Abstract
Restoration of epidermal barrier (epithelialization), is a major component of cutaneous response to stress imposed by wounding. Learning physiologic regulation of epithelialization may lead to novel treatments of chronic wounds. The non-canonical ligands of nicotinic acetylcholine receptors SLURP (secreted mammalian Ly-6/urokinase-type plasminogen activator receptor-related proteins)-1 and -2 are produced by keratinocytes (KCs) and inflammatory cells to augment physiologic responses to non-neuronal acetylcholine, suggesting that they can affect wound epithelialization and inflammation. In this study, recombinant (r)SLURP-1 and -2 exhibited dose dependent effects on migration of cultured KCs, and monoclonal antibodies inactivating auto/paracrine SLURPs in mouse skin delayed wound epithelialization. While effects of rSLURPs on migration were opposite, with rSLURP-1 inhibiting and rSLURP-2 stimulating migration of KCs, each anti-SLURP antibody produced a negative effect on epithelialization in vivo, suggesting their more extensive than regulation of keratinocyte migration involvement in wound repair. Since inflammation plays an important role in stress response to wounding, we measured inflammation biomarkers in wounds treated with anti-SLURP antibodies. Both anti-SLURP-1 and -2 antibodies, or their mixture, caused significant elevation of wound myeloperoxidase, IL-1β, IL-6 and TNFα. Taken together, results of this study demonstrated that SLURP-1 slows crawling locomotion of KCs, and exhibits a strong anti-inflammatory activity in wound tissue. In contrast, SLURP-2 facilitates lateral migration of KCs, but shows a lesser anti-inflammatory capacity. Thus, combined biologic activities of both SLURPs may be required for normal stress response to skin wounding, which favors clinical trial of rSLURP-1 and -2 in wounds that fail to heal.
Keywords: SLURP-1 and -2, keratinocyte migration, mouse skin, wound epithelialization, wound inflammation
Introduction
Restoration of the epidermal barrier, i.e., epithelialization, is a major component of the natural cutaneous response to a stress imposed by wounding. Epithelialization is just one step of a highly orchestrated process of wound repair that involves a multitude of cells and events.1,2 Initial stages (1–3 d post injury) involve the formation of a blood clot, crawling locomotion of epidermal keratinocytes (KCs) over the provisional matrix, and early inflammatory response mediated mainly by neutrophils. At 4–7 d, lymphocytes and macrophages are present in abundance. Formation of new epidermis over the dermal gap of < 2 cm is usually completed by days 10 to12, concomitant to granulation tissue formation. This phase is accompanied by attenuation of the inflammatory response. Subsequent healing stages are characterized by matrix remodeling, differentiation of the newly formed epidermis and increased elastic fiber content.
The main objective of our research is to identify novel approaches to facilitate epithelialization of wounds that fail to heal. Burn injuries, deep erosions, chronic ulcers, diabetic wounds, graft donor sites are but a few of the clinical situations that could benefit from development of novels approaches of pharmacologic control of epithelialization. We and others have generated an overwhelming evidence of the regulatory function of the auto/paracrine cutaneous acetylcholine (ACh) axis in KCs and inflammatory cells,3,4 which connects with the clinical arenas of wound repair. Already completed studies have demonstrated abundance of auto/paracrine of ACh and its receptors in epidermis and illustrated feasibility of treating mucocutaneous erosions and ulcers by a novel pharmacologic approach utilizing cholinergic drugs.5 Pyridostigmine bromide, which produces cholinomimetic effects due to both inhibition of ACh degradation and activation of nicotinic ACh receptors (nAChRs),6,7 healed skin erosion in patients with pemphigus.8,9 This justified the use of the solution of the cholinomimetic pilocarpine for topical treatment of pemphigus erosions.10,11 The nicotinic agonist nicotine has shown its efficacy in local treatment of cutaneous and oral ulcers.12-15 However, nicotine toxicity due to unavoidable off target effects in the neural system precludes its wide use and urges a search for non-toxic nicotinergic drugs that can mimic healing effects of nicotine in mucocutaneous wounds.
In a search for a non-toxic nicotinic agonist, we have identified rapid and profound effects on keratinocyte vital functions of the novel non-canonical ligands of nAChRs secreted mammalian Ly-6/urokinase-type plasminogen activator receptor-related protein (SLURP)-1 and -2 that are produced by skin cells to augment the physiologic effects of ACh at nAChRs.16-18 We cloned both SLURPs, produced recombinant proteins and generated monoclonal antibodies that visualized SLURP-1 and -2 in human epidermis and oral mucosa. In the intact epidermis, expression patterns of SLURP-1 and -2 are somewhat different, with SLURP-1 being predominant in the upper epithelial layers and SLURP-2—in the lower layers.17,18 As expected based on homology between human and murine SLURP molecules, the antibodies raised against human SLURP-1 and -2 visualized their murine counterparts in the epithelializing murine cutaneous wounds.19 Interestingly, KCs comprising epithelial tongues covering the epithelializing wounds abundantly expressed SLURP-2, but very little amounts of SLURP-1. The opposite was observed in perilesional epidermis, consistent with observations that SLURP-2 is important for keratinocyte survival,18 whereas SLURP-1—for keratinocyte maturation and terminal differentiation.17
The cell function and gene expression studies have shown that SLURPs play important roles in regulating vital functions of KCs and inflammatory cells,16,20-23 suggesting that during wound repair they may control both epithelialization and inflammation. Indeed, pilot studies showed feasibility to accelerate wound epithelialization due to synergistic regulation by recombinant (r)SLURP-1 and -2 of the integrin expression in KCs.19 rSLURP-1 upregulated expression of the “sedentary” integrins α2 and α3, and rSLURP-2 that of the “migratory” integrins α5 and αV. The effect of rSLURP-1 was predominantly mediated by keratinocyte α7 nAChR and that of rSLURP-2 by non-α7, such as α3 and α9, nAChRs. Since these same receptors modulate expression of the SLURP-1 and -2 genes,24 it appears that cutaneous stress response to wounding is mediated, in part, by a reciprocally arranged auto/paracrine cholinergic network.
In this study, we ultimately demonstrated the pharmacologic mechanism of action of rSLURP-1 and -2 on the crawling locomotion of human epidermal KCs in vitro, and identified a novel mechanism of protective action of the auto/paracrine SLURPs during cutaneous stress response to wounding mediated by their anti-inflammatory action. Taken together, these findings should justify a trial of rSLURP-1 and -2 in the treatment of chronic wounds.
Results
Dose dependent effects of rSLURP-1 and -2 on keratinocyte migration under agarose
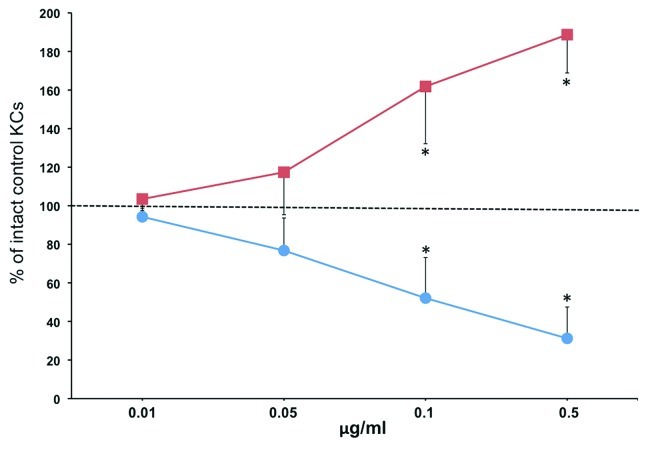
To determine direct effects of the auto/paracrine nicotinergic peptides SLURP-1 and -2 on lateral migration of KCs, we used the agarose gel keratinocyte outgrowth system (AGKOS), that proved to be a sensitive and reliable in vitro system for assessing pharmacologic effects of test compounds on keratinocyte activities mediating wound epithelialization.25 To ascertain that the observed changes in the migration distance of KCs under agarose are mediated by the specific, i.e., pharmacologic, effects of rSLURPs, each recombinant protein was used in the increasing concentrations ranging from 0.01 to 0.5 μg/ml. While rSLURP-1 decreased, rSLURP-2 increased the keratinocyte outgrowth rate in the dose-dependent fashion (Fig. 1). The differences from control became significant (p < 0.05) at the 0.1 μg/ml concentration of each rSLURP protein.
Figure 1. rSLURP-1 and -2 exhibit opposite effects on lateral migration of human KCs under agarose. Normal human KCs were seeded in the 3 mm wells of the AGKOS plates measuring random cell migration, exposed to increasing concentrations of rSLURP-1 (-●-) or -2 (-■-) dissolved in growth medium, and incubated for 10 d with daily refreshing the media. Migration distance was measured as described in Materials and Methods. Migration distance of control KCs incubated in growth medium without rSLURPs was taken as 100%, and the results expressed as percent of intact controls. Asterisks = p < 0.05 compared with control.
These results suggested that auto/paracrine SLURP-1 and -2 exhibit reciprocal regulation of lateral migration of KCs during cutaneous stress response to wounding.
Delayed epithelialization of cutaneous wounds treated with by anti-SLURP antibodies
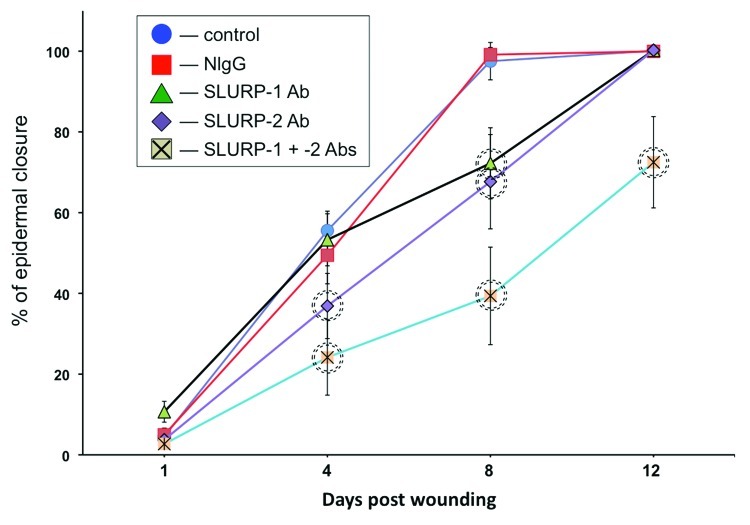
Next, we tested the hypothesis auto/paracrine SLURP proteins would exhibit opposite effects on wound epithelialization in vivo. We measured changes in the rate of epithelialization of excisional cutaneous wounds in BALB/c mice treated daily with microinjections of the antibodies to SLURP-1 and/or -2 that recognize their respective target SLURP proteins in murine skin.19 While the control mice had their wounds fully epithelialized by the 8th day post wounding, the experimental mice treated with anti-SLURP antibodies showed delay in epidermal closure (Fig. 2). The observed alterations in the epithelialization rate were specific to inactivation of endogenous SLURPs, because microinjections of normal mouse IgG (NIgG) did not cause any delays at same time points.
Figure 2. Anti-SLURP antibodies interfere with wound epithelialization. The excisional wounds in the skin of BALB/c mice were treated daily with microinjections of 10 μg/ml of anti-SLURP-1 or -2 antibodies or their mixture vs. 10 μg/ml of NIgG vs. plain saline (control) for the periods of time indicated in the graph. The wound epithelialization rate was measured as detailed in Materials and Methods. The percentage of epidermal closure was calculated as a ratio of the epithelialized to the initial wound area. Circles = p < 0.05 compared with control.
During the first 4 d post wounding, wounds treated with anti-SLURP-1 antibody healed as fast as control wounds, but exhibited a significant (p < 0.05) delay on the 8th day. In contrast, significantly (p < 0.05) inhibited epithelialization rate of the wounds treated with anti-SLURP-2 antibody was observed on both the 4th and the 8th days post wounding. A combination of both antibodies dramatically deteriorated epithelialization. The wounds treated with a mixture of anti-SLURP-1 and -2 antibodies failed to heal by the time points the wounds treated with each antibody separately had been complete covered by new epidermis (Fig. 2).
These observations indicated that despite their opposite effects on lateral migration of KCs, auto/paracrine functions of SLURP-1 and -2 during the cutaneous stress response to wounding extend beyond regulation of keratinocyte motility.
Increased inflammation of cutaneous wounds treated with anti-SLURP antibodies
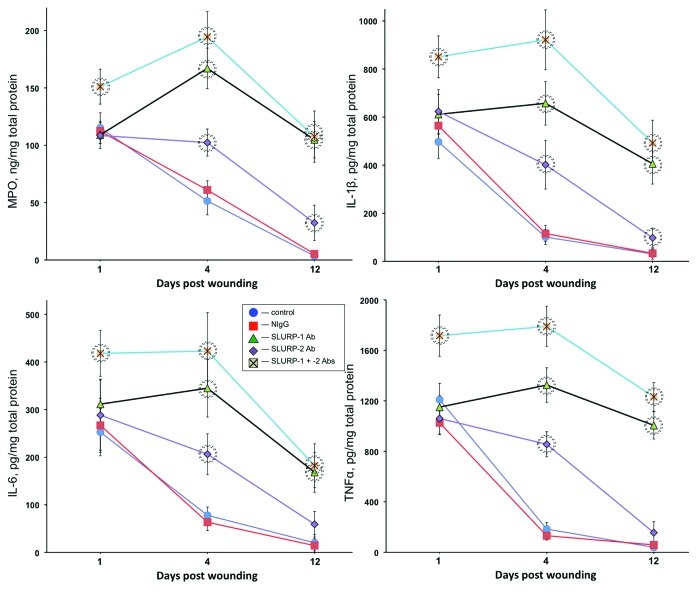
Since inflammation plays an important role in the cutaneous stress response to wounding,26-28 we measured inflammation biomarkers in wounds treated with anti-SLURP antibodies. The intensity of inflammation was judged from the amounts of myeloperoxidase (MPO), IL-1β, IL-6 and TNFα in the homogenates of excised wound tissues at 1, 4 and 12 d post wounding. Microinjections of anti-SLURP-1 or -2 antibody or their mixture caused significant (p < 0.05) elevation of all biomarkers on the 4th day post wounding (Fig. 3). By the 12th day, the amounts of IL-6 and TNFα in the wounds treated with anti-SLURP-2 antibodies decreased to the normal range and those of MPO and IL-1β were close to, yet significantly (p < 0.05) different from norm. Wounds treated with anti-SLURP-1 antibody or a combination of both anti-SLURP antibodies featured significantly (p < 0.05) increased levels of inflammation throughout the entire observation period, with the highest degree of inflammation observed in wounds treated with a mixture of both anti-SLURP antibodies (Fig. 3).
Figure 3. Anti-SLURP antibodies increase inflammation of cutaneous wounds. The murine cutaneous wounds treated as described in the legend to Figure 2 were harvested, homogenized and used in the ELISA assays of the biomarkers of inflammation MPO, IL-1β. IL-6 and TNFα. Circles = p < 0.05 compared with control.
These results indicated that auto/paracrine SLURPs control the inflammatory component of cutaneous stress response to wounding, and that SLURP-1 exhibits a stronger anti-inflammatory activity, compared with SLURP-2.
Discussion
In this study, we demonstrated for the first time that both nicotinergic peptides SLURP-1 and -2 produce in a complementary fashion a protective action against cutaneous stress caused by wounding, with each protein playing a distinctive biologic role. While SLURP-1 slows down crawling locomotion of KCs, it exhibits a strong anti-inflammatory activity in wound tissue. On the other hand, SLURP-2 facilitates lateral migration of KCs, but has a lesser anti-inflammatory capacity. Thus, combined biologic activities of both SLURPs appear to be required for normal epidermal closure.
Results of our previously completed studies suggested that auto/paracrine SLURP-1 and -2 exhibit reciprocal effects on the migratory function of KCs during wound epithelialization,19 Since accelerated migration of the KCs re-epithelializing a wound plays a pivotal role in wound repair,29 we determined in this study the dose-dependence of keratinocyte response to rSLURP-1 and -2 in vitro. The obtained results demonstrated the specificity of rSLURP actions, and suggested that anti-SLURP-2, but not anti-SLURP-1, antibody should delay epithelialization of skin wounds. However, despite opposite effects of rSLURP-1 and -2 on lateral migration of KCs in the AGKOS assay, simultaneous inhibition of auto/paracrine functions of SLURP-1 and -2 in skin wounds with a mixture of anti-SLURP-1 and -2 antibodies delayed epithelialization even more than the anti-SLURP-2 antibody given alone. This observation indicated that during the cutaneous stress response to wounding, the physiologic actions of auto/paracrine SLURPs extend beyond regulation of crawling locomotion of KCs. Hence, we hypothesized that SLURP-1 and -2 facilitate epidermal closure also by controlling inflammation.
In addition to extracellular matrix proteins and auto/paracrine growth factors, keratinocyte migration in wound bed is controlled by a large variety of cytokines and chemokines released by inflammatory cells, which can either stimulate or inhibit epithelialization.30-35 The role of inflammation in wound healing remains controversial. Although inflammation is an essential step of normal wound repair,26-28 the extent of wound inflammation appears to inversely correlate with the epithelialization rate.2,35,36 For instance, skin wounds in diabetic mice feature robust inflammation2,37 in association with overabundance of proliferating epidermis at the wound edge and insufficient migration of KCs, resulting in delayed epithelialization. Noteworthy, KCs themselves produce cytokines and chemokines that can attract inflammatory cells.38-40 Thus, the two processes—epithelialization and inflammation—are mutually interdependent and inseparable in wound repair. Therefore, to adequate evaluate the therapeutic potential of rSLURPs in chronic wounds, we measured the effect of inactivation of auto/paracrine SLURPs with specific antibodies on the degree of wound inflammation.
Treatments of cutaneous wounds with the antibodies inhibiting biologic activities of auto/paracrine SLURPs increased the amounts of inflammatory molecules in the wound specimens, indicating that these nicotinergic peptides indeed control an inflammatory arm of the cutaneous stress response to wounding. This observation is not surprising, because previous studies have documented that nicotinergic signaling inhibits cutaneous neutrophil infiltration,41 and pro-inflammatory functions of macrophages, and T- and B-cells.42-44 Functional inactivation of auto/paracrine SLURP-1 has a greater impact on the cutaneous immunoregulatory activity, in keeping with a report that patients with genetic SLURP-1 mutation have defective T-cell function.23 Indeed, both SLURP-1 and SLURP-2 are expressed in various immune cells and organs, and appear to be involved in regulating lymphocyte function via the nAChR-mediated pathways.22 Thus, rSLURPs could mimic both a stimulatory effect of the nAChR agonist nicotine on wound epithelialization45 and its inhibitory effect on wound inflammation,46-49 perhaps, because both SLURP peptides, just like nicotine, bind to and activate the same nAChR subtypes.17,18,22,50-52 However, in contrast to nicotine that activates all nAChR subtypes, the specific effects of each SLURP differ, because they preferentially activate distinct nAChRs that are selectively coupled to unique regulation of the epithelial and inflammatory cell functions.41,53-55
In conclusion, the results of this study established auto/paracrine SLURPs as physiologic regulators of cutaneous stress response to wounding and suggested that rSLURPs may become a prototype drugs for pharmacologic control of wounds that fail to heal. Therapeutic use of rSLURPs should avoid the systemic toxicity of the canonical agonists of nAChRs, such as nicotine, because SLURPs are cytokine-like physiologic substances normally present in blood, saliva, skin, sweat and urine.16-18,20,56
Materials and Methods
Chemicals
The full length rSLURP-1 and rSLURP-2 were manufactured at Virusys Corporation, as detailed by us before.57 Agarose type A was obtained from Accurate Chemical and Scientific Corporation. The anti-SLURP-1 and -2 monoclonal antibodies 336H12–1A3 and 341F10–1F12, respectively, characterized by us in previous studies,17,18 were from Research and Diagnostic Antibodies. NIgG was obtained from Santa Cruz Biotechnology, Inc. The ELISA kit for measuring mouse MPO was purchased from Hycult Biotech, IL-1β and IL-6 from Abcam, Inc. and TNFα from Thermo Fisher Scientific.
Keratinocyte migration assay
Human KCs were purchased from Invitrogen Life Technologies and grown in the serum-free keratinocyte growth medium containing 5 ng/ml EGF and 50 μg/ml bovine pituitary extract (GIBCO-BRL) in accordance to the protocol provided by the vendor. KCs were used in experiments between passages 2 and 4, grown in growth medium (GM) to approximately 80% confluence. The rate of epithelialization in vitro was measured by the random migration distance in AGKOS, as detailed by us elsewhere.58,59 Briefly, a confluent keratinocyte monolayer was formed by loading KCs at a high density (4 × 104 cells/10 μl) into 3 mm well in an agarose gel and incubating in GM to allow the cells to adhere to the dish bottom and form intercellular junctions. The cultures were incubated for 10 d in a humid CO2 incubator with daily changes of GM containing test concentrations of rSLURP-1 or -2 vs. GM given alone (control). To standardize results obtained in different experiments, the mean values of the migration distances were converted into the percentage of control. The control value in each experiment was determined by measuring the baseline migration distance (in μm) and taken as 100%.
In vivo wounding and morphometric and biochemical analyses of cutaneous wounds
This study was approved by the University California-Irvine Animal Care and Use Committee. Assay of the skin wound epithelialization rate was performed by histomorphometric analysis in accordance to our previously published protocol.25,60 Briefly, using a 5 mm in diameter Healthlink Biopsy Punch (MedPlusUSA Medical Supplies), full thickness excisions through the panniculus carnosus were made in the anesthetized skin of 6–7 weeks old BALB/c mice (The Jackson Laboratory), in whom the hair cycle had been synchronized by the anagen induction technique.61 Each animal received 2 wounds at the symmetric sites of the central back, 0.5 cm off the vertebral line. Wounds were dressed with the Tegaderm™ (3M™) and wounded animals were individually housed under aseptic conditions. Starting 1 h after wounding, we initiated daily treatments of experimental wounds by microinjections of 100 μl saline containing 1 μg of anti-SLURP-1 and/or -2 antibody. The wounds in control animals were similarly treated with plain saline solution alone or containing 1 μg of NIgG. Both experimental and control mice were euthanized on days 1, 4, 8 and 12 post wounding, and the wounds with 1 mm margin of surrounding skin were excised. One wound specimen from each animal was fixed, stained and used for the morphometric assay of epithelialization rate, whereas another one was freshly frozen and stored at -80°C until use in biochemical assays. The epithelialization rate was estimated by measuring the lengths of the tongues of new epithelium extending from either side of the central portion of the wound. The percentage epidermal closure was calculated as a ratio of epithelialized wound area (i.e., covered by new epithelium) to initial wound area that was identified by an abrupt change in the epidermal and dermal morphology at the incision site.62-64 For the ELISA assays of MPO, IL-1β, IL-6 and TNFα, the wound tissue was homogenized using TissueRuptor (Qiagen Inc.) and processed in accordance to the manufacturers’ protocols. The results were expressed as absolute values normalized to the total amount of protein determined by the Quick Start™ Bradford protein assay (BioRad Laboratories). At least 3 animals per time point were used in each experiment.
Statistical analysis
Statistical significance was determined using the Student’s t-test. The differences were deemed as significant when the calculated p value was < 0.05.
Acknowledgments
This work was supported by the NIH grant GM62136 S.A.G.
Glossary
Abbreviations:
- ACh
acetylcholine
- AGKOS
agarose gel keratinocyte outgrowth system
- GM
growth medium
- KCs
keratinocytes
- MPO
myeloperoxidase
- nAChR
nicotinic acetylcholine receptor
- NIgG
normal mouse IgG
- SLURP
secreted mammalian Ly-6/urokinase-type plasminogen activator receptor-related protein
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/dermatoendocrinology/article/22594
References
- 1.Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674–86, discussion 686. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 2.Braiman-Wiksman L, Solomonik I, Spira R, Tennenbaum T. Novel insights into wound healing sequence of events. Toxicol Pathol. 2007;35:767–79. doi: 10.1080/01926230701584189. [DOI] [PubMed] [Google Scholar]
- 3.Grando SA. Cholinergic control of epidermal cohesion. Exp Dermatol. 2006;15:265–82. doi: 10.1111/j.0906-6705.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 4.Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Metab Res. 2007;39:125–35. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- 5.Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126:1948–65. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- 6.Taylor P. Anticholinesterase agents. In: Gilman AG, Goodman LS, Rall TW, Murad F, editors. Goodman and Gilman's Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan, 1985:110-27. [Google Scholar]
- 7.Akaike A, Ikeda SR, Brookes N, Pascuzzo GJ, Rickett DL, Albuquerque EX. The nature of the interactions of pyridostigmine with the nicotinic acetylcholine receptor-ionic channel complex. II. Patch clamp studies. Mol Pharmacol. 1984;25:102–12. [PubMed] [Google Scholar]
- 8.Grando SA. New approaches to the treatment of pemphigus. J Investig Dermatol Symp Proc. 2004;9:84–91. doi: 10.1111/j.1087-0024.2004.00826.x. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen VT, Arredondo J, Chernyavsky AI, Pittelkow MR, Kitajima Y, Grando SA. Pemphigus vulgaris acantholysis ameliorated by cholinergic agonists. Arch Dermatol. 2004;140:327–34. doi: 10.1001/archderm.140.3.327. [DOI] [PubMed] [Google Scholar]
- 10.Namazi MR. Practice pearl: gargling with cholinergic ophthalmic drops for treating the oral lesions of pemphigus vulgaris. J Drugs Dermatol. 2004;3:484–5. [PubMed] [Google Scholar]
- 11.Iraji F, Yoosefi A. Healing effect of Pilocarpine gel 4% on skin lesions of pemphigus vulgaris. Int J Dermatol. 2006;45:743–6. doi: 10.1111/j.1365-4632.2006.02766.x. [DOI] [PubMed] [Google Scholar]
- 12.Patel GK, Rhodes JR, Evans B, Holt PJ. Successful treatment of pyoderma gangrenosum with topical 0.5% nicotine cream. J Dermatolog Treat. 2004;15:122–5. doi: 10.1080/09546630310019364. [DOI] [PubMed] [Google Scholar]
- 13.Hill SC, Stavrakoglou A, Coutts IR. Nicotine replacement therapy as a treatment for complex aphthosis. J Dermatolog Treat. 2010;21:317–8. doi: 10.3109/09546630903271563. [DOI] [PubMed] [Google Scholar]
- 14.Kaklamani VG, Markomichelakis N, Kaklamanis PG. Could nicotine be beneficial for Behçet’s disease? Clin Rheumatol. 2002;21:341–2. doi: 10.1007/s100670200090. [DOI] [PubMed] [Google Scholar]
- 15.Scheid P, Bohadana A, Martinet Y. Nicotine patches for aphthous ulcers due to Behçet’s syndrome. N Engl J Med. 2000;343:1816–7. doi: 10.1056/NEJM200012143432418. [DOI] [PubMed] [Google Scholar]
- 16.Chimienti F, Hogg RC, Plantard L, Lehmann C, Brakch N, Fischer J, et al. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet. 2003;12:3017–24. doi: 10.1093/hmg/ddg320. [DOI] [PubMed] [Google Scholar]
- 17.Arredondo J, Chernyavsky AI, Webber RJ, Grando SA. Biological effects of SLURP-1 on human keratinocytes. J Invest Dermatol. 2005;125:1236–41. doi: 10.1111/j.0022-202X.2005.23973.x. [DOI] [PubMed] [Google Scholar]
- 18.Arredondo J, Chernyavsky AI, Jolkovsky DL, Webber RJ, Grando SA. SLURP-2: A novel cholinergic signaling peptide in human mucocutaneous epithelium. J Cell Physiol. 2006;208:238–45. doi: 10.1002/jcp.20661. [DOI] [PubMed] [Google Scholar]
- 19.Chernyavsky AI, Kalantari-Dehaghi M, Phillips C, Marchenko S, Grando SA. Novel cholinergic peptides SLURP-1 and -2 regulate epithelialization of cutaneous and oral wounds. Wound Repair Regen. 2012;20:103–13. doi: 10.1111/j.1524-475X.2011.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mastrangeli R, Donini S, Kelton CA, He C, Bressan A, Milazzo F, et al. ARS Component B: structural characterization, tissue expression and regulation of the gene and protein (SLURP-1) associated with Mal de Meleda. Eur J Dermatol. 2003;13:560–70. [PubMed] [Google Scholar]
- 21.Tsuji H, Okamoto K, Matsuzaka Y, Iizuka H, Tamiya G, Inoko H. SLURP-2, a novel member of the human Ly-6 superfamily that is up-regulated in psoriasis vulgaris. Genomics. 2003;81:26–33. doi: 10.1016/S0888-7543(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 22.Moriwaki Y, Yoshikawa K, Fukuda H, Fujii YX, Misawa H, Kawashima K. Immune system expression of SLURP-1 and SLURP-2, two endogenous nicotinic acetylcholine receptor ligands. Life Sci. 2007;80:2365–8. doi: 10.1016/j.lfs.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Tjiu JW, Lin PJ, Wu WH, Cheng YP, Chiu HC, Thong HY, et al. SLURP1 mutation-impaired T-cell activation in a family with mal de Meleda. Br J Dermatol. 2011;164:47–53. doi: 10.1111/j.1365-2133.2010.10059.x. [DOI] [PubMed] [Google Scholar]
- 24.Arredondo J, Chernyavsky AI, Grando SA. SLURP-1 and -2 in normal, immortalized and malignant oral keratinocytes. Life Sci. 2007;80:2243–7. doi: 10.1016/j.lfs.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chernyavsky AI, Arredondo J, Wess J, Karlsson E, Grando SA. Novel signaling pathways mediating reciprocal control of keratinocyte migration and wound epithelialization through M3 and M4 muscarinic receptors. J Cell Biol. 2004;166:261–72. doi: 10.1083/jcb.200401034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Arbieva ZH, Guo S, Marucha PT, Mustoe TA, DiPietro LA. Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics. 2010;11:471. doi: 10.1186/1471-2164-11-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart J. Inflammation. 1: Its role in the healing of acute wounds. J Wound Care. 2002;11:205–9. doi: 10.12968/jowc.2002.11.6.26411. [DOI] [PubMed] [Google Scholar]
- 28.Tsirogianni AK, Moutsopoulos NM, Moutsopoulos HM. Wound healing: immunological aspects. Injury. 2006;37(Suppl 1):S5–12. doi: 10.1016/j.injury.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 29.Woodley DT. Reepithelialization. In: Clark RAF, editor. The Molecular and Cellular Biology of Wound Repair. 2nd ed. New York: Plenum Press, 1996:339-54. [Google Scholar]
- 30.Li W, Fan J, Chen M, Woodley DT. Mechanisms of human skin cell motility. Histol Histopathol. 2004;19:1311–24. doi: 10.14670/HH-19.1311. [DOI] [PubMed] [Google Scholar]
- 31.Zaja-Milatovic S, Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol. 2008;23:1399–407. doi: 10.14670/hh-23.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry G, Garner WL. Inflammatory mediators in wound healing. Surg Clin North Am. 2003;83:483–507. doi: 10.1016/S0039-6109(02)00200-1. [DOI] [PubMed] [Google Scholar]
- 33.Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–25. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 35.Szpaderska AM, DiPietro LA. Inflammation in surgical wound healing: friend or foe? Surgery. 2005;137:571–3. doi: 10.1016/j.surg.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound: adding insult to injury? Thromb Haemost. 2004;92:275–80. doi: 10.1160/TH03-11-0720. [DOI] [PubMed] [Google Scholar]
- 37.Goren I, Kämpfer H, Podda M, Pfeilschifter J, Frank S. Leptin and wound inflammation in diabetic ob/ob mice: differential regulation of neutrophil and macrophage influx and a potential role for the scab as a sink for inflammatory cells and mediators. Diabetes. 2003;52:2821–32. doi: 10.2337/diabetes.52.11.2821. [DOI] [PubMed] [Google Scholar]
- 38.Uchi H, Terao H, Koga T, Furue M. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(Suppl 1):S29–38. doi: 10.1016/S0923-1811(00)00138-9. [DOI] [PubMed] [Google Scholar]
- 39.Gröne A. Keratinocytes and cytokines. Vet Immunol Immunopathol. 2002;88:1–12. doi: 10.1016/S0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 40.Esche C, de Benedetto A, Beck LA. Keratinocytes in atopic dermatitis: inflammatory signals. Curr Allergy Asthma Rep. 2004;4:276–84. doi: 10.1007/s11882-004-0071-8. [DOI] [PubMed] [Google Scholar]
- 41.Gahring LC, Osborne AV, Reed M, Rogers SW. Neuronal nicotinic alpha7 receptors modulate early neutrophil infiltration to sites of skin inflammation. J Neuroinflammation. 2010;7:38. doi: 10.1186/1742-2094-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chernyavsky AI, Arredondo J, Galitovskiy V, Qian J, Grando SA. Structure and function of the nicotinic arm of acetylcholine regulatory axis in human leukemic T cells. Int J Immunopathol Pharmacol. 2009;22:461–72. doi: 10.1177/039463200902200223. [DOI] [PubMed] [Google Scholar]
- 43.Arredondo J, Omelchenko DM, Chernyavsky AI, Qian J, Skok M, Grando SA. Functional role of the nicotinic arm of the acetylcholine regulatory axis in human B-cell lines. J Exp Pharmacol. 2009;1:1–7. doi: 10.2147/jep.s7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chernyavsky AI, Arredondo J, Skok M, Grando SA. Auto/paracrine control of inflammatory cytokines by acetylcholine in macrophage-like U937 cells through nicotinic receptors. Int Immunopharmacol. 2010;10:308–15. doi: 10.1016/j.intimp.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morimoto N, Takemoto S, Kawazoe T, Suzuki S. Nicotine at a low concentration promotes wound healing. J Surg Res. 2008;145:199–204. doi: 10.1016/j.jss.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Mills CM, Hill SA, Marks R. Transdermal nicotine suppresses cutaneous inflammation. Arch Dermatol. 1997;133:823–5. doi: 10.1001/archderm.1997.03890430023004. [DOI] [PubMed] [Google Scholar]
- 47.Osborne-Hereford AV, Rogers SW, Gahring LC. Neuronal nicotinic alpha7 receptors modulate inflammatory cytokine production in the skin following ultraviolet radiation. J Neuroimmunol. 2008;193:130–9. doi: 10.1016/j.jneuroim.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–23. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sørensen LT, Toft B, Rygaard J, Ladelund S, Teisner B, Gottrup F. Smoking attenuates wound inflammation and proliferation while smoking cessation restores inflammation but not proliferation. Wound Repair Regen. 2010;18:186–92. doi: 10.1111/j.1524-475X.2010.00569.x. [DOI] [PubMed] [Google Scholar]
- 50.Chernyavsky AI, Arredondo J, Galitovskiy V, Qian J, Grando SA. Upregulation of nuclear factor-kappaB expression by SLURP-1 is mediated by α7-nicotinic acetylcholine receptor and involves both ionic events and activation of protein kinases. Am J Physiol Cell Physiol. 2010;299:C903–11. doi: 10.1152/ajpcell.00216.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horiguchi K, Horiguchi S, Yamashita N, Irie K, Masuda J, Takano-Ohmuro H, et al. Expression of SLURP-1, an endogenous alpha7 nicotinic acetylcholine receptor allosteric ligand, in murine bronchial epithelial cells. J Neurosci Res. 2009;87:2740–7. doi: 10.1002/jnr.22102. [DOI] [PubMed] [Google Scholar]
- 52.Moriwaki Y, Watanabe Y, Shinagawa T, Kai M, Miyazawa M, Okuda T, et al. Primary sensory neuronal expression of SLURP-1, an endogenous nicotinic acetylcholine receptor ligand. Neurosci Res. 2009;64:403–12. doi: 10.1016/j.neures.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80:2314–9. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 54.Grando SA. Basic and clinical aspects of non-neuronal acetylcholine: biological and clinical significance of non-canonical ligands of epithelial nicotinic acetylcholine receptors. J Pharmacol Sci. 2008;106:174–9. doi: 10.1254/jphs.FM0070087. [DOI] [PubMed] [Google Scholar]
- 55.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–71. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Favre B, Plantard L, Aeschbach L, Brakch N, Christen-Zaech S, de Viragh PA, et al. SLURP1 is a late marker of epidermal differentiation and is absent in Mal de Meleda. J Invest Dermatol. 2007;127:301–8. doi: 10.1038/sj.jid.5700551. [DOI] [PubMed] [Google Scholar]
- 57.Chernyavsky AI, Arredondo J, Putney DJ, Marsh JS, Grando SA. Potential role for epithelial nicotinic receptors in tobacco related oral and lung cancers. J Stomatol Invest. 2008;2:5–14. [Google Scholar]
- 58.Grando SA, Crosby AM, Zelickson BD, Dahl MV. Agarose gel keratinocyte outgrowth system as a model of skin re-epithelization: requirement of endogenous acetylcholine for outgrowth initiation. J Invest Dermatol. 1993;101:804–10. doi: 10.1111/1523-1747.ep12371699. [DOI] [PubMed] [Google Scholar]
- 59.Chernyavsky AI, Arredondo J, Marubio LM, Grando SA. Differential regulation of keratinocyte chemokinesis and chemotaxis through distinct nicotinic receptor subtypes. J Cell Sci. 2004;117:5665–79. doi: 10.1242/jcs.01492. [DOI] [PubMed] [Google Scholar]
- 60.Chernyavsky AI, Arredondo J, Vetter DE, Grando SA. Central role of α9 acetylcholine receptor in coordinating keratinocyte adhesion and motility at the initiation of epithelialization. Exp Cell Res. 2007;313:3542–55. doi: 10.1016/j.yexcr.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paus R, Stenn KS, Link RE. Telogen skin contains an inhibitor of hair growth. Br J Dermatol. 1990;122:777–84. doi: 10.1111/j.1365-2133.1990.tb06266.x. [DOI] [PubMed] [Google Scholar]
- 62.Rico RM, Ripamonti R, Burns AL, Gamelli RL, DiPietro LA. The effect of sepsis on wound healing. J Surg Res. 2002;102:193–7. doi: 10.1006/jsre.2001.6316. [DOI] [PubMed] [Google Scholar]
- 63.Fitzgerald DJ, Radek KA, Chaar M, Faunce DE, DiPietro LA, Kovacs EJ. Effects of acute ethanol exposure on the early inflammatory response after excisional injury. Alcohol Clin Exp Res. 2007;31:317–23. doi: 10.1111/j.1530-0277.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 64.Keylock KT, Vieira VJ, Wallig MA, DiPietro LA, Schrementi M, Woods JA. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R179–84. doi: 10.1152/ajpregu.00177.2007. [DOI] [PubMed] [Google Scholar]