Abstract
The development of efficient immunotherapies requires strong rationalization. We have recently implemented a large analysis of biomarkers in two studies involving the multi-peptide vaccine IMA901 and advanced renal cell cancer patients. Our findings demonstrate that the breadth of immune responses was associated with clinical benefits and that single-dose cyclophosphamide reduced the amount of regulatory T cells and was associated with prolonged survival after vaccination.
Keywords: biomarkers, cancer, immunomonitoring, immunotherapy, low-dose cyclophosphamide, peptides, randomized clinical trial, renal cell cancer, regulatory T cells, T cells, vaccine
The field of therapeutic anticancer vaccines has experienced a renaissance following the success of randomized Phase III clinical trials involving metastatic prostate cancer patients (leading to the first-in-history FDA approval of an anticancer vaccine in 2012),1 advanced-stage melanoma patients2 and follicular lymphoma patients.3 This recent success is contrasted by a large number of clinical trials based on anticancer vaccines that have failed in the past. The translational research and clinical development of therapeutic anticancer vaccines remains a highly complex process, requiring a strong rationalization based on a significant amount of biological data. We have recently demonstrated how markers of the immune response as well as biomarkers reflecting the immune regulatory environment can be utilized as guiding tools from the discovery of IMA901, a novel multi-peptide vaccine for the treatment of renal cell carcinoma (RCC), to its use in advanced clinical trials.4
XPRESIDENT is a platform that allows for the identification of thousands of tumor associated peptides (TUMAPs) from primary tumor tissues.5 We selected nine HLA-A*02- and one HLA-DR-restricted immunogenic TUMAPs derived from antigens overexpressed by RCC, and designated this multi-peptide multi-target composition IMA901.
We tested IMA901 in two clinical trials involving a total of 96 HLA-A*02+ subjects with advanced RCC. In a Phase I study, 28 HLA-A*02+ subjects were vaccinated repeatedly with granulocyte macrophage-colony stimulating factor (GM-CSF, 75 µg) followed by IMA901 (413 µg per peptide). Immunomonitoring was performed by human leukocyte antigen (HLA) multimer analysis and interferon γ (IFNγ) enzyme-linked immunospot (ELISPOT) from peripheral blood mononuclear cells collected at different time points pre- and post-vaccination. Among 27 subjects that were evaluable for immunomonitoring in the Phase I study, 20 showed a vaccine-induced T-cell response to at least one TUMAP, and 8 responded to multiple TUMAPs. Interestingly, this study showed a significant association between disease stabilization and T-cell responses to multiple TUMAPs (p = 0.019) on the one hand, and between the pre-vaccination levels of FOXP3+ regulatory T cells (Tregs) and multiple immune responses (p = 0.016) on the other hand. Based on these results, a Phase II study was designed to investigate whether an additional immunomodulator with the preclinically reported potential to reduce the number of Tregs could improve the outcome of vaccination (Fig. 1). Sixty eight HLA-A*02+ subjects were randomized to receive or not receive single-dose cyclophosphamide (Cy, 300 mg/m2), three days prior to the start of IMA901 + GM-CSF (which were given in the same doses employed in the Phase I study). While this study showed rates of immune responders that were comparable to those observed in the Phase I study (and were not influenced by the administration of Cy), we observed a trend for prolonged survival in subjects receiving Cy (hazard ratio, HR = 0.57, p = 0.090). Strikingly, among immune responders, patients that were pre-treated with Cy had a significantly longer survival that those that were not (HR = 0.38, p = 0.040). This was not the case for patients that did not develop immune responses (HR = 0.92, p = 0.870), suggesting that both, an immune response and the immunomodulatory mechanism of Cy are required for improved survival of cancer patients receiving IMA901, and strongly arguing against any clinical single-agent activity of Cy. In line with the observations of the Phase I trial, improved patient survival was associated with the number of immune responses (p = 0.023). As hypothesized in the design of the study, Cy resulted in a significant decrease of circulating FOXP3+ Tregs 3 d after infusion (p = 0.013), while no such effect was observed in patients not randomized to Cy.
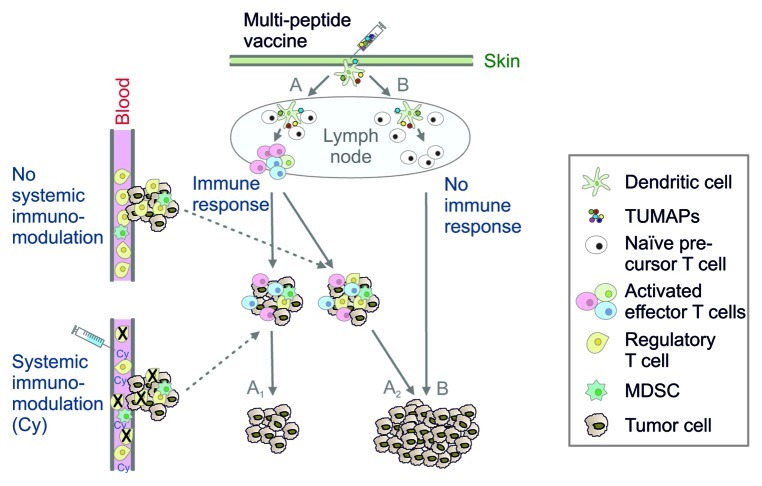
Figure 1. Potential cellular pathways underlying the clinical efficacy of IMA901 combined with low-dose cyclophosphamide. Intradermal vaccination with multiple tumor-associated peptides (TUMAPs) leads to the binding of peptides to HLA molecules on dendritic cells, which may (path A) or may not (path B) prime tumor-specific T cells. A relevant infiltration of the tumor with vaccine-induced effector T cells occurs only if a sufficient T-cell response is generated, otherwise the tumor continues to grow (path B). However, other cell types such as regulatory T cells (Tregs) are capable of inhibiting a beneficial antitumor immune response. Cyclophosphamide (Cy) reduces the systemic levels of Tregs. Immune responders that receive Cy (path A1) may survive longer than their non-Cy-receiving counterparts (path A2) due to a higher ratio of intratumoral effector cells to inhibitory Tregs. Additional factors including the expansion of myeloid-derived suppressor cells (MDSCs) also regulate the outcome of therapeutic anticancer vaccination.
The analysis of additional biomarkers pointed to specific patient subpopulations that may particularly benefit from immunotherapy. Five out of six phenotypes of myeloid-derived suppressor cells (MDSCs) sampled prior to immunotherapy were significantly increased in patients as compared with matched healthy donors, and two of those phenotypes were negatively associated with overall survival (p < 0.001 and p = 0.016 for CD14+ HLA-DR−/lo and CD11b+CD14−CD15+ MDSCs, respectively). Searching for easily assessable biomarkers that may predict a clinical response to immunotherapy, we studied more than 300 analytes in serum samples collected from RCC patients prior to IMA901 treatment. High concentrations of APOA1 and CCL17 identified patient subpopulations exhibiting a significant survival advantage (HR = 0.41, p = 0.007 and HR = 0.41, p = 0.011, respectively). This was only observed in patients receiving Cy. APOA1 and CCL17 were also positively associated with multi-peptide responses (p < 0.0001 and 0.0028, respectively). Of note, in both clinical studies, immunotherapy was safe and generally well tolerated.
What are the implications of these studies in the general context of anticancer vaccine development? First, our findings suggest that single-dose Cy may be a beneficial immunomodulator for anticancer vaccines in general. While this has previously been hypothesized, to the best of our knowledge, ours is the first randomized clinical study to provide evidence for this. Second, our results demonstrate that the systematic use of T-cell response monitoring and cellular biomarkers even beyond Phase I studies may constitute a valuable strategy to optimize regimens. Novel anticancer vaccines are likely to involve established therapies, and hence need to justify, with robust experimental data, dose and schedule selection for combinatorial regimens. An example of this is provided by the integration of the data on MDSCs gathered in the Phase II study with previous findings6,7 showing that sunitinib, a multi-kinase inhibitor, leads to a reduction of such MDSCs. Based on this knowledge, a Phase III study involving IMA901 in combination with sunitinib for the treatment of metastatic RCC has been designed and has recently completed patient recruitment. Third, in order to generate the opportunity of treating those patients that are most likely to respond to the vaccine, it is strongly recommended to perform systematic analyses of potentially predictive biomarkers as early as possible during the clinical development program, as this allows for validation during subsequent trials.
Glossary
Abbreviations:
- Cy
cyclophosphamide
- ELISPOT
enzyme-linked immunospot
- GM-CSF
granulocyte macrophage colony-stimulating factor
- MDSC
myeloid-derived suppressor cell
- IFN
interferon
- HLA
human leukocyte antigen
- HR
hazard ratio
- RCC
renal cell cancer
- Treg
regulatory T cell
- TUMAP
tumor-associated peptide.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22246
References
- 1.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. IMPACT Study Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–27. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29:2787–94. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012 doi: 10.1038/nm.2883. In Press. [DOI] [PubMed] [Google Scholar]
- 5.Weinschenk T, Gouttefangeas C, Schirle M, Obermayr F, Walter S, Schoor O, et al. Integrated functional genomics approach for the design of patient-individual antitumor vaccines. Cancer Res. 2002;62:5818–27. [PubMed] [Google Scholar]
- 6.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–82. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 7.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]