Abstract
We have recently reported that human natural killer (NK) cells release exosomes that express both NK-cell markers and cytotoxic molecules. Similar results were obtained with circulating exosomes from human healthy donors. Both NK-cell derived and circulating exosomes exerted a full functional activity and killed both tumor and activated immune cells. These findings indicate that NK-cell derived exosomes might constitute a new promising therapeutic tool.
Keywords: exosomes, natural killer, nanomedicine, perorin, theranostic
Exosomes are nanovesicles naturally released by almost all the cells of our body that—in both physiological and in pathological settings—deliver several molecules including proteins, lipids and nucleic acids to target cells. They are able to interact with target cells located in the close proximity or at distance using different mechanisms, including ligand-receptor interactions1,2 and plasma membrane fusion, leading to the transfer of their contents to the target cell cytoplasm.3 Thus, exosomes appear as a vectorized signaling system operating between the cytoplasm of a donor cell and either the extracellular compartment or potentially all the internal compartments of a target cell. These observations place exosomes at the center of current interests in translational research and point to exosomes as potential self-nanovectors for future nanomedicine approaches. Moreover, exosomes are attracting great consistent attention as tools for the identification of novel disease biomarkers. In fact, new tests offering the possibility to simultaneously characterize and quantify exosomes in human body fluids have been recently developed.4 Such a dual potential of exosomes suggest that they might consistute an ideal tool for “theranostic.” This new discipline of nanomedicine focuses on a multi-disciplinary research approach to build new systems for various nano-biomedical applications, ranging from the medical use of nanoplatform-based diagnostic agents to the development of therapeutic interventions, alone or combined to each other (therapy + diagnostic = theranostic).
We have recently shown that human natural killer (NK) cells release exosomes in both resting and activated condition.5 Moreover, we found that NK cell-derived exosomes not only express both typical NK markers (i.e., CD56) and killer proteins (i.e., FASL and perforin) but also exert antitumor and immune homeostatic activities. These findings demonstrate that — at odds with T and B cells6,7 —NK cells secrete exosomes in a constitutive way and independently from their activation status. This may suggest that NK cell-derived exosomes are involved in the control of the immune response without the need for specific stimuli. In fact, NK cell-derived exosomes contain both FASL and perforin when they are released by resting NK cells. However, NK-cell derived exosomes are functional exclusively against activated immune cells, suggesting that they have a control on the immune cell expansion only upon activation via cell-intrinsic or cell-extrinsic stimuli.
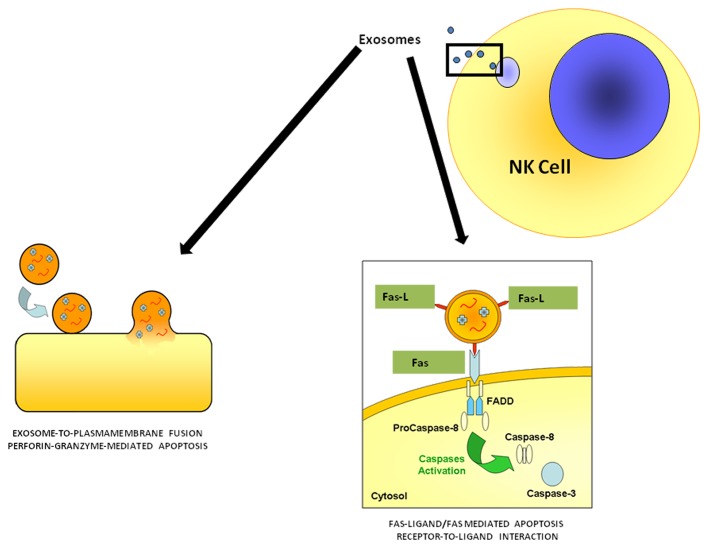
Our in vitro results were strongly supported by ex vivo findings on circulating exosomes obtained from healthy donors, showing expression of NK markers, such as CD56 and perforin, and exerting exosome-induced cytotoxicity. Thus, it appears conceivable that NK cell-derived exosomes control immune responses both in a paracrine fashion and sistemically. Together with CD56, NK cell-derived express detectable amounts of the activating receptor NKG2D, whereas natural cytotoxicity receptors (NCRs), the other NK-cell receptors that mediate cytotoxic functions (i.e., NKp30, NKp46 and NKp44), are less expressed. Intriguingly, we detected perforin in exosomes purified from both NK-cell culture supernatants and the plasma of healthy individuals, whereas FASL was undetectable in plasmatic exosomes.5 Moreover, perforin-containing plasmatic exosomes were exclusively associated with NK-cell but not CD8+ T-cell marker. This is in line with a previous report showing that perforin is highly expressed by resting NK cells, but not by resting CD8+ T lymphocytes.8 Of note, perforin is one of the most conserved proteins involved in immune responses, with a potential ancestral role in the immune system of heterogeneous organisms.9 The role of perforin in the control of tumor initiation, growth, and dissemination is well established and perforin-deficient mice are profoundly immunocompromised and more prone to develop spontaneous tumors.10 Hence, it appears conceivable that exosome-associated perforin may constitute a new target for the development of therapies against cancer and other diseases. In this context, it should be noted that the tumor microenvironment is acidic, which, on one hand, may impairs the effectiveness of chemotherapeutics and possibly of the antitumor immune response, but, on the other hand, may favor the accumulation and delivery of exosomes, as they are attracted by low pH and these conditions promote membrane fusion.3 Thus, paradoxically, differences in the electrostatic charges between exosomes and the plasma membrane of tumor cells might be more important than the specificity of ligand-receptor interactions. This said, based on the different mechanisms through which exosomes may interact with target cells,1-3 it is likely that NK cell-derived exosomes may interact with target cells with either a exosome-to-membrane fusion or a receptor-to-ligand interaction (Fig. 1).
Figure 1. Mechanisms through which NK-cell derived exosomes may interact with target cells. Natural killer (NK) cell-derived exosomes express two molecules involved in cytotoxicity: perforin, which may exert its action upon fusion of exosomes with the plasma membrane of target cells, and FASL, which mediates cytotoxic effects by interacting with FAS on the plasma membrane of target cells
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22337
References
- 1.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–16. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 3.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–22. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logozzi M, De Milito A, Lugini L, Borghi M, Calabrò L, Spada M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4:e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro F, et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189:2833–42. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 6.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters PJ, Geuze HJ, Van der Donk HA, Slot JW, Griffith JM, Stam NJ, et al. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur J Immunol. 1989;19:1469–75. doi: 10.1002/eji.1830190819. [DOI] [PubMed] [Google Scholar]
- 8.Obata-Onai A, Hashimoto S, Onai N, Kurachi M, Nagai S, Shizuno K, et al. Comprehensive gene expression analysis of human NK cells and CD8(+) T lymphocytes. Int Immunol. 2002;14:1085–98. doi: 10.1093/intimm/dxf086. [DOI] [PubMed] [Google Scholar]
- 9.Hwang JY, Ohira T, Hirono I, Aoki T. A pore-forming protein, perforin, from a non-mammalian organism, Japanese flounder, Paralichthys olivaceus. Immunogenetics. 2004;56:360–7. doi: 10.1007/s00251-004-0688-8. [DOI] [PubMed] [Google Scholar]
- 10.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–60. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]