Abstract
Metastases are responsible for more than 90% of cancer-related deaths. We have recently reported that miR-10b inhibits the expression of MICB, a stress-induced ligand of the activating natural killer (NK)-cell receptor NKG2D. Here, we discuss our findings, which link metastasis formation to immune evasion.
MicroRNAs (miRNAs) are small RNA molecules that regulate the majority of cellular processes, both in physiological conditions and in the course of multiple diseases including cancer.1 miR-10b, which is one of the most famous miRNAs, is critically involved in the formation of metastases, an activity for which it has been named “metastamir.”2,3
Natural killer (NK) cells kill virus-infected as well as malignant cells by using a limited number of NK killer receptors.4 Among these receptors a prominent role is played by NKG2D, which is also expressed by several T cell subsets. NKG2D has 8 known ligands: MICA, MICB and ULBP 1–6, all of which are exposed on the cell surface in response to a variety of stresses, including oncogenic transformation.5
Since we discovered that several miRNAs that are overexpressed in a variety of cancers are capable of targeting MICA and MICB,6 we speculated that miR-10b might also target stress-induced ligands. Using the TargetScan algorithm, several stress ligands were identified as possible candidates for miR-10b regulation. However, experiments performed in various cell lines demonstrated that miR-10b inhibits the expression of MICB only.7 In silico predictions, although very helpful, are often inaccurate and the rate of false-positive results can be as high as 70%. At least in part, this reflects the fact that the mechanisms of action of miRNAs are not completely understood. We therefore recommend performing bioinformatic target screens based on solid experimental hypotheses, which reduce the amount of false-positive results.
We demonstrated that MICB is a direct target for miR-10b by using luciferase reporter assays, which also allowed us to identify the miR-10b-binding site in the 3′ UTR of MICB. The miR-10b effect on the levels of MICB was moderate. However, such a moderate downregulation of MICB appeared to be sufficient for tumor cells overexpressing miR-10b to avoid NKG2D-mediated recognition and elimination.7
We also demonstrated that miR-10b endogenously controls the expression of MICB, by using a unique tool named anti-miRNA sponge that contains 6 repeats of the predicted target site fused to GFP and cloned into a lentiviral vector. This “sponge” has several advantages: it is stably expressed in cells, the targeting of relevant miRNAs is efficient and allows for functional determinations based on GFP fluorescence.
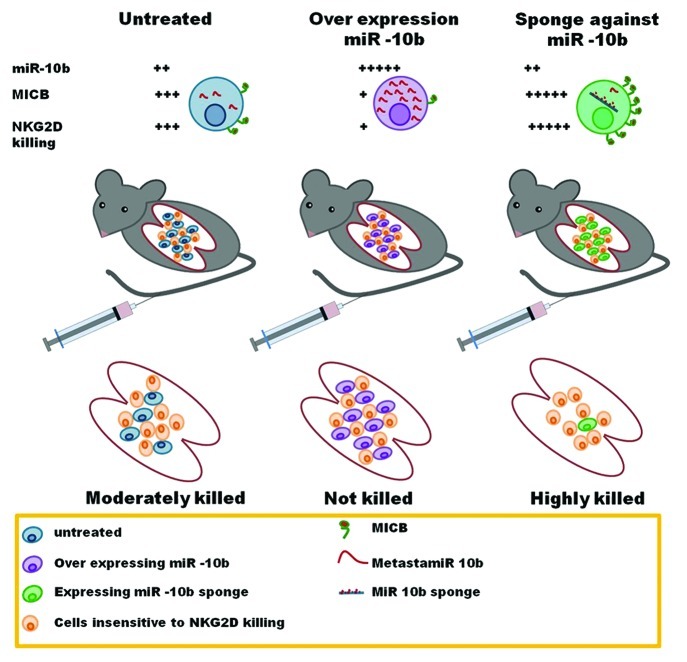
After establishing that miR-10b has immune evasive functions in vitro, we aimed at testing its functions in vivo. This was a challenging task as the murine stress-induced NKG2D ligands are different from their human counterparts and 3′ UTRs are not conserved. Furthermore, in vivo long-term assays are problematic as antagonizing or increasing miR-10b saffect not only MICB expression but also the metastatic properties of developing tumors.2,8 To bypass these obstacles, we took advantage of the fact that murine NKG2D recognizes human stress ligands and we examined tumors expressing various levels of MICB in a short-term in vivo lung clearance assay (Fig. 1). To this aim, we transduced tumor cells with a control vector (a setup in which miR-10b and MICB were expressed at moderate levels), with a miR-10b-coding vector (resulting in the expression of miR-10b at high levels and hence at the downregulation of MICB) or a with anti-miR-10b sponge (resulting in the inhibition of miR-10b and hence in high MICB expression levels). The involvement of NK cells and NKG2D was directly assessed by depleting NK cells and by blocking NKG2D function in vivo, respectively.7 Following a five hours period, in which the invading malignant cells are predominantly attacked by innate immune cells, we harvested the lungs and assessed tumor cell survival. The results were identical to those obtained in vitro: the downregulation of MICB from the cell surface (as promoted by miR-10b) facilitated the survival of cancer cells, while the tumor cells expressing the anti-mir-10b sponge were far more susceptible to NKG2D-mediated elimination, owing to an increased expression of MICB on their surface.
Figure 1. miR-10b facilitates cancer immune evasion in vivo. Mice were injected, intravenously in the tail vein, with cancer cells insensitive to NKG2D-dependent killing (orange) together with tumor cells that are sensitive to killing mediated by this receptor. Sensitive cells either expressed a control vector (resulting in moderate levels of miR-10b and MICB, blue, left), overexpressed miR-10b (leading to low levels of MICB, purple, middle) or expressed an anti-miR-10b sponge (resulting in high levels of MICB, green, right). Following injection, the cells reached the lungs and encountered immune cells. When cancer cells were harvested 5h post injection, the amounts of surviving NKG2D-sensitive and NKG2D-insensitive cells was calculated. Our results demonstrate that in vivo NKG2D-dependent immune elimination by NK cells is avoided by metastatic cells overexpressing miR-10b, which inhibits MICB translation and expression.
Malignant cells and the immune system are in a constant battle, which is also reflected by the so-called “tumor editing,” a process in which tumors develop mechanisms to escape immune attacks.9 The reduced immunogenicity of miRNAs and their target diversity (each miRNA is estimated to target hundreds of genes) make them perfect candidates as regulators of immune evasion, as the same miRNA (like miR-10b) can simultaneously promote tumor metastasis and decrease the immunogenicity of cancer cells. However, the target diversity of miRNAs also raises interesting questions such as: How do different genes targeted by the same miRNA relate to each other? Will the upregulation or the downregulation of a particular gene affect the expression of others? These questions are even more critical with regard to the control of MICB expression by miRNAs, as miR-10b is the 10th identified miRNA targeting MICB.6,10 Thus, we wonder why so many different miRNAs control MICB expression. Do they interact with each other? What are the functions of distinct MICB targeting miRNAs under normal conditions?
We think that the expression pattern of diverse MICB-targeting miRNAs is tissue-specific and this generates different thresholds underpinning the regulation of MICB expression. Normal cells use the miRNA-mediated threshold to maintain a delicate balance that limits MICB expression under normal conditions, on one hand, and allow for the rapid induction of MICB in response to stress, on the other hand. Tumor cells that elevate this threshold, by upregulating MICB-targeting miRNAs, are less susceptible to recognition and eradication by immune cells. Tilting the threshold of MICB-targeting miRNAs to increase MICB expression, for instance by means of specific miRNA inhibitors, may be a promising approach for cancer immunotherapy.
Glossary
Abbreviations:
- GFP
green fluorescent protein
- MICA
MHC class I polypeptide-related sequence A
- MICB
MHC Class I polypeptide-related sequence B
- NK
natural killer
- NKG2D
natural killer group 2, member D
- ULBP
UL16-binding protein
- UTR
untranslated region
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22245
References
- 1.Lovat F, Valeri N, Croce CM. MicroRNAs in the pathogenesis of cancer. Semin Oncol. 2011;38:724–33. doi: 10.1053/j.seminoncol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–8. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 3.Edmonds MD, Hurst DR, Welch DR. Linking metastasis suppression with metastamiR regulation. Cell Cycle. 2009;8:2673–5. doi: 10.4161/cc.8.17.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljunggren HG. Cancer immunosurveillance: NKG2D breaks cover. Immunity. 2008;28:492–4. doi: 10.1016/j.immuni.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, et al. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol. 2008;9:1065–73. doi: 10.1038/ni.1642. [DOI] [PubMed] [Google Scholar]
- 7.Tsukerman P, Stern-Ginossar N, Gur C, Glasner A, Nachmani D, Bauman Y, et al. MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-2671. In Press. [DOI] [PubMed] [Google Scholar]
- 8.Ma L. Role of miR-10b in breast cancer metastasis. Breast Cancer Res. 2010;12:210. doi: 10.1186/bcr2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–46. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachmani D, Lankry D, Wolf DG, Mandelboim O. The human cytomegalovirus microRNA miR-UL112 acts synergistically with a cellular microRNA to escape immune elimination. Nat Immunol. 2010;11:806–13. doi: 10.1038/ni.1916. [DOI] [PubMed] [Google Scholar]