Abstract
Although it has been shown in murine models that chemoradiotherapy may induce immunogenic tumor cell death, which could trigger T-cell immunity upon the released of high-mobility group box 1 protein (HMGB1), whether this also occurs in clinical settings remains unclear. Here, we discuss tumor-antigen specific T-cell responses in esophageal cancer patients receiving chemoradiotherapy. Our findings indicate that chemoradiation induces tumor antigen-specific T-cell responses and that the release of HMGB1 is related to clinical outcome.
Keywords: cancer-testis antigens, calreticulin, chemoradiation, esophageal squamous cell carcinoma, HMGB1, immunogenic cell death
Chemoradiotherapy (CRT) is thought to induce an immunosuppressive state involving both T cell- and natural killer (NK) cell-immunity. Moreover, CRT mediates its cytotoxic effects by inducing apoptosis, a form of cell death that is generally considered to be non-inflammatory and non-immunogenic.1,2 Recently, it has been shown that danger signals emitted by dying cells treated by specific chemotherapeutic drugs, such as oxaliplatin, or radiotherapy can induce Toll-like receptor (TLR)-dependent, antigen-specific T-cell immunity. In particular, the injection of tumor cells succumbing to chemoradiation has been shown to induced a protective antitumor immune response in wild type, but inTlr4 −/− mice.3,4 Among various danger signals released from dying cells, high-mobility group box 1 protein (HMGB1), but not other known TLR4 ligands, has been suggested to be a mandatory for such tumor antigen-specific T-cell responses.3,4 In addition, the early exposure of calreticulin on the plasma membrane of dying cancer cells, as induced by irradiation, reportedly enhances their phagocytosis by dendritic cells (DCs) and is required for tumor-specific T-cell responses in murine model.5-7 However, there is limited information describing whether immunogenic tumor cell death can be induced by CRT in clinical settings, due to the lack of accurate assays to evaluate antigen-specific T-cell responses in cancer patients.
We have recently established a reliable in vitro assay system based on peripheral blood lymphocytes (PBLs) to detect tumor-specific CTL responses against the panels of HLA Class I epitopes derived from cancer-testis antigens.8,9 By this method, we have shown for the first time in a human clinical study that tumor-antigen specific T-cell responses were induced in esophageal squamous cell carcinoma (ESCC) patients following chemoradiation, along with elevated HMGB1 levels in serum.10 Furthermore, our study clearly demonstrated that the presence of HMGB1 within the tumor microenvironment is significantly related to pre-operative CRT and that the levels of HMGB1 positively correlate with survival. Furthermore, we observed that the amount of CD8+ T-cells infiltrating the tumor microenvironment was comparatively higher in patients with high intratumoral levels of HMGB1.
Thus, immunogenic tumor cell death was induced by CRT in patients with ESCC, and HMGB1 turned out to be one important mediator linking chemoradiation-induced cell death to antigen-specific T-cell responses (Fig. 1). However, although it has been shown that both HMGB1 release and calreticulin expression are required for tumor-specific T-cell responses in murine models,5-7 we were unable to detect any significant differences in calreticulin exposure by the tumor cells of patients receiving or not chemoradiation, and there was no survival difference between calreticulin-strong and -weak groups. To clarify this aspect, further study is needed that is based upon a different technique to evaluate the cell surface exposure of calreticulin in clinical samples.
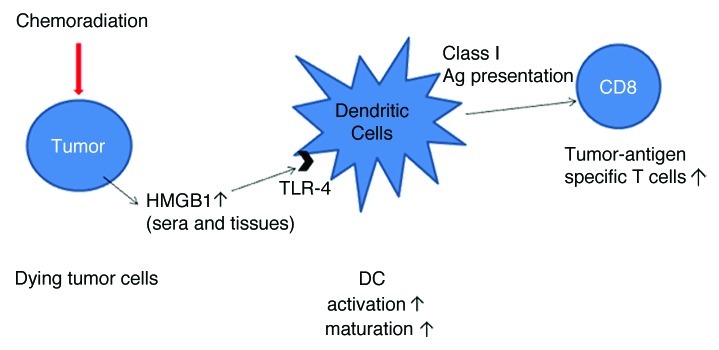
Figure 1. Schematic illustration of immunogenic tumor cell death as induced by chemoradiation. HMGB1, high-mobility group box 1; TLR, Toll-like receptor.
Interestingly, we showed that chemoradiation can induce the upregulation of HMGB1, with significant variations among ESCC patients, and that patients with high HMGB1 expression survived longer than patients with weak HMGB1 expression.10 Also, our in vitro studies indicate that there are substantial variations in chemoradiation-induced HMGB1 release among distinct ESCC cell lines, regardless of the amount of dying cells.10 These observations suggest that immune reactions related to HMGB1 release following chemoradiation may affect clinical outcomes in ESCC patients. Apetoh, et al. reported that patients with breast cancer bearing a TLR4 loss-of-function allele relapse more quickly after chemotherapy and radiotherapy than those with a normal TLR4 allele.4 Thus, HMGB1-related immune responses after CRT may play a critical role in the clinical outcome of cancer patients, and parameters such as HMGB1 expression levels and TLR polymorphisms may be able to predict clinical outcome after chemoradiation.
In conclusion, our study strongly suggests that tumor antigen-specific T-cell responses are induced following chemoradiation and that HMGB1 release is related to clinical outcome upon CRT.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22197
References
- 1.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–88. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 2.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 3.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 4.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 5.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100–4. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 6.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–91. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 7.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 8.Mizukami Y, Kono K, Daigo Y, Takano A, Tsunoda T, Kawaguchi Y, et al. Detection of novel cancer-testis antigen-specific T-cell responses in TIL, regional lymph nodes, and PBL in patients with esophageal squamous cell carcinoma. Cancer Sci. 2008;99:1448–54. doi: 10.1111/j.1349-7006.2008.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kono K, Mizukami Y, Daigo Y, Takano A, Masuda K, Yoshida K, et al. Vaccination with multiple peptides derived from novel cancer-testis antigens can induce specific T-cell responses and clinical responses in advanced esophageal cancer. Cancer Sci. 2009;100:1502–9. doi: 10.1111/j.1349-7006.2009.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72:3967–76. doi: 10.1158/0008-5472.CAN-12-0851. [DOI] [PubMed] [Google Scholar]


