Abstract
The immunogenicity of autologous tumor-associated antigens (TAAs) is markedly increased upon the intratumoral injection of α-gal glycolipids, which insert into tumor cell membranes. The binding of natural anti-Gal antibodies to these glycolipids activates the complement system and recruits antigen-presenting cells (APCs), which internalize anti-Gal-coated tumor cells upon Fc/FcγR interactions. Eventually, TAA-derived peptides presented by APCs activate a T cell-mediated tumor-specific immune response.
Keywords: anti-Gal, autologous tumor vaccine, cancer immunotherapy, α-gal epitopes
A major challenge in cancer therapy is the destruction of undetectable micrometastases that persist after tumor resection/ablation. In many patients, indeed, these micrometastases eventually develop into lethal lesions. A lasting protection against micrometastases may be achieved by immunotherapy, promoting the activation of the immune system against the one or many autologous tumor-associated antigens (TAAs). The immune system is capable of protecting against tumor cells presenting TAAs, as it can be inferred from the correlation between the extent of T-cell infiltration observed in resected tumors and patient survival.1,2 Many TAAs are unique to each cancer patient and are generated by coding mutations, owing to genomic instability.3 The identification of autologous TAAs on an individual basis and their synthesis for vaccination purposes are not feasible at present. Therefore, the tumor itself is a practical source for vaccinating patients with autologous TAAs. An effective immunization by TAAs expressed by autologous tumor cells requires the uptake of these cells (or their debris) by antigen-presenting cells (APCs), which present TAA-derived peptides on MHC molecules for activating tumor-specific T cells. In many patients, tumors evolve strategies to evade recognition and uptake by APCs. Thus, tumors are often “ignored” by the immune system and micrometastases can reside and proliferate in lymph nodes. Effective tumor vaccines require both the recruitment of APCs into the tumor and the active targeting of tumor cells for uptake by APCs.
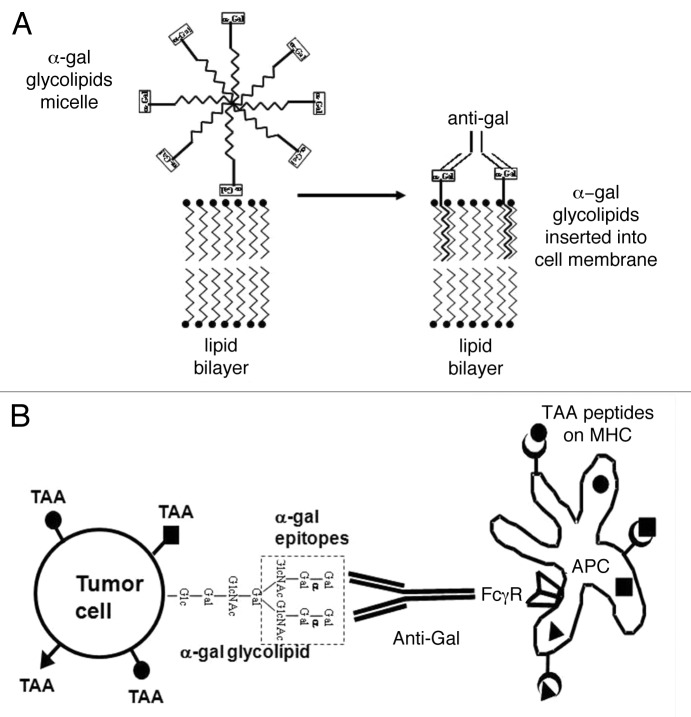
We have developed an immunotherapeutic regimen that promotes the recruitment of APCs into the tumor and in situ targets tumor cells for uptake by APCs, based on the intratumoral injection of α-gal glycolipids that interact with the natural anti-Gal antibody.4,5 Anti-Gal is the most abundant antibody in humans, constituting ~1% of immunoglobulins.6 Its ligand, the α-gal epitopes (Galα1–3Galβ1–4GlcNAc-R), is absent in humans and is produced in nonprimate mammals by the glycosylation enzyme α1,3-galactosyltransferase (α1,3GT).7,8 The anti-Gal antibody interacts very effectively in vivo with α-gal epitopes and activates the complement system, as indicated by the rapid rejection of pig xenografts following anti-Gal binding to α-gal epitopes on pig cells.9 Tumor cells can be manipulated to express α-gal epitopes by the intratumoral injection of α-gal glycolipids, hence becoming a target for anti-Gal antibodies. α-Gal glycolipids present linear or branched carbohydrate chains capped by α-gal epitopes.4,7 These glycolipids are extracted in large amounts from rabbit red cell membranes and dissolve in water as micelles. When injected into tumors, α-gal glycolipids insert into tumor cell membranes because their hydrophobic lipid “tail” is energetically much more stable when surrounded by cell membrane phospholipids than in micelles within aqueous environments (Fig. 1A). This spontaneous process results in the presentation of multiple α-gal epitopes on tumor cells.
Figure 1. Conversion of tumors into vaccines by the intratumoral injection of α-gal glycolipids. (A) Insertion of α-gal glycolipids into cell membranes of injected tumors. α-gal glycolipids dissolved in the form of micelles (hydrophobic ceramide tails form the core of the micelle and hydrophilic carbohydrate chains protrude into the surrounding aqueous environment) are injected into tumors. These glycolipids spontaneously insert into the outer lipid layer of the plasma membrane. Multiple α-gal epitopes (rectangles) bind natural anti-Gal antibodies, which reach the injection site from ruptured capillaries. This interaction activates the complement system and generates chemotactic peptides that promote the migration of antigen-presenting cells (APCs) to the treated tumor. (B) Anti-Gal mediated targeting of tumor cells for uptake by antigen-presenting cells. APCs bind via their Fcγ receptors (FcγRs) to the Fc portion of anti-Gal antibodies coating tumor cells with inserted α-gal glycolipids. This interaction stimulates APCs to internalize intact or lysed tumor cells and their TAAs. APCs transport internalized TAAs to regional lymph nodes, process them and present the multiple autologous and potentially immunogenic TAA-derived peptides in association with MHC Class I and Class II molecules for the activation of TAA-specific T CD8+ and CD4+ cells, respectively. These activated T cells mediate a protective antitumor immune response.
In vitro studies indicate that the incubation of tumor cells lacking α-gal epitopes with 0.1 or 1mg/mL α-gal glycolipids results in their extensive insertion into tumor cell membranes and cytolysis of these cells in the presence of anti-Gal antibodies and complement.4,5,10 The in vivo effects of α-gal glycolipids injected intratumorally were studied in a preclinical model involving α1,3GT deficient mice producing anti-Gal antibodies and carrying B16 melanoma tumors (which naturally lack α-gal epitopes). Injections of α-gal glycolipids into melanoma lesions resulted in tumor regression following complement-mediated cytolysis and antibody-dependent cell-mediated cytotoxicity (ADCC).4 The complement-derived chemotactic factors generated upon anti-Gal/α-gal glycolipid interactions induced an extensive recruitment of APCs, including dendritic cells and macrophages, within treated tumors.4 As a result, the Fc portion of anti-Gal antibodies coating tumor cells interacted with Fcγ receptors on APCs and stimulated them to internalize tumor cells, process TAAs and present TAA-derived peptides to tumor-specific T cells (Fig. 1B). These T cells mediated a systemic protective antitumor immune response that prevented tumor growth upon challenge at distant sites as well as the growth of established micrometastates.4,5 The antitumor immune response elicited by the intratumoral injection of α-gal glycolipids appears to be primarily mediated by CD8+ T cells and to be potent enough to overcome the immunosuppressive effect of regulatory T cells.5
The safety of α-gal glycolipids as an immunotherapeutic intervention was evaluated in a Phase I clinical trial in patients bearing various malignant solid tumors at an advanced stage of the disease.10 Patients received intratumorally 0.1 mg, 1 mg, or 10 mg α-gal glycolipids and kept under observation for 24 h. Subsequently, patients were monitored at regular intervals. None of the patients developed clinical or laboratory signs of toxicity, symptoms of allergic responses, autoimmune conditions, anti-nuclear antibodies or autoantibodies to normal tissue antigens.10 Thus, the treatment does not seem to cause a breakdown in immune tolerance to normal antigens. Injected tumors did not regress and patients developed evidence of disease progression at various time points after the four-week endpoint. However, several patients are alive with disease for 13+ to 48+ months, even though disease progression is evident by imaging. In addition, two patients with pancreatic adenocarcinoma had an unexpectedly long survival of 18 and 23 mo.10
The therapeutic effect of intratumoral α-gal glycolipids injection may not be limited to advanced diseases, but may improve outcome also when used as neoadjuvant immunotherapy. Injection of primary solid tumors such as colon or mammary carcinoma 2–4 weeks prior to resection may convert treated lesion into a temporary vaccine that “educates” the immune system to recognize and destroy metastatic cells presenting autologous TAAs. Such an induced immunosurveillance may protect against micrometastases long after the primary tumor is resected. This immunotherapeutic approach may synergize with treatments that nonspecifically expand the tumor-specific T-cell clones that are activated following the injection of α-gal glycolipids.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22449
References
- 1.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 2.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 3.Mumberg D, Wick M, Schreiber H. Unique tumor antigens redefined as mutant tumor-specific antigens. Semin Immunol. 1996;8:289–93. doi: 10.1006/smim.1996.0037. [DOI] [PubMed] [Google Scholar]
- 4.Galili U, Wigglesworth K, Abdel-Motal UM. Intratumoral injection of α-gal glycolipids induces xenograft-like destruction and conversion of lesions into endogenous vaccines. J Immunol. 2007;178:4676–87. doi: 10.4049/jimmunol.178.7.4676. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Motal UM, Wigglesworth K, Galili U. Intratumoral injection of α-gal glycolipids induces a protective anti-tumor T cell response which overcomes Treg activity. Cancer Immunol Immunother. 2009;58:1545–56. doi: 10.1007/s00262-009-0662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-α-galactosyl specificity. J Exp Med. 1984;160:1519–31. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galili U, Clark MR, Shohet SB, Buehler J, Macher BA. Evolutionary relationship between the natural anti-Gal antibody and the Gal α 1----3Gal epitope in primates. Proc Natl Acad Sci U S A. 1987;84:1369–73. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galili U, Shohet SB, Kobrin E, Stults CLM, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of α-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–62. [PubMed] [Google Scholar]
- 9.Galili U. Interaction of the natural anti-Gal antibody with α-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993;14:480–2. doi: 10.1016/0167-5699(93)90261-I. [DOI] [PubMed] [Google Scholar]
- 10.Whalen GF, Sullivan M, Piperdi B, Wasseff W, Galili U. Cancer Immunotherapy by intratumoral injection of α-gal glycolipids. Anticancer Res. 2012;32:3861–8. [PubMed] [Google Scholar]