Abstract
The accumulation of plasmacytoid dendritic cells (pDCs) within breast carcinoma lesions is associated with a poor clinical outcome. We demonstrated that the deleterious impact of tumor-associated pDCs (TApDCs) is due to their impaired capacity to produce Type I interferon, which in turn potentiates their ability to sustain the proliferation of immunosuppressive regulatory T cells.
Keywords: breast cancer, immunosuppression, plasmacytoid dendritic cells, regulatory T cells, tolerance
Breast cancers are the most common malignant tumors and the first leading cause of cancer-related deaths in women. Among several types of breast carcinoma, the so-called triple negative (TN)—that is, HER2/neu-, estrogen receptor- and progesterone receptor- is generally viewed as the most aggressive one. Breast cancers are considered immunogenic tumors as CD8+ T-cell as well as humoral responses against specific tumor-associated antigens have been reported. Nevertheless, in a large majority of cases, natural immunity against breast cancer is not protective, suggesting that breast tumors (BTs) escape immunosurveillance. In addition, several studies have demonstrated that the BT microenvironment subverts the function of immune cells and favor immunosuppression, avoiding the establishment of antitumor immunity.1 In this context, we have observed that BT infiltration by plasmacytoid dendritic cells (pDCs) is associated with an adverse clinical outcome,2 suggesting that pDCs might contribute to the immune evasion of BTs and ultimately to their outgrowth.
pDCs are well known for their role in antiviral immunity as they massively produce Type I interferons (IFNα/β/ω) in response to viral nucleic acids recognized by Toll-like receptor 7 and 9 (TLR7 and TLR9). They are also involved in the pathogenesis of autoimmune/inflammatory disorders, owing to the chronic production of Type I IFN in response to self nucleic acids.3 In addition to their innate immunostimulatory functions, pDCs exhibit adaptive tolerogenic properties by favoring the differentiation and expansion of immunosuppressive FOXP3+ regulatory T cells (Treg) (for a review see ref. 4). Recent works (for a review see ref. 5) including one from our group6 have shown that pDCs accumulate within several types of solid tumors, but very limited studies have analyzed their function in the tumor microenvironment so far. Thus, the aim of our study was to decipher the mechanistic basis for the deleterious impact of pDCs on the clinical outcome of breast carcinoma patients.7
In this study, including 60 newly diagnosed breast cancer patients, we observed a preferential accumulation of pDCs in aggressive BTs with a high mitotic index and a TN phenotype. These results strengthen our previous observation on the deleterious impact of tumor-associated pDCs (TApDCs) on the clinical outcome of breast cancer patients.2 Such TApDCs exhibited a partially activated phenotype (CD40, CD83, CD86 and HLA-DR intermediate levels) when compared with patient-derived circulating pDCs. In addition, similar to tonsil-derived pDCs, TApDCs responded in vitro to TLR7 and TLR9 agonists by maturing and by promoting the proliferation and differentiation of naive CD4+ T cells, which resulted in interleukin-10 (IL-10) and IFNγ production. These observations indicate that the adaptive immune function of TApDCs is unaffected.
In contrast, TApDCs were strongly impaired in their ability to secrete IFNα upon TLR7/9 stimulation in vitro. This functional defect was specific to IFNα and occurred selectively at the tumor site. Indeed, the production of (1) inflammatory molecules such as IP-10/CXCL10 by TApDCs as well as (2) IFNα by patient-derived circulating pDCs was not affected. Using BT-derived supernatants that inhibit IFNα but not IP-10/CXCL10 production by circulating pDCs obtained from healthy donors (HDs), we have recently identified a major role for transforming growth factor β (TGFβ) and tumor necrosis factor α (TNFα) within the BT microenvironment in the functional dysfunction of TApDCs (Sisirak, in revision). Moreover, we have demonstrated that TGFβ and TNFα affect both the expression and the nuclear translocation of interferon regulatory factor 7 (IRF7), the master regulator of Type I IFN production in pDCs, explaining the specific impairment in Type I IFN production. As Type I IFN plays important antitumor functions, either directly or by promoting immunosurveillance (for a review see ref. 8), our work suggests that the BT-mediated inhibition of Type I IFN production by TApDCs confer a selective advantage to tumor cells. In this context, it has recently been shown that the downregulation of the IRF7 pathway in BTs is associated with increased bone metastasis and, hence, reduced patients survival.9 The similarity in the biology of breast7 and ovarian6 carcinoma-associated pDCs strengthens the importance of our work and indicates that common inhibitory mechanisms might be established by epithelial cancers of different histological origin.
Of note, we also observed that (1) TApDCs were in close contact with tumor-associated Tregs (TATregs) within lymphoid aggregates in BTs and (2) the frequency of TApDCs strongly correlates with that of TATregs within BT, especially TN BTs.7 As we previously reported an accumulation of activated and proliferating TATregs that is associated with poor prognosis in BTs,10 we wondered whether TApDCs might contribute to TATreg expansion within the BT microenvironment. We observed that TApDCs as well as HD-derived pDCs preconditioned with BT-derived supernatants are very potent in supporting (1) a selective expansion of FOXP3+ Tregs and (2) a robust production of immunosuppressive IL-10 by memory CD4+ T cells.7 Interestingly, exogenous IFNα reverted immunosuppressive CD4+ T cell responses induced by TApDCs and HD-derived pDC preconditioned with BT supernatants, indicating that the induction of tolerance is strongly amplified in tumors as a result of impaired IFNα production by pDCs. Ongoing studies from our group aim at identifying the molecular mechanisms involved in TApDC-triggered TATreg proliferation (Faget, in revision).
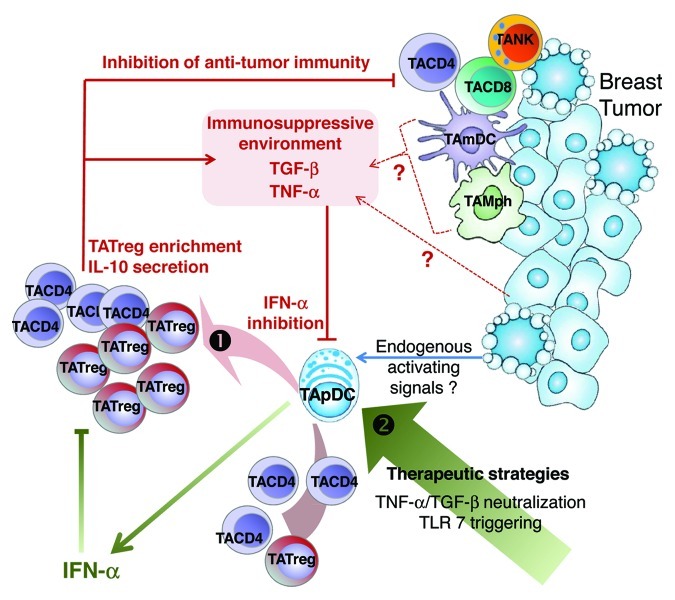
Collectively, our findings show that the BT microenvironment inhibits Type I IFN production by TApDCs through TGFβ and TNFα, hence conferring pDCs an enhanced capacity to promote the expansion of FOXP3+ TATregs and IL-10-secreting T cells. This favors the accumulation of immunosuppressive CD4+ T cells at the tumor site, thus dampening antitumor immune responses (Fig. 1). Our observations pave the way for the development of new therapeutic strategies aimed at restoring IFNα production by TApDCs by means of a combination of TLR7/9 agonists and TGFβ/TNFα antagonists, resulting in the induction of a potent antiviral-like immunity against breast cancer.
Figure 1. Schematic view of immune escape mechanisms involving IFNα-deficient TApDCs, IL-10-secreting CD4+ T cells, and TATregs in breast tumors. (1) Transforming growth factor β (TGFβ) and tumor necorsis factor α (TNFα) produced by the tumor microenvironment inhibit the production of Type I interferon (IFN) by tumor-associated plasmacytoid dendritic cells (TApDCs) that may be triggered by endogenous activating signals. Such functionally impaired TApDCs support the proliferation of tumor-associated FOXP3+ regulatory T cells (TATregs) and the production of interleukin-10 (IL-10) by tumor-associated memory CD4+ T cells, establishing an immunosuppressive environment. This enables breast tumors to escape immunosurveillance and outgrow. As the proliferation of TATregs is strongly inhibited by exogenous IFNα, our data collectively suggest that restoring Type I IFN production by TApDCs may favor antitumor immunity in breast cancer. (2) Therapeutic strategies leading to the restoration of IFNα production by TApDCs need to be investigated.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22338
References
- 1.Hurwitz AA, Watkins SK. Immune suppression in the tumor microenvironment: a role for dendritic cell-mediated tolerization of T cells. Cancer Immunol Immunother. 2012;61:289–93. doi: 10.1007/s00262-011-1181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–74. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 3.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011;29:163–83. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–76. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermi W, Soncini M, Melocchi L, Sozzani S, Facchetti F. Plasmacytoid dendritic cells and cancer. J Leukoc Biol. 2011;90:681–90. doi: 10.1189/jlb.0411190. [DOI] [PubMed] [Google Scholar]
- 6.Labidi-Galy SI, Sisirak V, Meeus P, Gobert M, Treilleux I, Bajard A, et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 2011;71:5423–34. doi: 10.1158/0008-5472.CAN-11-0367. [DOI] [PubMed] [Google Scholar]
- 7.Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, et al. Impaired IFN-a production by Plasmacytoid dendritic cells favors regulatory T cell expansion and contributes to breast cancer progression. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-3468. In Press. [DOI] [PubMed] [Google Scholar]
- 8.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 9.Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med. 2012 doi: 10.1038/nm.2830. In Press. [DOI] [PubMed] [Google Scholar]
- 10.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–9. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]