Abstract
One of the driving forces of oncogenesis is tetraploidy, a duplication of the DNA content that, upon asymmetric cell division or progressive chromosome loss, can originate aneuploidy. Recent findings from our group indicate the existence of an immunosurveillance system that eliminates tetraploid cancer cells. We surmise that tetraploidy-inducing chemotherapeutic agents may elicit potent anticancer responses by re-activating this immunosurveillance system.
Keywords: breast carcinoma, calreticulin, HMGB1, hyperploidy, immunogenic cell death, mitotic catastrophe
When a pathologist focuses the microscope on a hematoxylin/eosin-stained tissue section, cancer cells can be detected owing to their peculiar morphological appearance. One of the cardinal cytological hallmarks of cancer is indeed anisokaryosis, an intercellular variation in nuclear size and shape that is particular pronounced in high-grade and anaplastic neoplasms, correlating with dismal prognosis. The causes underlying this nuclear pleiomorphism are now unfolding, thanks to recent advances in our understanding of the fundamental cell biology of oncogenesis.
One of the initiating events of carcinogenesis is tetraploidization, i.e., the generation of cells that contain twice as much nuclear DNA and chromosomes than their normal, diploid counterparts. Such an increase in nuclear DNA content may result from endomitosis (an abortive variant of mitosis that fails either before anaphase or at cytokinesis, generating mononucleated or polynucleated tetraploid cells, respectively), cell-to-cell fusion (among somatic cells that express fusogenic proteins, most often of viral origin) or endocycling (DNA replication not followed by mitosis, leading to the formation of polytenic chromosomes).1,2 In most cell types, significant variations from the diploid status are not tolerated, and tetraploid as well as higher-order polyploid cells die as soon as they are generated.3 However, tetraploidy has been observed in the early stages of bronchial, esophageal, gastric, mammary, colorectal, ovarian, cervical and prostate cancer, correlating with the inactivation of the tumor suppressors TP53 and RB.1 Indeed, a large number of oncoproteins and oncosuppressor proteins regulate the generation, survival and propagation of tetraploid cells.1,2,4
Tetraploid cells can give rise to an aneuploid offspring, either via the progressive loss of chromosomes during subsequent rounds of normal mitosis or as a consequence of multipolar mitoses, asymmetric cell divisions in which chromosomes are randomly distributed among three or more daughter cells.4,5 In most cases, this sort of genomic lottery results in the generation of daughter cells that lack essential chromosomes and hence are destined either to undergo an irreversible growth arrest or to succumb to cell death upon the activation of mitotic catastrophe.4,5 However, in a few instances, aneuploid cells can acquire a proliferative advantage as compared to their tetraploid progenitors, thus becoming able to progressively transform into tumorigenic cells. Altogether, these phenomena generate the histopathological aspects of pleiomorphism, ultimately constituting a snapshot of malignant transformation and tumor progression that morphologically reflects genetic heterogeneity.
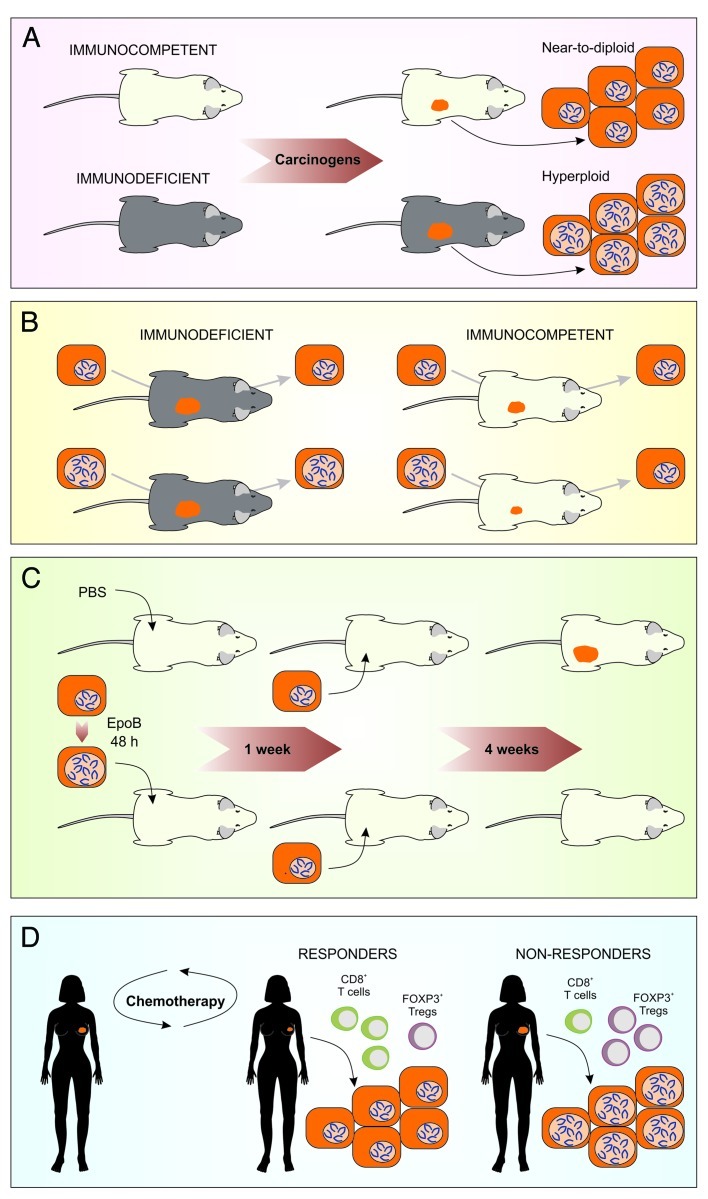
The aforementioned polyploidization/depolyploidization cascade is not only regulated by cell-autonomous mechanisms,4,5 but also subjected to an extrinsic control that is operated by the immune system.6 Thus, fibrosarcomas induced by the chemical carcinogen methylcholanthrene, B-cell lymphomas caused by the transgenic expression of the MYC oncogene, as well as mammary carcinomas generated by the synthetic hormone medroxyprogesterone acetate, develop more rapidly in immunodeficient (Rag2−/− γc−/−, Stat1−/− or Dnam1−/−) than in immunocompetent mice. The neoplastic lesions that develop in immunocompromised mice exhibit larger nuclei, correlating with a higher DNA content, than those developing in immunocompetent hosts (Fig. 1A).6 Murine colorectal carcinoma CT26, fibrosarcoma MCA205 and Lewis lung carcinoma (LLC) cells essentially fail to proliferate in vivo when they are rendered hyperploid in vitro before inoculation into syngenic immunocompetent mice. In sharp contrast, there is no difference in the proliferation rates of parental (near-to-diploid) and hyperploid CT26, MCA205 or LLC cells growing in immunodeficient mice. Of note, tumors that develop (invariably after some latency) from hyperploid cells inoculated into immunocompetent hosts exhibit marked signs of immunoselection, including reduced nuclear size, DNA content and chromosome number as compared to non-immunoselected hyperploid cells that were cultured in vitro or allowed to grow in vivo in immunodeficient hosts (Fig. 1B). Spectral karyotyping and comparative genomic hybridization revealed that the reduction in chromosome content as induced by immunoselection is general, in that it does not affect a specific chromosome or chromosome set.6 Altogether, these results clearly indicate that the immune system can control the growth of (and likely eliminate) tetraploid cancer cells. In line with this hypothesis, primary epithelial cells from Tp53−/− mice can be transformed in vitro by a combination of hyperploidizing agents and chemical mutagens, yet only form tumors in vivo if they are injected into severely immunodeficient mice.3
Figure 1. A therapy-elicited anticancer immunosurveillance system. (A) A variety of neoplasms as induced by chemical or genetic interventions grow more rapidly in immunodeficient than in immunocompetent hosts. Contrarily to lesions developing in immunocompetent animals, tumors arising in an immunodeficient setting exhibit increased ploidy and nuclear size. (B) Immunodeficient mice do not counteract tumor growth, irrespective of cancer cell ploidy. Conversely, immunocompetent mice are resistant to the development of hyperploid neoplastic lesions. When such tumors develop (rarely and invariably after some latency), they exhibit marked signs of immunoselection, including reduced nuclear size, DNA content and chromosome number. (C) Cancer cells responding to hyperploidizing agents such as epothilone B (EpoB) are capable of vaccinating immunocompetent mice against a subsequent challenge with untreated cancer cells of the same type. (D) Breast cancer patients that respond to chemotherapy often exhibit an early intratumoral accumulation of cytotoxic CD8+ T lymphocytes, which efficiently control cancer cell ploidy and tumor growth. Conversely, the lesions of patients who do not benefit from chemotherapy are robustly infiltrated by immunosuppressive FOXP3+ regulatory T cells (Tregs). In this latter setting, neoplastic lesions grow unrestrained and accumulate hyperploid cells, as they are not subjected to control from the immune system.
Intriguingly, two classes of microtubular inhibitors that are currently used as anticancer chemotherapeutics, namely vinca alkaloids (e.g., vincristine, vinblastine, vindesine and vinorelbine) and taxanes (e.g., paclitaxel and docetaxel), induce the hyperploidization of proliferating cells by virtue of their capacity to block the microtubule-dependent progression of mitosis beyond metaphase. By triggering hyperploidization, such agents stimulate an endoplasmic reticulum stress response that culminates in the exposure of the immunostimulatory protein calreticulin on the outer leaflet of the plasma membrane.6 With regard to this, microtubular inhibitors operate similar to prototypic inducers of immunogenic cell death such as anthracyclines and oxaliplatin.7,8 In addition, microtubular inhibitors mimic bona fide immunogenic cell death inducers as they are capable of triggering significant extents of cell death, in turn allowing for the release of the non-histone chromatin-binding protein HMGB1 into the extracellular space.9 However, both vinca alkaloids and taxanes are relatively inefficient in stimulating the secretion of ATP from dying cells, which is also required for immunogenic cell death.10 In fact, microtubular poisons block the last step of the autophagic pathway (the fusion between autophagosomes and lysosomes), hence actively preventing the release of ATP from dying cells.11In spite of this, cancer cells treated with some microtubular inhibitors (such as epothilone B) can induce anticancer immune responses in assays specifically designed to detect immunogenic cell death.6 In this context, cells succumbing to a lethal stimulus are injected subcutaneously into syngenic immunocompetent mice (vaccine), in the absence of any adjuvant, and, one week later, live tumor cells of the same type are injected into the opposite flank (challenge). Tumor growth is then monitored routinely and its absence is interpreted as a sign of a protective anticancer immune response, suggesting that the vaccine cell population was succumbing to bona fide immunogenic cell death.12Thus, microtubular inhibitors may stimulate anticancer immune response via their capacity to cause the hyperploidization of tumor cells (Fig. 1C).
Currently available data indicate that anthracyclines, which are very efficient at triggering immunogenic cell death, mediate a therapeutic effect mostly as they elicit anticancer immune responses that control the growth of residual tumor cells.9-11 In line with this notion, we found that the efficacy of anthracycline-based neo-adjuvant chemotherapy in breast cancer patients correlates with the composition of the immune infiltrate. Thus, in patients manifesting a pathological complete response (i.e., a consistent reduction in tumor volume and virtually no residual disease) after six cycles of anthracycline-based chemotherapy, the first cycle of therapy was sufficient to induce a statistically significant increase in the ratio between cytotoxic CD8+ T lymphocytes and immunosuppressive FOXP3+ regulatory T cells (Tregs) in the tumor bed. In contrast, no such change in the immune infiltrate could be detected in patients who failed to respond to anthracycline-based chemotherapy.6,13 Correlating with the elicitation of antitumor immunity, patients who benefitted from chemotherapy also exhibited a reduction in the mean nuclear size of residual tumor cells (Fig. 1D).6 These results suggest that chemotherapy-elicited immune responses are particularly efficient at eliminating tumor cells characterized by an elevated DNA content.
Altogether, our findings point to the existence of a natural immunosurveillance system for the elimination of cells with supranormal ploidy, which, at least in some circumstances, can be stimulated or reactivated by specific chemotherapeutic agents.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22409
References
- 1.Davoli T, de Lange T. The causes and consequences of polyploidy in normal development and cancer. Annu Rev Cell Dev Biol. 2011;27:585–610. doi: 10.1146/annurev-cellbio-092910-154234. [DOI] [PubMed] [Google Scholar]
- 2.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17:157–62. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–7. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 4.Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12:385–92. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- 5.Vitale I, Galluzzi L, Senovilla L, Criollo A, Jemaà M, Castedo M, et al. Illicit survival of cancer cells during polyploidization and depolyploidization. Cell Death Differ. 2011;18:1403–13. doi: 10.1038/cdd.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–84. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 7.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 8.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–90. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 10.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 11.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–7. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 12.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–33. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 13.Ladoire S, Mignot G, Dabakuyo S, Arnould L, Apetoh L, Rébé C, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol. 2011;224:389–400. doi: 10.1002/path.2866. [DOI] [PubMed] [Google Scholar]