Abstract
Age-associated skin lesions linked to UV radiation (UVR) include actinic keratosis, non-melanoma skin cancer—such as basal cell carcinoma and squamous cell carcinoma—lentigo senilis and lentigo maligna. Their incidence is increasing worldwide, mainly due to exaggerated UV exposure and to an aging population. Early diagnosis and therapy of pre-malignant cutaneous lesions is crucial for the secondary prophylaxis of invasive and highly aggressive skin cancers. Combined efforts to increase public awareness, patient education about self-examination, prophylactic modalities, such as consistent and sufficient UV protection, and rigorous follow-up of high-risk groups are of highest importance.
Keywords: skin aging, carcinogenesis, skin cancer, actinic keratosis, field cancerization, basal cell carcinoma, squamous cell carcinoma, lentigo maligna, photoprotection
Introduction
Age-related skin changes are induced by chronological aging, also known as intrinsic aging, and photoaging or extrinsic aging. Extrinsic skin aging is characterized by elastosis in the upper dermis, destruction of its fibrilar structure, augmented intercellular substance and moderate inflammatory infiltrate. With accelerating age, skin functions deteriorate due to structural and morphologic changes. Skin is prone to the development of several diseases, varying from benign to malignant.1
Clinical signs associated with aged skin include wrinkling, fine telangiectasia, irregular or blotchy pigmentation, skin coarseness, laxity, atrophy, dryness. These changes result from intrinsic aging associated with reduced cellular proliferative capacity, but are accelerated by extrinsic factors, such as chronic sun exposure and other environmental factors, particularly smoking.2
The cumulative UV exposure correlates in general with the site-specific incidence of skin cancer. Anatomic location, UV radiation and senescence can influence the expression of apoptosis-related molecules resulting in cancer cell development and tumor progression. Anti-tumor immune response, which plays an important role in the elimination of cancer cells, is modified by UV radiation and aging, further contributing to cancer progression and its development.3
Age-associated skin lesions linked to UVR include actinic keratosis, non-melanoma skin cancer, such as basal cell carcinoma and squamous cell carcinoma, lentigo senilis and lentigo maligna. Their prevalence rises throughout the world, making it necessary to increase the level of attention from a diagnostic and a preventive point of view.
The aim of this paper is to present the relationship between skin aging and skin carcinogenesis, key features of epidemiology, major risk factors and possible pathogenetic mechanisms associated with the development of UV- and age-related skin cancer. Basic clinical and histopathological features, as well as the course of UV-associated skin lesions and their prognosis are presented.
Actinic Keratosis and Field Cancerization
Actinic keratoses or solar keratoses or senile keratoses are intraepithelial skin neoplasms constituted by atypical proliferation of keratinocytes. AKs were previously considered precancerous or premalignant lesions with a potential for evolving into SCCs. In recent years, they have been redefined as malignant neoplasms, since they are squamous cell carcinomas in situ, and thus precursors of invasive squamous cell carcinoma.4
Epidemiology: Risk factors
AKs develop in photoexposed skin areas of elderly individuals; they are induced mainly by UVR and are considered cutaneous markers of chronic exposure to sunlight. They can less often be seen in younger individuals with sufficient sun exposure.5 Incidence appears to be rising worldwide due to increasing levels of UVR exposure, lifestyle changes and an aging population.
The most important susceptibility risk factors for the development of AKs are cumulative exposure to UVR and age. Epidemiological studies indicate that AKs increase in prevalence with increasing age, with rates ranging from 10% in Caucasians aged 20–29 y to 80% in those 60–69 y 6. Male gender is an important risk factor for their development, which probably reflects a greater cumulative sun exposure in males. Other susceptibility risk factors include skin phototypes I to III, continued residence in a rural area after the age of 30 y, practice of outdoor sports, history of episodes of sunburn, especially in childhood, immunosuppression, certain genetic syndromes, such as albinism and xeroderma pigmentosum.5,6
Etiology: Pathogenesis
Habitual exposure to UVR is the most important contributing factor for the development of AKs. Apart from melanin synthesis, active repair mechanisms play the most important protective role for human skin against UVR. UV-induced mutations in the p53 tumor suppressor gene are thought to be the most common event, as approximately 80% of AKs reveal UV-specific p53 mutations.7 Moreover, UVR acts as an immunosuppressant preventing tumor rejection. AKs on photodamaged skin represent expanded clones of mutated cells that have escaped apoptosis and immune surveillance and have proceeded to proliferate to clinically evident premalignant lesions.5,7
It is currently unclear whether tissue changes surrounding multifocal epithelial tumors are a cause or consequence of cancer. It has been shown in human skin samples that decreased signaling of certain pathways, such as mesenchymal Notch/CSL signaling, causes molecular changes and in result tissue alterations, including stromal atrophy and inflammation, which precede and are potent triggers for epithelial tumors. These changes in gene expression are also induced by UVA, a known environmental cause of cutaneous field cancerization and skin cancer.8
Clinical findings
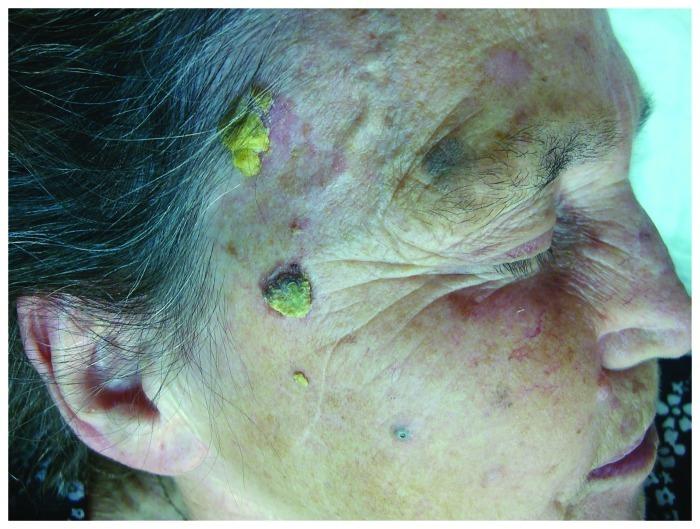
AKs are ill-defined pink to skin-colored hyperkeratotic papules found on chronically sun-exposed areas, most frequently on the face, scalp, ears, forearms, décolleté, dorsal hands. There are several clinical subtypes of actinic keratoses, including the erythematous and the hypertrophic one (Fig. 1). The cutaneous horn or cornu cutaneum is considered by some a type of hypertrophic AK. Also actinic cheilitis represents confluent AKs on the lips, usually the lower lip.9 They are often asymptomatic, but common signs and symptoms include pruritus, burning or stinging pain, bleeding and crusting.5,9
Figure 1. Histologically confirmed hypertrophic actinic keratoses on right facial side of an elderly woman on a background of dermatoheliosis.
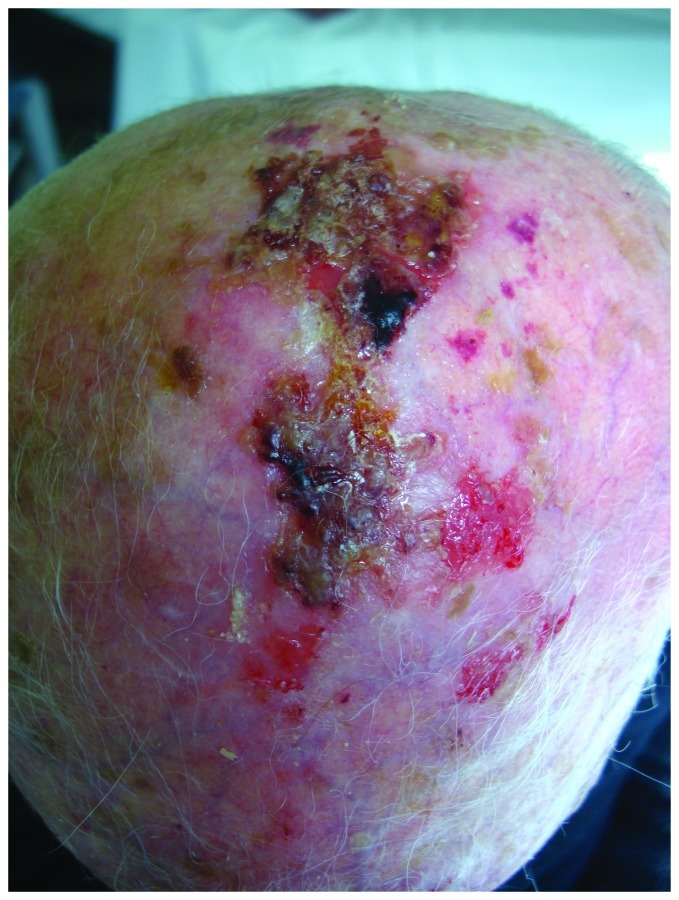
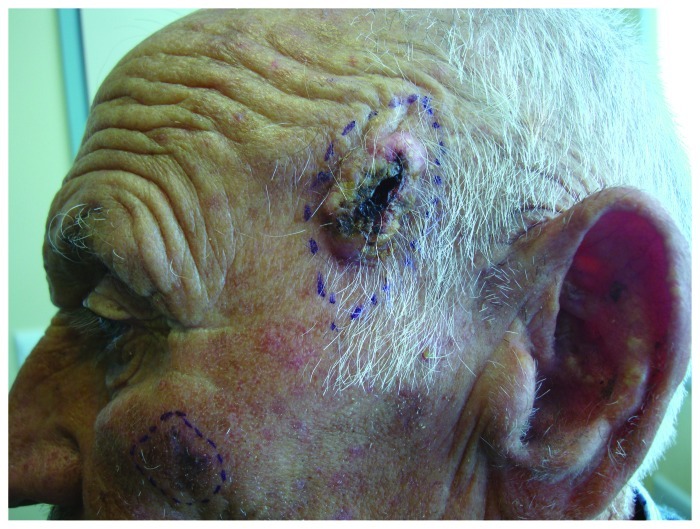
AKs are most often seen on a background of dermatoheliosis with solar elastosis, yellow discoloration, dyspigmentation, telangiectasias, ephelides, lentigos, sagging skin or on skin areas with field cancerization.10 Field cancerization is a term that describes the presence of genetic abnormalities in a tissue chronically exposed to a carcinogen. These abnormalities are responsible for the presence of multilocular clinical and sub-clinical cancerous lesions that explains the increased risks of multiple cancers in this area. With respect to the skin, this term is used to define the presence of multiple NMSC lesions, its precursors, actinic keratoses and dysplastic keratinocytes in sun exposed areas11,12 (Fig. 2).
Figure 2. Field cancerization on scalp of an elderly man, with multiple actinic keratoses and histologically confirmed SCCs.
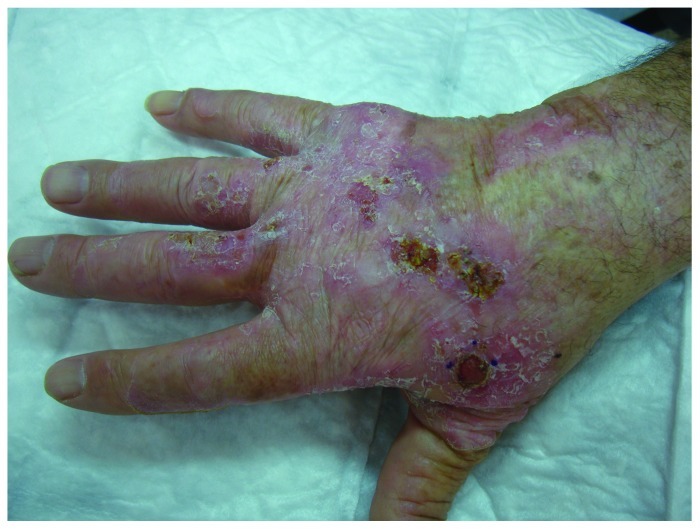
Bowen’s disease is also considered an intraepidermal form of squamous cell carcinoma. It typically presents as an asymptomatic, gradually enlarging, well demarcated, erythematous plaque with an irregular border and surface crusting or scaling13 (Fig. 3). It is most commonly found in patients aged over 60 and occurs predominantly in women (70–85% of cases). In the majority of patients it is located on sun-exposed sites, in 60–85% of cases on the lower leg.13,14 Etiological factors include UVR, but also immunosuppression, previous ionizing radiation, previous therapy with psoralen and UVA (PUVA), infection with HPV. If left untreated, lesions can progress to invasive SCC, called Bowen's carcinoma (BC), in 3–5% of cases. The presence of BD marks a high-risk individual for developing non-melanoma skin cancer (NMSC).9,13
Figure 3. Histologically confirmed Bowen’s disease at right dorsal hand of an elderly patient existing for many years with a recently growing histologically confirmed Bowen’s carcinoma at the base of the thumb.
Diagnosis
Diagnosis is primarily made clinically. Skin biopsy is sometimes necessary to rule out invasive SCC in patients with field cancerization, immunosuppressed patients and in high-risk areas such as lip or ears. Indications for biopsy include tenderness, rapid lesion growth, bleeding and failure to respond to treatment.9
Histopathology
Histological features of AK include:9
• Hyperkeratosis and/or ulceration
• Columns of parakeratosis, overlying atypical keratinocytes, separated by areas of orthokeratosis
• Basal atypical keratinocytes with varying degrees of overlying loss of maturation, hyperchromatism, pleomorphism, increased and abnormal mitoses, dyskeratosis.
• Variable superficial perivascular or lichenoid chronic inflammatory infiltrate
• Solar elastosis
• Lack of dermal invasion
Disease progression: Prognosis
AKs represent one of the strongest predictors of NMSC. Their presence indicates long-term sun damage and identifies a group of individuals at high risk for developing SCC, BCC and to a lesser extent melanoma.9 Several studies have shown that pain and inflammation are signs of AK progression to SCC. The risk of progression is difficult to assess and varies in the literature from less than 1 to 20%.4 Since AKs are in situ SCCs that may progress to invasive tumors, they must be managed using one of the available approved therapeutic alternatives. The inability to predict which AKs will persist, regress or proceed to SCCs makes treatment of all AKs indispensable. Treatment aims to clinically evident lesions but also non-apparent lesions in the cancerization field.15 The concept of field cancerization has been instructive in broadening treatments to include entire affected areas rather than individual lesions given that the areas with significant photodamage will continue to develop numerous individual precancerous and cancerous lesions. Field therapy is especially recommended for the treatment of these large clinically asymptomatic fields containing tumor cells.16
Non-Melanoma Skin Cancer
Non-melanoma skin cancer represents the most common form of cancer, encompassing BCC and SCC. Its incidence is increasing worldwide. It primarily afflicts geriatric patients, shown by the fact that 80% of all non-melanoma skin cancers are diagnosed in patients aged over 60 y.17,18
Basal cell carcinoma
Epidemiology: Risk factors
Basal cell carcinoma is the most common malignant neoplasm in humans. It accounts for 75% of cases of NMSC, whereas squamous cell carcinoma accounts for the remaining majority of NMSC cases.17 Its incidence has been increasing over the last decades due to an aging population and sun exposure habits.19 Incidence of BCC increases steeply with increasing age; it is, however, becoming increasingly frequent in people younger than 50 y of age.20 BCCs are more commonly found in males. The most common localization is the head and neck area in 80% of cases, but anatomical site distribution is different for its histological subtypes. Studies support that in females BCC begins at a younger age.21
Exposure to UVR is the main environmental risk factor associated with its cause. It has been mostly associated with intermittent and childhood sun exposure. Other elements of risk include light skin phototypes and light eyes, freckles in childhood, advanced age, family history of skin carcinoma and immunosuppression. BCC is rare in dark skin and is approximately 19 times more common in white that black individuals. The tumor is commonly found in concomitance with skin lesions related to chronic sun exposure, such as actinic keratoses, solar lentigines and facial telangiectasias.22 Exposure to ionizing radiation or chemical carcinogens are also involved in BCC pathogenesis. Rare skin diseases exhibiting BCC as a prominent feature include basal cell naevus syndrome (BCNS), Bazex-Dupré-Christol syndrome, Rombo syndrome and xeroderma pigmentosum. BCC may represent a relatively common, although less specific finding in many other genodermatoses, such as Bloom, Werner, Muir-Torre, Cowden syndromes, oculocutaneous albinism and some epidermal naevus syndromes.23
Etiology: Pathogenesis
UVR, particularly the UVB spectrum (290–320 nm) is accepted as the most important causal factor for BCC development. UV light is thought to directly induce DNA mutations via covalent bonding between adjacent pyrimidines (UVB) and formation of reactive oxygen species (UVA).17
Discovery of the mutation of Patched gene (PTCH) on chromosome 9q22 underlying basal cell naevus syndrome, a genodermatosis associated with multiple BCCs, has greatly forwarded the understanding of the genetics underlying BCC appearance.24 Patched1, the protein product of PTCH, is a cell surface receptor, inhibiting smoothened (SMO), a G-protein-coupled receptor. Cessation of SMO inhibition by patched 1 initiates a signal cascade that leads to the activation of transcription factor Gli1. Dysregulation of this pathway by either the loss of PTCH or uncontrolled expression of SMO results in cell proliferation and differentiation.25 Mutations of either PTCH or SMO have been found in 70% of sporadic human BCCs.26 Other reported genes associated with the pathogenesis of BCC are cytochrome 450 (CYP), glutathione S-transferase (GST) and p53. CYP and GST are known to detoxify mutagens, while p53 has an important function as a tumor suppressor gene regulating the cell cycle.24 UV-induced mutations in the p53 tumor suppressor gene have been detected in almost 50% of BCC cases.
Particular attention must be held for organ transplant recipients (OTR) and immunosuppressed patients, who are predisposed to NMSC. Incidence of BCC is increased 10-fold in this group of patients.27,28
Clinical findings
BCC is a slow-growing, locally invasive epidermal tumor with characteristic clinical features that depend on the clinical subtype. These include:29
• Nodular BCC (Fig. 4A) the most common subtype, which occurs most commonly on the head and neck. It appears as a well-defined, firm, translucent or pearly papule or nodule, with telangiectasias and a rolled border at the periphery. Differential diagnoses include dermal naevus and amelanotic melanoma.
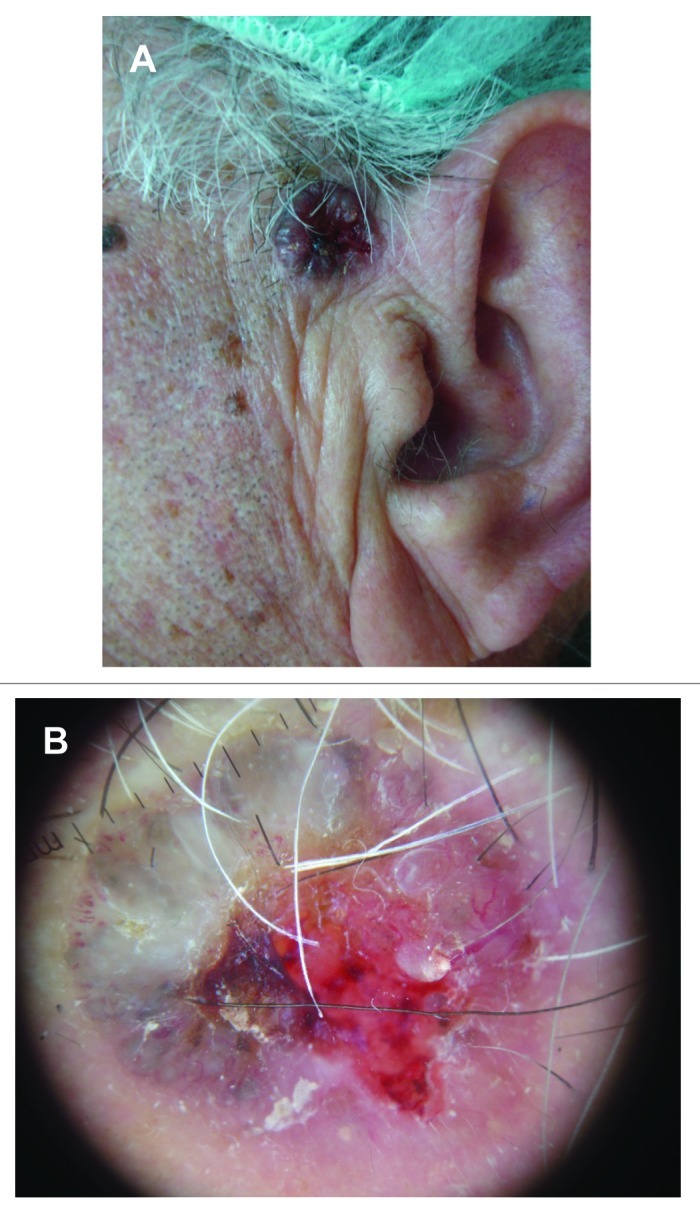
Figure 4. (A) Histologically confirmed nodular BCC in left preauricular area of a male patient. (B) Dermoscopic picture on same patient, showing central necrosis, maple leaf-like structures, blue-ovoid nests, blue-gray globules, and arborising vessels.
• Pigmented BCC, a subtype of nodular BCC, appearing as a hyperpigmented, translucent nodule. Differential diagnosis includes nodular melanoma.
• Ulcerated BCC which shows central necrosis and is referred to as ulcus rodens or rodent ulcer.
• Superficial BCC, which occurs predominantly on the trunk. It appears as an erythematous plaque that must be distinguished differentially from eczema.
• Morphea-like (or morpheaform or sclerosing) BCC, which has an ivory-white appearance and has to be differentiated from scar tissue or a morphea-plaque.
• Fibroepithelioma of Pinkus, which presents as a pink papule, usually on the lower back, that must be distinguished from an acrocordon.
Diagnosis
Diagnosis is primarily clinical and is completed with histological confirmation. Punch biopsy is the preferred biopsy method; sometimes a shave biopsy is, however, also adequate. Dermoscopy is used as an aid for diagnosis of BCC, with maple leaf-like structures, blue-ovoid nests, blue-gray globules, spoke-wheel structures, and arborizing blood vessels seen upon examination30 (Fig. 4B).
Histopathology
Features vary according to the subtype, but common histological findings include a tumor consisting of proliferating atypical basal cells, which appear large, oval and stain deep blue on hematoxylin-eosin. Cells show little anaplasia, infrequent mitoses and have a palisading arrangement at the periphery.29
Disease progression: Prognosis
BCC is a slow-developing malignant skin tumor, infiltrating the adjacent tissues. If left untreated, it progresses to invade subcutaneous tissue, muscle and even bone. It causes significant patient morbidity, due to local tissue destruction and disfigurement. Perineural invasion is an uncommon feature of BCC. When present, it is associated with larger, histologically aggressive tumors, and the risk of 5-y recurrence is higher.31
BCC has a metastatic rate of < 0.1%. Metastases correlate to the size and depth of the tumor, and less to its histological subtype. They are expected to occur when large tumors greater than 3 cm in diameter are present. Whereas the risk of metastases is 1–2% in tumors of 3 cm in diameter, it increases to 20–25% in tumors 5 cm in diameter, and up to 50% in lesions greater than 10 cm in diameter. If a BCC is greater than 10 cm in diameter, it is called “giant” and poses a significant risk of morbidity and mortality.32 In case metastases occur, involvement of regional lymph nodes and lungs is most common. Metastases to the bone and bone marrow have also been reported. Aggressive histological characteristics, such as morpheaform features, squamous differentiation and perineural invasion are risk factors for metastasis.33
With appropriate treatment, prognosis is excellent. However, patients must be closely monitored for recurrence or development of new BCCs, the risk for development of a second primary BCC varying from 36 to 50%. Patients with metastatic disease have a poor prognosis, with a mean survival of 8–10 mo from time of diagnosis.34
Squamous cell carcinoma
Cutaneous SCC is a malignant neoplasm derived from suprabasal epidermal keratinocytes.17 It has a lifetime incidence of approximately 10% in the general population and is the second most common type of NMSC.35
Epidemiology: Risk factors
Although the precise incidence of NMSC is not known and SCC is less common than BCC, SCC carries a risk of metastasis, accounting for a considerable number of deaths from NMSC each year. SCC is strongly associated with advanced age. A sharp increase in incidence is seen after the age of 40 y. Increased exposure to UVR, through greater use of tanning salons, increased time spent outdoors, changes in clothing style, and ozone depletion, have been implicated for the increase in the lifetime risk for SCC among Caucasians, which has doubled over the last two decades to approximately 15%.36
SCC is twice as common in men as it is in women, probably as a result of greater lifetime UV exposure through profession, clothing and shorter hairstyles. Chronic exposure to UVR is the main environmental risk factor associated with SCC evolution. There is actually a linear correlation between the incidence of SCC and exposure to UVR.17,36 Other elements of risk include light skin phototype and blue eyes, advanced age, family history of skin carcinoma, increasing number of melanocytic naevi, freckles in childhood and immunosuppression. Behavioral aspects such as occupational sun exposure, rural labor and sunburns at a young age also play a role. Further predisposing factors include exposure to ionizing radiation, exposure to environmental and occupational carcinogens, such as arsenic and aromatic hydrocarbons, exposure to chemical carcinogens, to thermal radiation, presence of scars, chronic inflammation and ulcers. Human papillomavirus has also been reported to be pathogenic for SCC and shown to prolong keratinocyte cell cycle, with increased degradation of p53. HPV-16 and HPV-18 infection has been associated with increased risk of oral, as well as head and neck SCC development.36-39
In a large prospective study seropositivity for HPV-76 was significantly associated with an increased risk for future development of SCC.40 Smoking and alcohol abuse have been strongly associated with SCCs of the oral cavity.41
A dose-dependent increase in the risk of squamous cell carcinoma of the skin was found to be associated with exposure to Psoralen and UVA radiation. Risk is not as strongly associated with narrowband UVB. Use of tanning devices is associated with 2.5-fold increase in SCC risk.42 Moreover, a number of hereditary skin disorders predispose to SCC development, in particular oculocutaneous albinism and xeroderma pigmentosum.43
Particular attention must be attributed to solid organ transplant recipients (SOTRs) and immunosuppressed patients. Skin cancer constitutes the most frequently reported post-transplant malignancy in solid organ transplant recipients (OTR) worldwide. Whereas the risk for malignant melanoma is only moderately increased, non-melanoma skin cancers (NMSC) seem to thrive on chronic immunosuppression and account for up to 95% of post-transplant cutaneous malignancies.44 These patients have a 50 to 250 times greater risk of developing a squamous cell carcinoma than the general population and experience higher rates of invasive and metastatic disease, due to the tumorigenic effects of their immunosuppressive medication. As the number of transplantations and life expectancy of SOTRs increase, SCCs are becoming a major source of morbidity and mortality.45 SCC is the most common NMSC that develops after transplantation. Compared with the general population cutaneous squamous cell carcinoma and actinic keratoses characteristically show even higher incidences than basal cell carcinoma and act as an indicator for the development of multiple primary cutaneous neoplasias and locally recurrent cancers (field cancerization).44 In this group of patients, the risk of developing a second NMSC within 5 y following a primary SCC is 66%. At presentation, the tumors are often more deeply invasive, with decreased histological differentiation and, have, therefore, a greater risk of metastasis.46,47
Etiology: Pathogenesis
While sporadic BCC develops de novo, SCC arises from precursor lesions of actinic keratosis and Bowen's disease, and represents a multistep accumulation of genetic damage. Over 70% of SCC develop within AK or actinically damaged skin on a background of field cancerization. Significant histological and genetic mimicry exists between AK and SCC and errors of p53 signaling have been implicated in both. Mutations in p53 have been identified in 69% to over 90% of invasive SCC. Other reported mutations for SCC include WNT, Ras, p16INK4, NFκB and c-Myc.17,48,49
Clinical findings
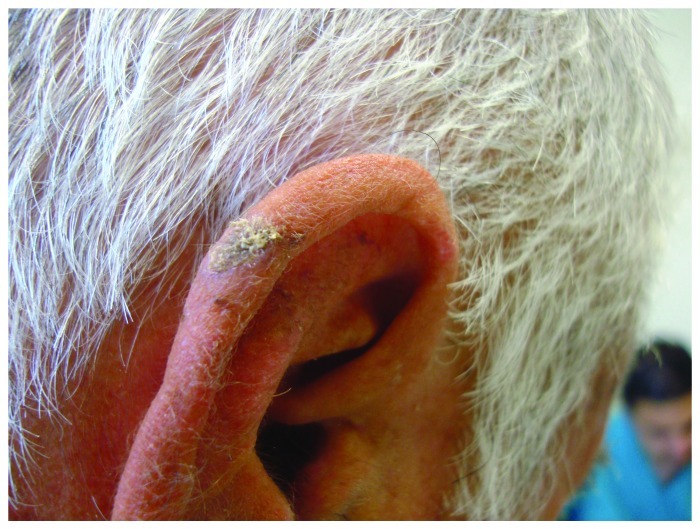
SCC tends to present as a rapidly growing pink or red nodule or plaque, which may be hyperkeratotic or ulcerated (Figs. 3,5,6). It may also be pigmented, verrucous or appear as a thick cutaneous horn. Progressive tumor invasion results in tenderness and fixation to underlying tissues. Especially in the head and neck region, an enlarged lymph node may indicate tumor metastasis.36,50
Figure 5. Histologically confirmed SCC on right helix of an elderly man.
Figure 6. Histologically confirmed SCC on left temporal area of an elderly man, multiple actinic keratoses on left ear and lentigo maligna on his left cheek.
Particular SCC morphologies include the SCC of the oral cavity, SCC of the lower lip, SCC of the genitalia, SCC developing on scar tissue, verrucous carcinoma and keratoakanthoma. Oral SCC shows a male predominance and is commonly associated with cigarette smoking, tobacco chewing or alcohol abuse. It usually evolves from erythroplakia. Lower lip SCC begins as actinic cheilitis or scaly leukoplakia and slowly progresses to a tumor nodule. Genital SCC (vulvar, anal, penile) is often associated with lichen sclerosus and atrophicans, whereas cervical SCC with HPV-infection, most commonly type 16. A precursor of penile SCC is erythroplasia of Queyrat. Keratoacanthoma is considered to be a clinical subtype of SCC.36,51
Diagnosis
Clinical suspicion must always be verified by skin biopsy. Any persistent, enlarging, or non-healing lesion, particularly on a sun-exposed site, must be evaluated histologically.
Histopathology
The hallmark of invasive SCC is the extension of atypical keratinocytes beyond the basement membrane and into the dermis. The presence of solar elastosis or keratinocyte atypia at tumor margins suggest that the SCC is actinically derived.36
Disease progression: Prognosis
Cutaneous SCC shows a significant rate of metastasis (0.3–3.7%), the majority from high-risk tumors.17 This risk is determined by clinical and histological elements, which are individually recognized. So far, staging systems do not allow the assessment of a risk score or a standardized therapeutical approach.52
Risk factors for metastasis include tumor size > 2 cm, depth > 2mm, Clark level ≥ IV, perineural invasion, lymphovascular invasion, poor differentiation, certain histological subtypes (desmoplastic or adenosquamous carcinoma, invasive Bowen’s carcinoma, SCC arising in areas of chronic inflammation), immunosuppression, human papillomavirus infection, high-risk anatomic location (pinna of the ear, labial mucosa), expression of certain tumor genes and inadequate tumor resection. The most important risk factors for development of metastatic disease are poor histological differentiation and perineural/lymphovascular infiltration.53-55
Lentigo Senilis
Epidemiology: Risk factors
The prevalence of lentigo senilis (or solar lentigo, or lentigo solaris) correlates with increasing age. They are present in 90% of white individuals over 60 y of age. They appear most commonly in sun-exposed areas, such as the face and back of the hands.56 Solar lentigines are common in fair-skinned individuals, but uncommon among people with dark pigmented skin. Their presence is a risk factor for melanoma and NMSC.57
Etiology: Pathogenesis
Solar lentigines represent a marker of intermittent high intensity and of cumulative UVR. They are characterized by epidermal hyperplasia, concerning both keratinocytes and melanocytes. The primary defect could be in either cell type eliciting a secondary proliferative response in the other.56,57 Lentigines may be induced by photochemotherapy (PUVA-lentigines), occurring in 40–50% of patients receiving a PUVA therapy. Phototoxic doses of PUVA may lead to the development of lentigines 6–8 mo after therapy. Their appearance has also been associated with UVA tanning bed use for cosmetic tanning.57
Clinical findings
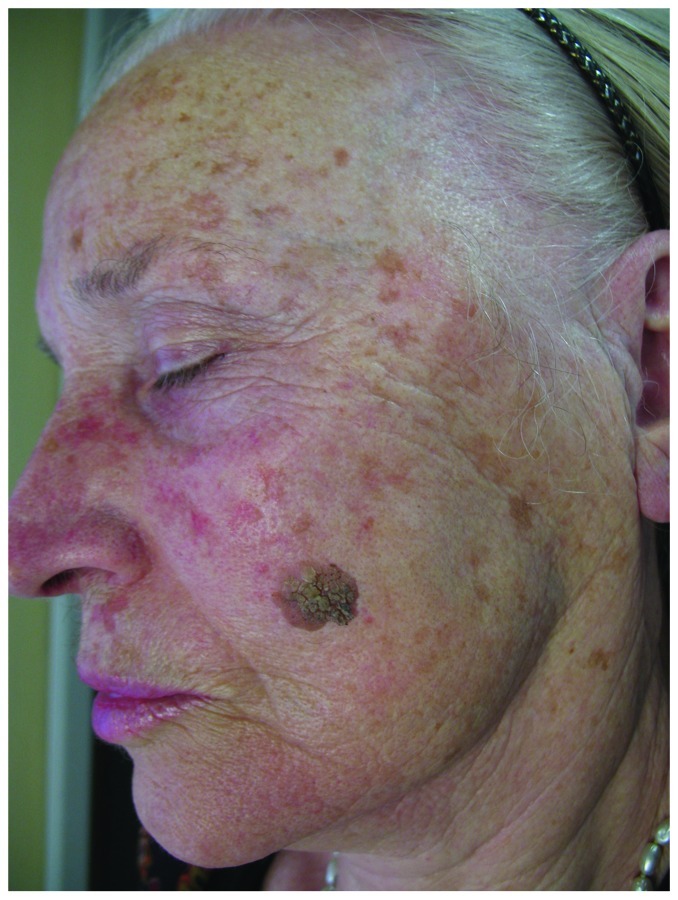
Lentigo solaris is a benign, acquired, circumscribed pigmented macule with a smooth or irregular border that appears in sun-exposed skin, such as face and back of hands.56 Its color varies from light brown to black (Fig. 7). It presents on skin exposed to natural or artificial UVR, but may also appear in sun-protected areas. Children with xeroderma pigmentosum develop lentigines solaris even in the early months of life after minimal sun exposure. Variants of lentigo senilis with acute onset after intense UVR are sunburn freckles. To differentiate from solar lentigines, ephelids are common genetically determined pigment spots appearing during childhood in a distinct photodistribution.58
Figure 7. Multiple lentigines senilis on face of an elderly woman and a seborrheic keratosis on her left cheek.
Diagnosis
Diagnosis is primarily clinical. Dermoscopy reveals a uniform reticular network.58
Histopathology
A solar lentigo can be hard to distinguish from a flat seborrhoic keratosis. It also has to be differentiated from an ephelis, a lentigo simplex, a pigmented actinic keratosis, a pigmented BCC and a lentigo maligna. A lentigo solaris shows increased basal melanocytes, while an ephelid increased pigmentation but no increase in melanocytes.59 The absence of cellular atypia may account for a favorable prognosis and enables clear distinction from more serious diagnoses, such as lentigo maligna melanoma.57 Histological features of solar lentigines include hyperpigmented elongated epidermal rete ridges, a thinned epidermis in between, and keratinocyte and melanocyte proliferation without nesting.58
Lentigo Maligna: Lentigo Maligna Melanoma
Epidemiology-Risk factors
Lentigo maligna (LM) is a subtype of melanoma in situ with a prolonged radial growth phase. Lentigo maligna melanoma (LMM) is the most common subtype of melanoma on the face. Presentation of LMM may be quite subtle, particularly in early stages and delayed diagnosis is common.60 If left untreated, LM may evolve into the invasive form of LMM.61 LM and LMM are most often diagnosed in patients in their seventh to eighth decade of life. They are rarely seen before the age of 40. Epidemiology of these tumors progressively concerns younger patients.62 The most common location is on the chronically sun-exposed face, most commonly cheeks and nose, then neck, scalp and ears.63 Lentigo maligna and LMM account for about 10% of all melanomas.64
Etiology: Pathogenesis
LM pathogenesis is thought to be associated with cumulative, and not intermittent, sun exposure.63,64 A stronger association of lentigo maligna melanoma is noted with intermittent sun exposure.65 The epidermis in lentigo maligna shows overall low proliferation and an apparently low apoptotic tendency. It is thought that the dysfunctional epidermis in chronically sun-damaged skin may be permissive to aberrant melanocyte proliferation in the early stages of melanoma development. Keratinocytes influence the number, morphology, and proliferation of melanocytes. An interference in the melanocyte-keratinocyte relationship may contribute to LMM development.66
Clinical findings
Lentigo maligna presents as a flat, slowly enlarging macular lesion with poorly defined irregular borders, prominent asymmetry and pigment variation, persisting for years on chronically sun-exposed skin of elderly individuals (Fig. 6). LMM is frequently larger than LM and may continue to be macular, although a nodular portion is often seen within the macule as the lesion progresses61 (Fig. 8A).
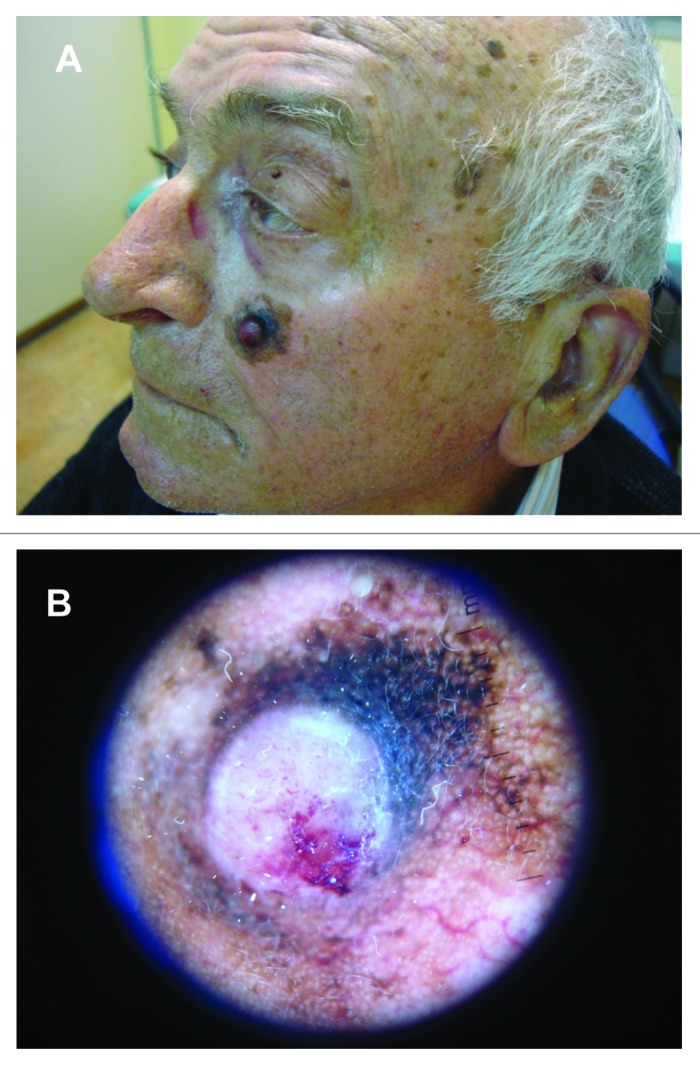
Figure 8. (A) Histologically confirmed lentigo maligna melanoma on left cheek of an elderly male patient, arising from a lentigo maligna after many years. (B) Dermoscopic picture of LMM on same patient.
Diagnosis
Early clinical detection of LM is imperative, though often very difficult. Differential diagnoses include solar lentigo, early lesions of seborrheic keratosis, lentigo maligna melanoma, lichen planus-like keratosis, pigmented actinic keratosis and melanocytic naevus.67
Dermoscopy has been shown to have higher diagnostic accuracy. Dermoscopically, LM is often associated with one or two pigment colors and few distinctive dermoscopic features. Initially, LM shows asymmetrical pigmented follicular openings and/or annular-granular structures, then expands and develops rhomboidal structures. Dermoscopic features of LMM include a large number of colors, an annular-granular pigment pattern, asymmetrical pigmented follicular openings, pigmented rhomboidal structures, obliterated hair follicles, gray pseudo-network. Also, an increased density of the vascular network, red rhomboidal structures and target-like patterns can be commonly seen in invasive LMMs. Vertical growth phase-associated dermoscopic criteria (ulceration, blue papular areas and black structureless areas) are rarely seen68,69 (Fig. 8B). Annular-granular structures and gray pseudo-network seem to be observed also in regressive areas of solar lentigo/initial seborrheic keratosis, lichen planus-like keratosis and pigmented actinic keratosis.67
Dermatoscopic diagnosis of a pigmented skin lesion cannot be based on the presence of a single criterion, and therefore histopathology still remains the gold standard for correct diagnosis. Histological differentiation of LM and LMM can be difficult due to widespread atypical melanocytes that are present in an area of chronically sun damaged skin. Limited sampling may be inadequate for an accurate diagnosis of pigmented melanocytic lesions on actinically damaged skin. Areas chosen for biopsy may not contain the most advanced areas histologically and may fail to detect foci of invasive melanoma elsewhere within the lesion.70
Disease progression: Prognosis
Lentigo maligna may evolve into LMM after many years. Prognosis for invasive lentigo maligna melanoma does not differ from that for other histogenetic types of melanoma after controlling for tumor thickness.71,72
Conclusion
For all above mentioned UV-associated skin lesions, prevention of further exposure to UVR and close patient monitoring for melanoma or NMSC development is of greatest importance. Patient education and increased public awareness may potentially aid in their early detection and treatment.73 It is pivotal to protect the skin from UVR in order to prevent the de novo appearance and progression of preinvasive to invasive malignancies.
The most effective preventive measure is photoprotection, including broad-spectrum sunscreen use starting from an early age, use of UV-protective clothing, and behavioral adjustments, such as avoidance of tanning beds. Sun avoidance and protection methods should be rigorous. Other preventive measures include treatment of precursor lesions, such as individual actinic keratoses, or field cancerization), HPV transmission prevention, smoking and alcohol consumption cessation.74,75
Patient education is of highest importance. Except for photoprotection, skin self-examination in order to achieve early lesion detection is imperative. After diagnosis of one NMSC all patients should be considered at high risk for developing another tumor. In patients with a history of NMSC, monthly self examination of all skin surfaces is recommended. Individual risk assessment is necessary and should be discussed. In case of organ transplant patients, patient education should begin at transplantation, whereas in case of xeroderma pigmentosum or albinism, at birth or diagnosis.75,76
Patient follow-up is a second-line prevention mechanism for UV-associated skin lesions. In patients with a history of BCC, complete skin examination should occur every 6–12 mo for life.77 In patients with a history of SCC, complete skin and regional lymph node examination every 3–6 mo for 2 y, then every 6–12 mo for 3 y, then annually for life are recommended. As for patients with LM, at least an annual skin examination for life is recommended. For invasive LMM, follow-up is recommended according to the stage of the disease. Follow-up schedule is influenced by risk of recurrence, prior primary melanoma, family history of melanoma, and includes other factors, such as presence of dysplastic nevi and patient/physician concern.78
Patients with actinic keratoses, field cancerization or any predisposing risk factors for the development of a NMSC should be monitored on a regular basis, at least annually. Early diagnosis and therapy of pre-malignant cutaneous lesions is crucial for the secondary prophylaxis of further invasive and highly aggressive skin cancers. In case of field cancerization, field directed therapies are more worthwhile than lesion targeted approaches. In organ transplant patients, long-term skin surveillance, early diagnosis and aggressive treatment of any suspicious lesion, tapering immunosuppressive treatment or the selection of immunosuppressants with proposed antiangiogenic properties like mTor-inhibitors may help to reduce the multiplicity of subsequent primary skin cancers in high-risk patients.44,76
NMSC chemoprevention is a promising intervention for early management, and has made enormous progresses over the past years due to the large number of research studies including many randomized clinical trials.79 Retinoids including vitamin A (retinol), 13-cis-retinoic acid (isotretinoin), N-(4-hydroxyphenyl)retinamide (fenretinide) and vitamin A precursor β-carotene, are most extensively studied. Chemoprevention with systemic retinoids has demonstrated promise in decreasing the incidence of new primary NMSCs in immunocompromised post-transplantation recipients. There is limited evidence for the use of systemic retinoids in the non-transplantation patient, indicating that despite short-time effectiveness, systemic retinoids do not prevent tumor recurrence. Use of oral retinoids (acitretin, isotretinoin) has been effective in reducing the development of precancerous lesions, but side effects may be significant and therapeutic effects disappear shortly after cessation of the drug. Topical tretinoin failed to show a chemopreventive benefit for NMSC.80-83
Chemopreventive activity has also been studied in various agents including COX inhibitors, vitamin E forms, green tea extracts and other plant-derived compounds like resveratrol, protocatechuic, ellagic and caffeic acids. Encouraging outcomes were found with most natural extracts.81 Further study is warranted for validation or improvement of treatment efficacy with current agents and strategies. Future research may include identification of new biomarkers/targets, improvement of bioavailability or tissue penetration, combinations of different compounds.79
Glossary
Abbreviations:
- AK
actinic keratosis
- BCC
basal cell carcinoma, BC, Bowen's carcinoma
- BD
Bowen’s disease
- HPV
human papilloma virus
- LM
lentigo maligna
- LMM
lentigo maligna melanoma
- NMSC
non-melanoma skin cancer
- SCC
squamous cell carcinoma
- SOTR
solid organ transplant recipient
- UV
ultraviolet
- UVR
ultraviolet radiation
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/22519
References
- 1.Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3–14. doi: 10.1016/j.clindermatol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Suppa M, Elliott F, Mikeljevic JS, Mukasa Y, Chan M, Leake S, et al. The determinants of periorbital skin ageing in participants of a melanoma case-control study in the U.K. Br J Dermatol. 2011;165:1011–21. doi: 10.1111/j.1365-2133.2011.10536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manestar-Blazić T, Batinac T, Hadzisejdić I, Brajac I. Apoptosis and immune response are responsible for the site-specific incidence of non-melanoma skin cancer. Med Hypotheses. 2007;68:853–5. doi: 10.1016/j.mehy.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg LH, Mamelak AJ. Review ofactinic keratosis. Part I: etiology, epidemiology and clinical presentation. J Drugs Dermatol. 2010;9:1125–32. [PubMed] [Google Scholar]
- 5.Schmitt JV, Miot HA. Actinic keratosis: a clinical and epidemiological revision. An Bras Dermatol. 2012;87:425–34. doi: 10.1590/S0365-05962012000300012. [DOI] [PubMed] [Google Scholar]
- 6.Trakatelli M, Charalampidis S, Novakovic LB, Patsatsi A, Kalabalikis D, Sotiriadis D. Photodermatoses with onset in the elderly. Br J Dermatol. 2009;161(Suppl 3):69–77. doi: 10.1111/j.1365-2133.2009.09452.x. [DOI] [PubMed] [Google Scholar]
- 7.Rass K, Reichrath J. UV damage and DNA repair in malignant melanoma and nonmelanoma skin cancer. Adv Exp Med Biol. 2008;624:162–78. doi: 10.1007/978-0-387-77574-6_13. [DOI] [PubMed] [Google Scholar]
- 8.Hu B, Castillo E, Harewood L, Ostano P, Reymond A, Dummer R, et al. Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell. 2012;149:1207–20. doi: 10.1016/j.cell.2012.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan KO, Geisse JK, Leffell DJ. Epithelial precancerous lesions. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, ed. Fitzpatrick's Dermatology in General Medicine. 8th ed. Columbus: McGraw-Hill, 2012: 1261-1283. [Google Scholar]
- 10.Szeimies RM, Torezan L, Niwa A, Valente N, Unger P, Kohl E, et al. Clinical, histopathological and immunohistochemical assessment of human skin field cancerization before and after photodynamic therapy. Br J Dermatol. 2012;167:150–9. doi: 10.1111/j.1365-2133.2012.10887.x. [DOI] [PubMed] [Google Scholar]
- 11.Braathen LR, Morton CA, Basset-Seguin N, Bissonnette R, Gerritsen MJ, Gilaberte Y, et al. International Society for Photodynamic Therapy in Dermatology Photodynamic therapy for skin field cancerization: an international consensus. J Eur Acad Dermatol Venereol. 2012;26:1063–6. doi: 10.1111/j.1468-3083.2011.04432.x. [DOI] [PubMed] [Google Scholar]
- 12.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part I. Epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:253–61, quiz 262. doi: 10.1016/j.jaad.2010.11.062. [DOI] [PubMed] [Google Scholar]
- 13.Patel KB. Bowen’s Disease Treated with Imiquimod and Cryotherapy. Indian J Dermatol. 2012;57:239–41. doi: 10.4103/0019-5154.96217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kossard S, Rosen R. Cutaneous Bowen’s disease. An analysis of 1001 cases according to age, sex, and site. J Am Acad Dermatol. 1992;27:406–10. doi: 10.1016/0190-9622(92)70208-W. [DOI] [PubMed] [Google Scholar]
- 15.Rossi R, Calzavara-Pinton PG, Giannetti A, Peserico A, Santucci M, Vena GA, et al. Italian guidelines and therapeutic algorithm for actinic keratoses. G Ital Dermatol Venereol. 2009;144:713–23. [PubMed] [Google Scholar]
- 16.Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354–76. doi: 10.1007/s11864-012-0195-3. [DOI] [PubMed] [Google Scholar]
- 17.Samarasinghe V, Madan V. Nonmelanoma skin cancer. J Cutan Aesthet Surg. 2012;5:3–10. doi: 10.4103/0974-2077.94323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spandau DF, Lewis DA, Somani AK, Travers JB. Fractionated laser resurfacing corrects the inappropriate UVB response in geriatric skin. J Invest Dermatol. 2012;132:1591–6. doi: 10.1038/jid.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasper M, Jaks V, Hohl D, Toftgård R. Basal cell carcinoma - molecular biology and potential new therapies. J Clin Invest. 2012;122:455–63. doi: 10.1172/JCI58779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trakatelli M, Ulrich C, del Marmol V, Euvrard S, Stockfleth E, Abeni D. Epidemiology of nonmelanoma skin cancer (NMSC) in Europe: accurate and comparable data are needed for effective public health monitoring and interventions. Br J Dermatol. 2007;156(Suppl 3):1–7. doi: 10.1111/j.1365-2133.2007.07861.x. [DOI] [PubMed] [Google Scholar]
- 21.de Vries E, Micallef R, Brewster DH, Gibbs JH, Flohil SC, Saksela O, et al. EPIDERM Group Population-based estimates of the occurrence of multiple vs first primary basal cell carcinomas in 4 European regions. Arch Dermatol. 2012;148:347–54. doi: 10.1001/archdermatol.2011.2244. [DOI] [PubMed] [Google Scholar]
- 22.Chinem VP, Miot HA. Epidemiology of basal cell carcinoma. An Bras Dermatol. 2011;86:292–305. doi: 10.1590/S0365-05962011000200013. [DOI] [PubMed] [Google Scholar]
- 23.Castori M, Morrone A, Kanitakis J, Grammatico P. Genetic skin diseases predisposing to basal cell carcinoma. Eur J Dermatol. 2012;22:299–309. doi: 10.1684/ejd.2011.1633. [DOI] [PubMed] [Google Scholar]
- 24.Tang JY. Elucidating the role of molecular signaling pathways in the tumorigenesis of basal cell carcinoma. Semin Cutan Med Surg. 2011;30(Suppl):S6–9. doi: 10.1016/j.sder.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Takebe N, Lorusso P. Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol. 2010;2:237–50. doi: 10.1177/1758834010366430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low JA, de Sauvage FJ. Clinical experience with Hedgehog pathway inhibitors. J Clin Oncol. 2010;28:5321–6. doi: 10.1200/JCO.2010.27.9943. [DOI] [PubMed] [Google Scholar]
- 27.Ritchie SA, Patel MJ, Miller SJ. Therapeutic options to decrease actinic keratosis and squamous cell carcinoma incidence and progression in solid organ transplant recipients: a practical approach. Dermatol Surg. 2012;38:1604–21. doi: 10.1111/j.1524-4725.2012.02452.x. [DOI] [PubMed] [Google Scholar]
- 28.Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1–17, quiz 18-20. doi: 10.1067/mjd.2002.125579. [DOI] [PubMed] [Google Scholar]
- 29.Carucci JA, Leffell DJ, Pettersen JS. Basal cell carcinoma. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, ed. Fitzpatrick's Dermatology in General Medicine. 8th ed. Columbus: McGraw-Hill, 2012: 1294-1303. [Google Scholar]
- 30.Puig S, Cecilia N, Malvehy J. Dermoscopic criteria and basal cell carcinoma. G Ital Dermatol Venereol. 2012;147:135–40. [PubMed] [Google Scholar]
- 31.Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R. Basal cell carcinoma treated with Mohs surgery in Australia III. Perineural invasion. J Am Acad Dermatol. 2005;53:458–63. doi: 10.1016/j.jaad.2005.04.089. [DOI] [PubMed] [Google Scholar]
- 32.Hakverdi S, Balci DD, Dogramaci CA, Toprak S, Yaldiz M. Retrospective analysis of basal cell carcinoma. Indian J Dermatol Venereol Leprol. 2011;77:251. doi: 10.4103/0378-6323.77483. [DOI] [PubMed] [Google Scholar]
- 33.Gropper AB, Girouard SD, Hojman LP, Huang SJ, Qian X, Murphy GF, et al. Metastatic basal cell carcinoma of the posterior neck: case report and review of the literature. J Cutan Pathol. 2012;39:526–34. doi: 10.1111/j.1600-0560.2012.01871.x. [DOI] [PubMed] [Google Scholar]
- 34.Weinstock MA, Still JM. Assessing current treatment options for patients with severe/advanced basal cell carcinoma. Semin Cutan Med Surg. 2011;30(Suppl):S10–3. doi: 10.1016/j.sder.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Martorell-Calatayud A, Sanmartín Jimenez O, Cruz Mojarrieta J, Guillén Barona C. Cutaneous Squamous Cell Carcinoma: Defining the High-Risk Variant. Actas Dermosifiliogr. 2012 doi: 10.1016/j.adengl.2011.12.012. In Press. [DOI] [PubMed] [Google Scholar]
- 36.Grossman D, Leffell DJ. Squamous cell carcinoma. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, ed. Fitzpatrick's Dermatology in General Medicine. 8th ed. Columbus: McGraw-Hill, 2012: 1283-94. [Google Scholar]
- 37.Lee LA, Huang CG, Liao CT, Lee LY, Hsueh C, Chen TC, et al. Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS One. 2012;7:e40767. doi: 10.1371/journal.pone.0040767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marques-Silva L, Farias LC, Fraga CA, de Oliveira MV, Cardos CM, Fonseca-Silva T, et al. HPV-16/18 detection does not affect the prognosis of head and neck squamous cell carcinoma in younger and older patients. Oncol Lett. 2012;3:945–9. doi: 10.3892/ol.2012.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arron ST, Jennings L, Nindl I, Rosl F, Bouwes Bavinck JN, Seçkin D, et al. Viral Working Group of the International Transplant Skin Cancer Collaborative (ITSCC) & Skin Care in Organ Transplant Patients, Europe (SCOPE) Viral oncogenesis and its role in nonmelanoma skin cancer. Br J Dermatol. 2011;164:1201–13. doi: 10.1111/j.1365-2133.2011.10322.x. [DOI] [PubMed] [Google Scholar]
- 40.Andersson K, Michael KM, Luostarinen T, Waterboer T, Gislefoss R, Hakulinen T, et al. Prospective study of human papillomavirus seropositivity and risk of nonmelanoma skin cancer. Am J Epidemiol. 2012;175:685–95. doi: 10.1093/aje/kwr373. [DOI] [PubMed] [Google Scholar]
- 41.Allam E, Zhang W, Al-Shibani N, Sun J, Labban N, Song F, et al. Effects of cigarette smoke condensate on oral squamous cell carcinoma cells. Arch Oral Biol. 2011;56:1154–61. doi: 10.1016/j.archoralbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Spandau DF, Lewis DA, Somani AK, Travers JB. Fractionated laser resurfacing corrects the inappropriate UVB response in geriatric skin. J Invest Dermatol. 2012;132:1591–6. doi: 10.1038/jid.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Hawary AK, Yassin E, Khater A, Abdelgaber S. Expression of Matrix Metalloproteinase-13 and Ki-67 in Nonmelanoma Skin Cancer in Xeroderma Pigmentosum and Non-xeroderma Pigmentosum. Am J Dermatopathol. 2012 doi: 10.1097/DAD.0b013e31825aa334. In Press. [DOI] [PubMed] [Google Scholar]
- 44.Lonsdorf AS, Becker MR, Stockfleth E, Schäkel K, Ulrich C. [Primary and secondary prevention of skin cancer in organ transplant recipients] Hautarzt. 2010;61:195–206. doi: 10.1007/s00105-009-1858-2. [DOI] [PubMed] [Google Scholar]
- 45.Ritchie SA, Patel MJ, Miller SJ. Therapeutic options to decrease actinic keratosis and squamous cell carcinoma incidence and progression in solid organ transplant recipients: a practical approach. Dermatol Surg. 2012;38:1604–21. doi: 10.1111/j.1524-4725.2012.02452.x. [DOI] [PubMed] [Google Scholar]
- 46.Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354–76. doi: 10.1007/s11864-012-0195-3. [DOI] [PubMed] [Google Scholar]
- 47.Signorell J, Hunziker T, Martinelli M, Koestner SC, Mohacsi PJ. Recurrent non-melanoma skin cancer: remission of field cancerization after conversion from calcineurin inhibitor- to proliferation signal inhibitor-based immunosuppression in a cardiac transplant recipient. Transplant Proc. 2010;42:3871–5. doi: 10.1016/j.transproceed.2010.07.090. [DOI] [PubMed] [Google Scholar]
- 48.Brash DE, Ziegler A, Jonason AS, Simon JA, Kunala S, Leffell DJ. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136–42. [PubMed] [Google Scholar]
- 49.Ziegler A, Jonason A, Simon J, Leffell D, Brash DE. Tumor suppressor gene mutations and photocarcinogenesis. Photochem Photobiol. 1996;63:432–5. doi: 10.1111/j.1751-1097.1996.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 50.Johnson TM, Rowe DE, Nelson BR, Swanson NA. Squamous cell carcinoma of the skin (excluding lip and oral mucosa) J Am Acad Dermatol. 1992;26:467–84. doi: 10.1016/0190-9622(92)70074-P. [DOI] [PubMed] [Google Scholar]
- 51.Alam M, Ratner DN. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–83. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 52.Chollet A, Hohl D, Perrier P. [Risk of cutaneous squamous cell carcinomas: the role of clinical and pathological reports] Rev Med Suisse. 2012;8:743–6. [PubMed] [Google Scholar]
- 53.Nuño-González A, Vicente-Martín FJ, Pinedo-Moraleda F, López-Estebaranz JL. High-risk cutaneous squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:567–78. doi: 10.1016/j.adengl.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Peat B, Insull P, Ayers R. Risk stratification for metastasis from cutaneous squamous cell carcinoma of the head and neck. ANZ J Surg. 2012;82:230–3. doi: 10.1111/j.1445-2197.2011.05994.x. [DOI] [PubMed] [Google Scholar]
- 55.Brougham ND, Dennett ER, Cameron R, Tan ST. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol. 2012 doi: 10.1002/jso.23155. In Press. [DOI] [PubMed] [Google Scholar]
- 56.Situm M, Bulat V, Buljan M, Puljiz Z, Situm V, Bolanca Z. Senile lentigo--cosmetic or medical issue of the elderly population. Coll Antropol. 2010;34(Suppl 2):85–8. [PubMed] [Google Scholar]
- 57.Peter RU, Gottlöber P, Nadeshina N, Krähn G, Plewig G, Kind P. Radiation lentigo. A distinct cutaneous lesion after accidental radiation exposure. Arch Dermatol. 1997;133:209–11. doi: 10.1001/archderm.1997.03890380081012. [DOI] [PubMed] [Google Scholar]
- 58.Grichnik JM, Rhodes AR, Sober AJ. Benign neoplasias and hyperplasias of melanocytes. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, ed. Fitzpatrick's Dermatology in General Medicine. 8th ed. Columbus: McGraw-Hill, 2012: 1377-1410. [Google Scholar]
- 59.Hölzle E. Pigmented lesions as a sign of photodamage. Br J Dermatol. 1992;127(Suppl 41):48–50. doi: 10.1111/j.1365-2133.1992.tb16989.x. [DOI] [PubMed] [Google Scholar]
- 60.Pralong P, Bathelier E, Dalle S, Poulalhon N, Debarbieux S, Thomas L. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280–7. doi: 10.1111/j.1365-2133.2012.10932.x. [DOI] [PubMed] [Google Scholar]
- 61.Kvaskoff M, Siskind V, Green AC. Risk factors for lentigo maligna melanoma compared with superficial spreading melanoma: a case-control study in Australia. Arch Dermatol. 2012;148:164–70. doi: 10.1001/archdermatol.2011.291. [DOI] [PubMed] [Google Scholar]
- 62.Le Gal FA, Toutous-Trellu L, Kaya G, Salomon D. [Lentigo maligna: a special melanoma] Rev Med Suisse. 2011;7:765–6, 768-71. [PubMed] [Google Scholar]
- 63.Situm M, Bolanca Z, Buljan M. Lentigo maligna melanoma--the review. Coll Antropol. 2010;34(Suppl 2):299–301. [PubMed] [Google Scholar]
- 64.Naldi L, Altieri A, Imberti GL, Gallus S, Bosetti C, La Vecchia C, Oncology Study Group of the Italian Group for Epidemiologic Research in Dermatology Sun exposure, phenotypic characteristics, and cutaneous malignant melanoma. An analysis according to different clinico-pathological variants and anatomic locations (Italy) Cancer Causes Control. 2005;16:893–9. doi: 10.1007/s10552-005-2300-4. [DOI] [PubMed] [Google Scholar]
- 65.Walter SD, King WD, Marrett LD. Association of cutaneous malignant melanoma with intermittent exposure to ultraviolet radiation: results of a case-control study in Ontario, Canada. Int J Epidemiol. 1999;28:418–27. doi: 10.1093/ije/28.3.418. [DOI] [PubMed] [Google Scholar]
- 66.Feinmesser M, Tsabari C, Fichman S, Hodak E, Sulkes J, Okon E. Differential expression of proliferation- and apoptosis-related markers in lentigo maligna and solar keratosis keratinocytes. Am J Dermatopathol. 2003;25:300–7. doi: 10.1097/00000372-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka M, Sawada M, Kobayashi K. Key points in dermoscopic differentiation between lentigo maligna and solar lentigo. J Dermatol. 2011;38:53–8. doi: 10.1111/j.1346-8138.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 68.Pralong P, Bathelier E, Dalle S, Poulalhon N, Debarbieux S, Thomas L. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280–7. doi: 10.1111/j.1365-2133.2012.10932.x. [DOI] [PubMed] [Google Scholar]
- 69.Akay BN, Kocyigit P, Heper AO, Erdem C. Dermatoscopy of flat pigmented facial lesions: diagnostic challenge between pigmented actinic keratosis and lentigo maligna. Br J Dermatol. 2010;163:1212–7. doi: 10.1111/j.1365-2133.2010.10025.x. [DOI] [PubMed] [Google Scholar]
- 70.Somach SC, Taira JW, Pitha JV, Everett MA. Pigmented lesions in actinically damaged skin. Histopathologic comparison of biopsy and excisional specimens. Arch Dermatol. 1996;132:1297–302. doi: 10.1001/archderm.1996.03890350035006. [DOI] [PubMed] [Google Scholar]
- 71.Piérard-Franchimont C, Piérard GE. [Melanoma of older subjects] Rev Med Liege. 2011;66:34–40. [PubMed] [Google Scholar]
- 72.Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med. 2011;135:838–41. doi: 10.5858/2011-0051-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 73.Gillen W, Forman SB, Nunley JR, Bhole S, Eliason K, Fox P, et al. Check your skin: insights regarding skin cancer education. J Am Acad Dermatol. 2011;65:427–8, 428, e1. doi: 10.1016/j.jaad.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 74.Surber C, Pittelkow M, Lautenschlager S. Photoprotection in transplant recipients. Curr Probl Dermatol. 2012;43:171–96. doi: 10.1159/000335799. [DOI] [PubMed] [Google Scholar]
- 75.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263–79, quiz 280. doi: 10.1016/j.jaad.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 76.Tessari G, Girolomoni G. Nonmelanoma skin cancer in solid organ transplant recipients: update on epidemiology, risk factors, and management. Dermatol Surg. 2012;38:1622–30. doi: 10.1111/j.1524-4725.2012.02520.x. [DOI] [PubMed] [Google Scholar]
- 77.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Basal Cell and Squamous Cell Skin Cancers. 2011;v.1: Available at http://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf [DOI] [PubMed]
- 78.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology,Melanoma.Version1.2013.http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf
- 79.Feng L, Wang Z. Clinical trials in chemoprevention of head and neck cancers. Rev Recent Clin Trials. 2012;7:249–54. doi: 10.2174/157488712802281349. [DOI] [PubMed] [Google Scholar]
- 80.Kadakia KC, Barton DL, Loprinzi CL, Sloan JA, Otley CC, Diekmann BB, et al. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (North Central Cancer Treatment Group Study 969251) Cancer. 2012;118:2128–37. doi: 10.1002/cncr.26374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szumiło J, Podlodowska J, Podlodowski W, Starosławska E, Burdan F. [Chemoprevention of oral cancer--clinical and experimental studies] Pol Merkur Lekarski. 2012;32:138–42. [PubMed] [Google Scholar]
- 82.Wu PA, Stern RS. Topical tretinoin, another failure in the pursuit of practical chemoprevention for non-melanoma skin cancer. J Invest Dermatol. 2012;132:1532–5. doi: 10.1038/jid.2012.136. [DOI] [PubMed] [Google Scholar]
- 83.Weinstock MA, Bingham SF, Digiovanna JJ, Rizzo AE, Marcolivio K, Hall R, et al. Veterans Affairs Topical Tretinoin Chemoprevention Trial Group Tretinoin and the prevention of keratinocyte carcinoma (Basal and squamous cell carcinoma of the skin): a veterans affairs randomized chemoprevention trial. J Invest Dermatol. 2012;132:1583–90. doi: 10.1038/jid.2011.483. [DOI] [PubMed] [Google Scholar]