Abstract
The clinical use of lymphocytes engineered to express high affinity T-cell receptors (TCRs) specific for two broadly expressed tumor-associated antigens is strongly limited by MHC-restricted fratricide of lymphocytes and TCR-mediated killing of hematopoietic stem cells. Specific clinical applications must therefore be conceived to bypass these limitations.
Keywords: hematopoietic stem cell toxicity, hyluronan-mediated motility receptor, MHC-restricted fratricide, survivin, TCR gene therapy
Adoptive T-cell therapy using T-cell receptor (TCR)-engineered lymphocytes has great potential for treatment of patients with advanced cancer. The potential of adoptive T cells to cure cancer is best seen with donor lymphocyte infusions (DLIs) to leukemic patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) and with the transfer of tumor-infiltrating lymphocytes in patients affected by solid tumors. In both these settings, the T-cell specificities that cause tumor rejection are not fully defined, since T-cell mixtures are most frequently used. This is not the case in TCR gene therapy, a situation in which the TCR specificity must be optimally matched to tumor antigens, as the clinical success of this approach critically depends on tumor antigen recognition. To date, clinical trials have used the TCR of patient-derived cells that were previously described and readily available. In addition, alterations have been introduced to augment the TCR affinity.1-3 TCRs for gene therapy have also been obtained from HLA-transgenic mice, despite their immunogenic potential as xenogeneic proteins. Alternative methods allow for the isolation of native TCRs that naturally display a high affinity,1 enabling a relatively fast acquisition of TCRs to treat various malignancies.
The selection of target tumor antigens is of pivotal importance since the transfer of high-avidity T cells has the potential to cause dangerous on- as well as off-target toxicity.1-3 In this setting, antigen selection can be guided by candidates ranked for use in cancer vaccines,4 since the common goal of adoptive T-cell transfer and vaccination is an antitumor T-cell response of maximum efficacy and minimal toxicity. Here, we emphasize two additional characteristics that should be heeded during antigen selection for TCR gene therapy that are currently not included in the NCI taskforce evaluation.4 These two new parameters are based on a study evaluating survivin as a universal cancer antigen5 as well as on our more recent findings on hyaluronan-mediated motility receptor (HMMR, also known as Rhamm) as a candidate antigen for the immunotherapy of leukemia.6
We developed a dendritic cell (DC)-based priming method to obtain high affinity allo-MHC-restricted TCRs recognizing peptides derived from different tumor-associated antigens.7 As a first example, we derived numerous self-MHC- and allo-MHC-restricted cytotoxic T lymphocytes (CTLs) specific against tyrosinase.8 As expected, allo-restricted clones showed greater peptide sensitivity and superior function, both as original clones and after TCR transfer into recipient lymphocytes, than self-restricted CTLs.8,9
Surprisingly, when survivin was substituted as the priming antigen, DC priming failed to yield self-MHC-restricted CTLs.5 In contrast, many allo-restricted peptide-specific CTL clones were obtained that recognized a survivin-derived peptide in association with HLA-A2. When the TCR of high avidity CTLs was transferred into recipient lymphocytes, they efficiently killed HLA-A2+survivin+ tumor cells.
For gene therapy, a survivin-specific TCR would be expressed in lymphocytes of an HLA-A2+ patient enabling autologous tumor cells to be killed. Unexpectedly, when HLA-A2-restricted survivin-specific TCR were transferred into HLA-A2+ recipient lymphocytes, CTLs failed to expand because of extensive apoptosis. Lymphocyte death was caused by MHC-restricted fratricide, since apoptosis occurred only in HLA-A2+ recipient cells. Lymphocyte apoptosis correlated with presence of the survivin mRNA in peripheral blood lymphocytes (PBLs) and CTLs, making them direct targets for high-affinity survivin-specific TCR-expressing lymphocytes. Based on these results, the overexpression of multiple antigens was assessed in activated PBLs and several candidates were identified that may also allow for self-destruction.5 Since MHC-restricted fratricide strongly limits the clinical usefulness of a specific TCR, antigen expression in activated lymphocytes needs to be excluded when target candidates are selected for TCR gene therapy.
HMMR (CD168) is considered to be a good target for the treatment of leukemia, in particular acute myeloid leukemia (AML).10 Low levels of the HMMR mRNA as found in normal HSCs have been considered to be inconsequential when compared with the very high levels detected in AML blasts. We isolated allo-restricted HMMR-specific CTLs and studied TCR-engineered lymphocytes for their capacity to specifically recognize tumor cells in vitro and to retard the outgrowth of AML cells in vivo in a humanized mouse model.6 The results indicate that this TCR would be well suited for the immunotherapy of AML.
Driven by the low levels of HMMR mRNA detected in HSCs, we examined whether the pre-culture of healthy CD34+ HSCs with TCR-engineered lymphocytes would disturb myeloid-derived colony formation in vitro. Indeed, inhibition of colony formation occurred but only when HSCs were obtained from HLA-A2+ donors, demonstrating that this toxicity was MHC-restricted. We then assessed the myeloreconstitution capacity of HSCs obtained from HLA-A2-transgenic mice and exposed to HMMR-specific TCR-engineered lymphocytes before adoptive transfer into lethally irradiated syngeneic mice. HSCs exposed to HMMR-specific TCR-engineered lymphocytes failed to reconstitute irradiated animals. These results demonstrate that a potent TCR can efficiently recognize low levels of antigen expressed by HSCs and cause life-threatening on-target toxicity. Thus, antigen expression by HSCs must also be considered during the selection of tumor antigens for TCR gene therapy.
Both MHC-restricted fratricide and TCR-mediated HSC toxicity became apparent when recipient lymphocytes were engineered to express high affinity TCRs. Thus, increases in TCR affinity that allow for a better recognition of tumor cells may shift the balance toward a dangerous toxicity for normal cells, as previously reported for other antigens in clinical studies.3 Importantly, low level antigen expression by HSCs and lymphocytes also needs to be considered in the selection of antigens employed for the development of anticancer vaccines, since the ultimate clinical efficacy of these interventions depends upon the generation of potent T-cell responses.
Does this mean that TCR specific for survivin and HMMR have no therapeutic use? Lymphocyte fratricide and HSC toxicity were strictly HLA-A2-restricted, implying that survivin- and HMMR-specific TCRs could be used in the setting of HLA-A2-mismatched transplantation, as illustrated in Figure 1. In this context, TCR gene therapy may provide two major benefits: the elimination of residual HLA-A2+ patient-derived HSCs, leading to faster donor chimerism, and the elimination of residual tumor cells. The use of a reduced number of adoptively transferred cells may avoid graft-vs.-host-disease (GvHD). This strategy relies on MHC-restriction to compensate for the detrimental expression of target antigens by lymphocytes and HSCs.
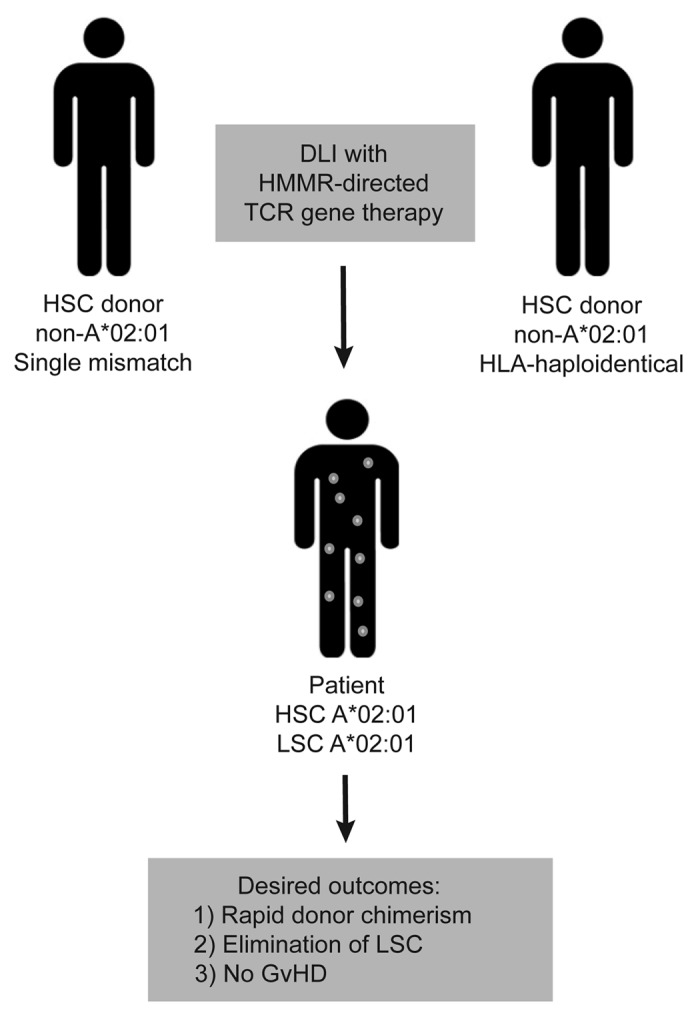
Figure 1. HLA-A2-mismatched donor-recipient pairs can be used to bypass MHC-restricted fratricide and TCR-mediated hematopoietic stem cell toxicity. In the setting of allogeneic hematopoietic stem cell transplantation (HSCT), two kinds of healthy individuals can be selected to serve as HLA-A*02:01-mismatched donors. Unrelated donors who are HLA-A*02:01-negative but otherwise patient-matched can be used for HSCT and their peripheral blood lymphocytes (PBLs) can be subsequently modified with the HMMR-specific TCR for use in donor lymphocyte infusions (DLIs). Alternatively, HLA-haplotype-mismatched family members who are HLA-A*02:01-negative can be selected for use in HLA-A*02:01-positive acute myeloid leukemia (AML) patients. This therapy could provide three advantages for the patient: (1) a relatively rapid development of donor chimerism through the elimination of residual HSCs; (2) the elimination of leukemic stem cells (LSCs); and (3) the avoidance of graft-vs.-host disease (GvHD) in the presence of potent antitumor responses, through the use of low numbers of adoptively transferred cells expressing a high-avidity HMMR-specific TCR.
Glossary
Abbreviations:
- AML
acute myeloid leukemia
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- DLI
donor lymphocyte infusion
- GvHD
graft versus host disease
- HLA
human leukocyte antigen
- HMMR
hyaluronan-mediated motility receptor
- HSC
hematopoietic stem cell
- HSCT
HSC transplantation
- LSC
leukemic stem cell
- MHC
major histocompatibility complex
- PBL
peripheral blood lymphocyte
- TCR
T-cell receptor
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22410
References
- 1.Nicholson E, Ghorashian S, Stauss H. Improving TCR gene therapy for treatment of haematological malignancies. Adv Hematol 2012; 2012:404081;; 10.1155/2012/404081 [DOI] [PMC free article] [PubMed]
- 2.Jorritsma A, Schotte R, Coccoris M, de Witte MA, Schumacher TN. Prospects and limitations of T cell receptor gene therapy. Curr Gene Ther. 2011;11:276–87. doi: 10.2174/156652311796150390. [Review] [DOI] [PubMed] [Google Scholar]
- 3.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. doi: 10.1038/nri3191. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–37. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leisegang M, Wilde S, Spranger S, Milosevic S, Frankenberger B, Uckert W, et al. MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J Clin Invest. 2010;120:3869–77. doi: 10.1172/JCI43437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spranger S, Jeremias I, Wilde S, Leisegang M, Stärck L, Mosetter B, et al. TCR-transgenic lymphocytes specific for HMMR/Rhamm limit tumor outgrowth in vivo. Blood. 2012;119:3440–9. doi: 10.1182/blood-2011-06-357939. [DOI] [PubMed] [Google Scholar]
- 7.Wilde S, Geiger C, Milosevic S, Mosetter B, Eichenlaub S, Schendel DJ. Generation of allo-restricted peptide-specific T cells using RNA-pulsed dendritic cells: A three phase experimental procedure. OncoImmunology. 2012;1:129–40. doi: 10.4161/onci.1.2.18216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilde S, Sommermeyer D, Frankenberger B, Schiemann M, Milosevic S, Spranger S, et al. Dendritic cells pulsed with RNA encoding allogeneic MHC and antigen induce T cells with superior antitumor activity and higher TCR functional avidity. Blood. 2009;114:2131–9. doi: 10.1182/blood-2009-03-209387. [DOI] [PubMed] [Google Scholar]
- 9.Wilde S, Sommermeyer D, Leisegang M, Frankenberger B, Mosetter B, Uckert W, et al. Human antitumor CD8+ T cells producing Th1 polycytokines show superior antigen sensitivity and tumor recognition. J Immunol. 2012;189:598–605. doi: 10.4049/jimmunol.1102165. [DOI] [PubMed] [Google Scholar]
- 10.Greiner J, Bullinger L, Guinn BA, Döhner H, Schmitt M. Leukemia-associated antigens are critical for the proliferation of acute myeloid leukemia cells. Clin Cancer Res. 2008;14:7161–6. doi: 10.1158/1078-0432.CCR-08-1102. [Review] [DOI] [PubMed] [Google Scholar]


