Abstract
High amounts of adenosine are released in the tumor mass. Depending on the levels of adenosine, as well as on the receptor subtypes that are expressed by immune cells, adenosine can affect tumor growth in different fashions. Specifically targeting CD73, the rate-limiting enzyme for the extracellular generation of adenosine, or the A3 receptor offers new therapeutic strategies to limit tumor progression.
Keywords: adenosine, adenosine receptors, ATP, CD73, tumor immunity
Adenosine is an important factor that controls antitumor immunity, conditioning both the innate and adaptive immune responses.1,2 The biological effects of adenosine on the immune system, which can be stimulatory or inhibitory, are mediated by four receptor subtypes. A2a and A2b are Gs-coupled receptors that increase intracellular cyclic AMP (cAMP) levels, hence suppressing immune responses. A1 and A3 are Gi/o-coupled receptors that decrease intracellular cAMP, thereby favoring cell activation. The A2a and A2b receptors (A2aR and A2bR, respectively) play a dominant role in adenosine-induced immunosuppression in a cAMP-dependent manner (Fig. 1). The stimulation of A2aR critically impairs T-cell function during activation, by reducing the production of important cytokines and chemokines, and promotes the accumulation of regulatory T cells (Tregs).1,2 In addition, A2bR activation stimulates the expansion of myeloid-derived suppressor cells (MDSCs) and induces the release of angiogenic factors.1,2
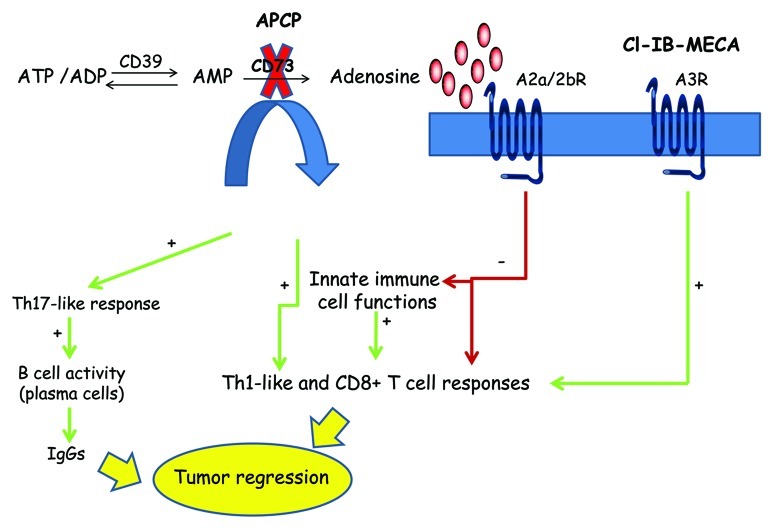
Figure 1. Involvement of the adenosinergic signaling in cancer progression. Adenosine is produced to high levels in the tumor microenvironment thanks to sequential action of by the ecto-ATPase CD39 and the ecto-5′-nucleotidase CD73. By binding to the A2aR/A2bR, adenosine exerts immunosuppressive functions, in a cAMP-dependent manner. Conversely, A3R activation by adenosine can induce an efficient T-cell response. Inhibition of CD73 with the selective compound adenosine 5′-(α,β-methylene) diphosphate (APCP) as well as the activation of A3R with the agonist Cl−IB-MECA reduce tumor growth. APCP, by lowering intratumoral adenosine levels, facilitates the release of Th1 and Th17 cytokines, which stimulates B cells to produce antitumor immunoglobulins. The administration of Cl−IB-MECA enhances antitumor CD8+ T-cell responses.
Extracellular adenosine results from the degradation of ATP by the sequential action of two cell-surface ectonucleotidases: CD39, which hydrolyzes ATP and ADP into AMP, and CD73, which hydrolyzes AMP into adenosine (Fig. 1). CD73 is highly expressed on the surface of several types of cancer cells and immunosuppressive cells, including Tregs and MDSCs.2,3 Therefore, ATP, which is released in high quantity from malignant cells succumbing to chemotherapy or other stressful conditions, is rapidly converted into adenosine, which accumulates in the tumor microenvironment. By activating A2aR/A2bR, intratumoral adenosine favors the escape of cancer cells from immune surveillance, hence promoting tumor progression. Hence, targeting CD73 may represent one potential strategy to increase the efficacy of anticancer therapy.1-3 Reduction of extracellular adenosine as produced from CD73 on tumor or host cells improves antitumor T-cell responses (Fig. 1).1-3 In this regard, we have recently provided new insights into the mechanisms underlying the antitumor activity of adenosine 5′-(α,β-methylene) diphosphate (APCP), a selective inhibitor of CD73, which—in mice—promotes both a T-cell and B-cell antitumor response by lowering intratumoral adenosine levels (Fig. 1).4
Several studies report that the majority of B cells do not express CD73, although some authors have demonstrated that CD73 is expressed on a subset of memory B cells,5 suggesting that CD73-derived adenosine might regulate memory B-cell function.5,6 Although the role of CD73 in regulating B-cell function has not yet been clearly defined, we found that CD73 inhibition in mice can indirectly affect B-cell activity in vivo, via a mechanism that relies on T cell-derived interleukin-17A (IL-17A).4 Indeed, the antitumor activity of APCP was associated with the release of Th17-like cytokines into the tumor, in turn activating B cells to produce antitumor immunoglobulins (IgGs) (Fig. 1).4 Despite the controversial role of Th17 immunity in cancer, in our model the inhibition of CD73 skewed the tumor microenvironment toward a Th17-like antitumor immune response (Fig. 1).4 Accordingly, the neutralization of IL-17A blocked the ability of APCP to inhibit tumor growth.4 Of note, in addition to a Th17 response, Th1 and CD8+ T cell-mediated immune responses were essential for APCP to reduce tumor growth in melanoma-bearing mice (Fig. 1).4 These results provide further support to the notion that CD73-generated adenosine critically regulates the function of immune cells. However, although it has been clearly demonstrated that adenosine dampens the immune response, and hence favors tumor progression, upon ligation of A2aR/A2bR, the activation of different adenosine receptors may be associated with opposite outcomes on tumor growth. In other two reports, indeed, we have observed that NK1.1+ cells7 and CD8+ T cells7,8 mediate the antitumor activity of another agent, the A3R agonist Cl−IB-MECA, which—contrarily to A2aR/A2bR agonists—exerts immunostimulatory effects in melanoma-bearing mice (Fig. 1).
Therefore, the modulation of the adenosinergic system can influence tumor growth in opposite ways (Fig. 1). On one hand, the pharmacological inhibition of CD73 alters the extracellular balance between ATP and adenosine. The accumulation of extracellular ATP in pathological conditions has been shown to operate as an endogenous stimulus for the activation of the inflammasome, which in turn promotes anticancer immune responses.9 Accordingly, the success of some agents currently used in anticancer chemotherapy might depend—at least in part—on their capacity to promote ATP release from cancer cells.1,9 On the other hand, an efficient antitumor immune response can also be achieved by targeting specific adenosine receptors, such as A3R. In conclusion, the adenosinergic system opens novel therapeutic strategies, aimed at limiting immunosuppression and potentiating protective antitumor immune responses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22448
References
- 1.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–58. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 2.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–7. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Zhang B. CD73: a novel target for cancer immunotherapy. Cancer Res. 2010;70:6407–11. doi: 10.1158/0008-5472.CAN-10-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forte G, Sorrentino R, Montinaro A, Luciano A, Adcock IM, Maiolino P, et al. Inhibition of CD73 improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol. 2012;189:2226–33. doi: 10.4049/jimmunol.1200744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–14. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minguet S, Huber M, Rosenkranz L, Schamel WW, Reth M, Brummer T. Adenosine and cAMP are potent inhibitors of the NF-kappa B pathway downstream of immunoreceptors. Eur J Immunol. 2005;35:31–41. doi: 10.1002/eji.200425524. [DOI] [PubMed] [Google Scholar]
- 7.Morello S, Sorrentino R, Montinaro A, Luciano A, Maiolino P, Ngkelo A, et al. NK1.1 cells and CD8 T cells mediate the antitumor activity of Cl-IB-MECA in a mouse melanoma model. Neoplasia. 2011;13:365–73. doi: 10.1593/neo.101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montinaro A, Forte G, Sorrentino R, Luciano A, Palma G, Arra C, et al. Adoptive Immunotherapy with Cl-IB-MECA-Treated CD8+ T Cells Reduces Melanoma Growth in Mice. PLoS One. 2012;7:e45401. doi: 10.1371/journal.pone.0045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855–8. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]