Abstract
RAS is constitutively active in multiple types of tumor cells. We have recently demonstrated that H-RASV12 enhances the translation of ligands for the activating immune receptor NKG2D, hence rendering cells more susceptible to natural killer (NK) cell-mediated lysis. This effect depends on MAPK and PI3K signaling, but not on the DNA damage response.
Keywords: cytotoxicity, NK cells, signal transduction, tumor immunology
The transformation of normal cells is often associated with mutations in genes that regulate cell proliferation and differentiation.1 Deregulated cell proliferation as a result of oncogene activation is an early hallmark of tumorigenesis and is thought to induce “replication stress,” causing DNA replication forks to progress slowly or stall. The factors that promote replication stress are not well understood, but may include the depletion of nucleotides and other molecules that are required for DNA synthesis, a deregulated firing of DNA replication origins, as well as the accumulation of DNA lesions that block replication forks, in particular in genomic regions that are intrinsically difficult to replicate.2 Oncogene-induced replication stress and the consequent collapse of replication forks result in DNA damage and the activation of the DNA damage response (DDR), a conserved signaling pathway that preserves genome integrity.3 Two key DNA damage sensor kinases of the DDR are ataxia telangiectasia and Rad3 related (ATR), and the ataxia telangiectasia, mutated (ATM), which are preferentially activated by single-stranded and double-stranded DNA breaks, respectively.4 In turn, ATR and ATM activate many downstream mediators including the Ser/Thr kinases CHK1 and CHK2, which phosphorylate effector proteins such as BRCA1, CCD25 and p53 family members that inhibit cell cycle progression and activate DNA repair systems or apoptosis, if the DNA damage cannot be repaired.
RAS
RAS is a membrane-associated GTP-binding protein that is activated by many growth factors and regulates various cellular functions, including proliferation, apoptosis and migration.5 In multiple human cancer, the activity of RAS is deregulated as a result of mutations that lock RAS in an perpetual “on state,” resulting in the activation of a number of signaling pathways, including the RAF-MAPK/MEK and the PI3K pathway. Oncogenic RAS initially drives a quick proliferation phase, which is followed by cell cycle arrest (senescence) due to replication stress and the activation of the DDR.6
NKG2D
The DDR and other pathways activated in response to malignant transformation have been shown to upregulate ligands for the activating immune receptor NKG2D.7 NKG2D is expressed by all natural killer (NK) cells as well as by CD8+ T cells in humans, activated CD8+ T cells in mice and subsets of both γδ and NK T cells.9 Multiple NKG2D ligands have been identified in humans and mice, all of which are distantly related to MHC Class I molecules.8 Human NKG2D ligands include MICA, MICB and 6 different UL-16 binding proteins (ULBPs), otherwise known as RAET1 proteins. In mice, NKG2D ligands include 5 different isoforms of RAE-1 proteins, MULT1 and 3 different isoforms of H60 proteins.
Posttranscriptional Regulation of NKG2D Ligand Expression by RAS
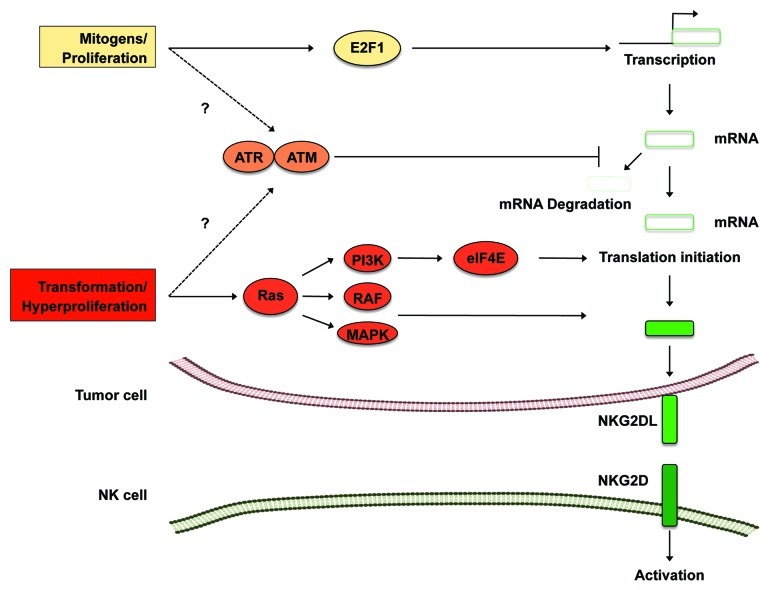
We have recently demonstrated that the expression of NKG2D ligands is intimately linked to RAS activation.9 Overexpression of the RAS isoform H-RASV12 resulted in the upregulation of NKG2D ligands in mouse and human cell lines, and rendered tumor cells more susceptible to NK cell-mediated lysis. H-RASV12 expression induced the DDR, as indicated by an increased phosphorylation of p53. Surprisingly, inhibition of ATM and ATR, which we have previously shown to regulate NKG2D ligand expression, did not abrogate the effects of H-RASV12 (Fig. 1). Instead, H-RASV12-elicited signals depended on RAF, MAPK/MEK and PI3K, in both mouse and human tumor cell lines. In contrast to other mitogenic signals that induce the transcription of Raet1 genes by E2F transcription factors, H-RASV12 only modestly increased the levels of Rae1-encoding transcripts.10 However, the turnover of Rae1 at the cell surface was significantly increased in H-RASV12-expressing cells, suggesting that H-RASV12 regulates the expression of NKG2D ligands by post-transcriptional mechanisms. Consistent with this possibility, we found that some Rae1 isoforms have an unusually long 5′ UTR, which may adopt a complex secondary structure. eIF4e participates in the unwinding of complex secondary structures at 5′ UTRs and is a limiting factor for the initiation of mRNA translation. We found that the overexpression of eIF4e induced the expression of NKG2D ligands. This effect depended on RAS and PI3K, which were shown to regulate eIF4e. Our data indicate that expression of NKG2D ligands is regulated at several stages of biogenesis, including transcription and translation. Enhanced translation of NKG2D ligands in tumor cells expressing hyperactivated RAS is expected to activate immune cells and may provide a barrier against tumorigenesis. In summary, NKG2D ligands expression may be a consequence of the activation of several oncogenic signaling pathways, including those involving RAS. NKG2D ligands may therefore constitute useful markers for detecting or targeting tumor cells that bear RAS activating mutations.
Figure 1. Regulation of NKG2D ligand expression by mitogens and RAS. Mitogenic signals transactivate various NKG2D ligands and have also been shown to induce the DNA damage response, which stabilizes transcripts coding for NKG2D ligands (NKG2DL) (Hsiung and Raulet, personal communication). RAS-transduced signals increase translation at least in part by stimulating the activity of the rate-limiting translation initiation factor eIF4e.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22244
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Burhans WC, Weinberger M. DNA replication stress, genome instability and aging. Nucleic Acids Res. 2007;35:7545–56. doi: 10.1093/nar/gkm1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartkova J, Horejsí Z, Koed K, Krämer A, Tort F, Zieger K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–70. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 4.Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 5.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–31. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–42. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 7.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 9.Liu XV, Ho SS, Tan JJ, Kamran N, Gasser S. Ras activation induces expression of raet1 family NK receptor ligands. J Immunol. 2012;189:1826–34. doi: 10.4049/jimmunol.1200965. [DOI] [PubMed] [Google Scholar]
- 10.Jung H HB, Procyk E, Raulet DH. E2F Transcription Factors Regulate Expression of RAE1 Ligands for NKG2D, an Immune Cell Receptor Implicated in Tumor Surveillance. Submitted. [Google Scholar]