. doi: 10.1007/s00403-010-1062-3. [DOI] [PubMed] [Google Scholar]
- 32.Makrantonaki E, Brink TC, Zampeli V, Elewa RM, Mlody B, Hossini AM, et al. Identification of biomarkers of human skin ageing in both genders. Wnt signalling: a label of skin ageing? PLOS One. 2012;7:e50393. doi: 10.1371/journal.pone.0050393. [DOI] [PMC free article] [PubMed] [Google Scholar]