Abstract
Breast cancer spread to distant sites is often incurable. Our recent findings demonstrate that Type I interferons secreted by tumor cells induce anti-metastatic immune responses that prevent breast cancer metastasis to the bone. This provides novel insights into the importance of the crosstalk between neoplastic and immune cells in the metastatic process.
Keywords: bone metastasis, breast cancer, interferon, tumor immune surveillance
Despite improvements in anticancer-targeted therapies, there are still limited options for patients that develop metastatic disease in organs such as the bone. Metastasis is a complex process initiated by tumor cell invasion of the surrounding stroma, intravasation into the circulation, extravasation and lodging in distant organs. At distant sites, tumor cells overcome dormancy and induce angiogenesis before they eventually invade the tissue and outgrow into macrometastases. This multistep process is dynamic, involving interactions between tumor cells and other host cells, including immune cells.1 Perhaps the most important steps in the metastatic cascade from a therapeutic perspective are the survival and outgrowth of disseminated cells in distant organs. Many breast cancer patients already have neoplastic cells in their bloodstream or lodged in distant tissues at the time of diagnosis.2 Although there are reports indicating that the immune system regulates the survival and growth of some cancers,3 there is little evidence on the role of the immune system in controlling these critical steps of metastasis.
The contribution of immune surveillance in regulating the metastatic spread to the bone has been somewhat ignored, as most experimental models of bone metastasis involve injection of human breast cancer lines into immunocompromised hosts. To address this issue, we used the syngeneic 4T1.2 model of spontaneous mammary metastasis,4 allowing us to determine the changes that occur both in tumor cells and in the host immune system that may contribute to tumor cell survival and outgrowth in the bone. Comparison of the gene signatures from tumor cells derived from primary tumors and bone metastases revealed that malignant cells at the primary site express genes associated with elements of a Type I interferon (IFN) innate immune pathway. In contrast, upon spread to the bone, the expression of this gene signature is significantly decreased. In particular, an important regulator of the Type I IFN system, IFN regulatory factor 7 (Irf7)5 and 208 of its predicted target genes were suppressed in the tumor cells that had formed metastases in the bone. The large representation of Irf7-regulated genes in the downregulated gene list suggested that tumor-derived Type I IFNs suppress bone metastasis.6
Type I IFNs are a family of cytokines well known for their function in antiviral immune responses.5 Secretion from immune cells like dendritic cells can shape host immunity through direct and indirect actions on both the innate and adaptive immune systems, including the enhancement of natural killer (NK) cell expansion, survival and cytotoxicity as well as the enhancement of T lymphocyte clonal expansion and differentiation.7 In addition, Type I IFNs are capable of reducing myeloid-derived suppressor cell (MDSC) number and of limiting their immunosuppressive functions.8 Although a role for Type I IFNs in anticancer immune responses have previously been proposed,9 their cellular source, antitumor response mechanisms, and importantly, their role in metastasis remained unclear. Based on the detection of an active IFN signaling pathway in mammary tumors, we hypothesized that the activation of this pathway could stimulate an antitumor immune response, suppressing the development of bone metastases.
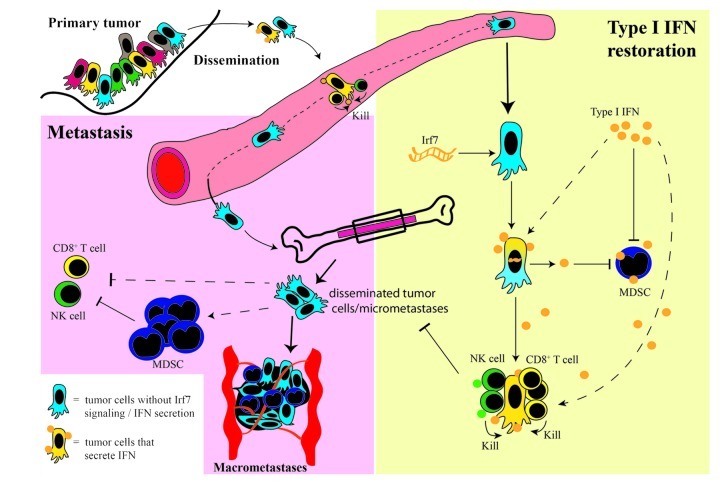
The restoration of Type I IFN signaling in mice bearing 4T1.2 tumors by either enforced expression of Irf7 by tumor cells or by the systemic administration of Type I IFN, dramatically decreased metastasis to the bone and prolonged metastasis-free survival. This was associated with an increased abundance of immune effector cells (CD8+ T cells and NK cells) and reduced numbers of immunosuppressive MDSCs in the blood and bone marrow, pointing to the stimulation of anti-metastatic immune responses. An immune-dependent mechanism was confirmed using mice lacking the the Type I IFN receptor (Ifnar1−/−), mice lacking functional innate and adaptive immune responses (NOD SCID Il2rγ−/− mice) and wild type mice depleted of CD8+ T-cell and NK-cell populations. These approaches confirmed a critical role for IFN-driven immune responses in Irf7-induced metastasis suppression (Fig. 1), as bone metastasis was accelerated in all settings. This indicates that the anti-metastatic effects of Type I IFN are not intrinsic to tumor cells but are mediated by the immune system.
Figure 1. Mechanisms of Type I IFN-mediated bone metastasis suppression. During metastasis, tumor cells disseminate from the primary site into the circulation and the bone marrow. In order for macrometastases to form in the bone, disseminated tumor cells or micrometastases need to escape immune recognition and elimination and to initiate angiogenesis and metastatic growth. One mechanism of immune escape is the downregulation of Type I IFN secretion by tumor cells, preventing a direct activation of immune cells such as CD8+ T cells and NK cells, and/or allowing the accumulation of immunosuppressive MDSCs. We propose that elevated Type I IFN, as obtained either by the molecular restoration of Irf7 expression in tumor cells or by the systemic administration of Type I IFN, could activate an antitumor immune response that prevents tumor cell outgrowth in the bone. Our studies suggest that Type I IFN influences the immune system in multiple ways, including the expansion of CD8+ T cells and NK cells as well as the inhibition of MDSC immunosuppressive functions.
Importantly, suppression was only observed in bone metastasis, as Irf7 expression or IFN treatment did not significantly alter primary tumor growth or metastasis to the lungs.6 This observation in animal models was confirmed by clinical data. Analysis of over 800 patient samples confirmed that the Irf7 signature could predict bone metastatic relapse. Patients with low expression of this signature in the primary tumors were more likely to develop bone metastasis as the first site of relapse, yet their risk for developing metastasis to other sites including the lung, liver and brain was unchanged.6 Altogether, these results suggest that the IFN-driven immune control of breast cancer is specific for metastases and organ-dependent.
Understanding the role of Type I IFN in metastasis-specific antitumor immune responses has important mechanistic and prognostic implications. Our study suggests that immune surveillance has an important role in regulating metastatic spread and hence demonstrates that the use of metastasis models that lack a competent immune system ignores critical tumor-immune cell crosstalk. Additionally, our study supports the use of the Irf7 signature to predict bone metastatic relapse in patients that may benefit from therapies aimed at restoring IFN-driven immune responses to prevent this. Although the clinical application of Type I IFN-based therapies has been tested unsuccessfully in small cohorts of breast cancer patients that already developed macrometastases,10 we hypothesize that the best therapeutic window for investigating such therapies is in the adjuvant setting, when disseminated breast cancer cells have not yet formed macrometastases. Future studies based on the patient population that would most likely benefit from IFN-based therapies and the most appropriate therapeutic setting for administering such treatments are imperative.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22339
References
- 1.Eckhardt BL, Francis PA, Parker BS, Anderson RL. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012;11:479–97. doi: 10.1038/nrd2372. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-1587. In press. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 4.Eckhardt BL, Parker BS, van Laar RK, Restall CM, Natoli AL, Tavaria MD, et al. Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol Cancer Res. 2005;3:1–13. [PubMed] [Google Scholar]
- 5.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–7. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 6.Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med. 2012 doi: 10.1038/nm.2830. In press. [DOI] [PubMed] [Google Scholar]
- 7.Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17:2619–27. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- 8.Zoglmeier C, Bauer H, Nörenberg D, Wedekind G, Bittner P, Sandholzer N, et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17:1765–75. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- 9.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 10.Repetto L, Giannessi PG, Campora E, Pronzato P, Vigani A, Naso C, et al. Tamoxifen and interferon-beta for the treatment of metastatic breast cancer. Breast Cancer Res Treat. 1996;39:235–8. doi: 10.1007/BF01806190. [DOI] [PubMed] [Google Scholar]