Abstract
Recently, we reported the accumulation of CD4+FOXP3+ regulatory T cells (Tregs) within the tumor mass of patients bearing liver cancer. Tumor-infiltrating Tregs (TiTregs) are active and potent suppressors of antitumor immunity. Importantly, treatment with GITRL reduced the immunosuppression mediated by TiTregs.
Keywords: colorectal cancer, GITRL, immunotherapy, hepatocellular carcinoma, liver cancer, liver metastasis, regulatory T cells, T cells
The liver constitutes a relatively immunoprivileged microenvironment, as demonstrated by the fact that it accepts antigenic material (bacterial components and food antigens through blood supply from the portal vein) in the absence of immune responses, as well as by the relatively high rate of acceptance of allogeneic transplants, in many species including humans.1 In addition, the liver is the site of persistence of common pathogens including the hepatitis B and C viruses, which exploit the relative immunological tolerance of this organ.1 Cancer cells may also take advantage of the immunoregulatory mechanisms that are established in the liver. Indeed, the liver is one of the most common sites of metastatic dissemination.
Hepatocellular carcinoma (HCC) and liver metastases from colorectal cancer (LM-CRC) are the main malignancies affecting the liver and are among the most common cancers and leading causes of cancer mortality.2,3 Curative treatment options, like liver resection, liver transplantation and image-guided percutaneous ablation, are limited to very early stages of the malignant disease and cannot prevent recurrence. In addition, HCC is extremely resistant to chemotherapy. Immunotherapy represents an attractive alternative approach because of the specificity and memory of the immune system. The main goal of anticancer immunotherapy is to induce a strong immune response that can efficiently attack and eliminate tumor cells. Several attempts to design such a therapy have been evaluated in clinical trials (reviewed in refs. 3,4). However, although vaccination therapies induced detectable numbers of circulating tumor-specific T cells, they have shown limited efficacy in terms of disease progression and patient survival. The failure of immunotherapeutic approaches against hepatic neoplasms may be related to intra-hepatic immunological tolerance, but the tumor microenvironment can also play a major role in hampering the antitumor immunity, as it has been shown in many others oncological settings.5,6
Recently, in patients affected by primary or metastatic liver cancer, we observed that the high frequency of CD8+ T cells, natural killer (NK) and NKT cells that are detected in normal liver tissue are significantly decreased in the tumor bed, while the amount of intratumoral CD4+ T cells is increased. Furthermore, we found that tumor-infiltrating CD4+ and CD8+ T cells display an impaired antitumor response.7 These dramatic changes in the composition and function of intratumoral lymphocytes indicate that hepatic tumors generate an immunopermissive environment, which may contribute to tumor progression. Importantly, we showed that CD4+FOXP3+ regulatory T cells (Tregs) accumulate in the tumors of these patients. Tumor-infiltrating Tregs (TiTregs) were highly activated as compared with Tregs isolated from tumor-free liver tissue, and showed a potent capacity to inhibit tumor-specific T-cell responses ex vivo as compared with circulating Tregs from the same patient. Therefore, we conclude that Tregs locally suppress antitumor immunity within hepatic tumors.
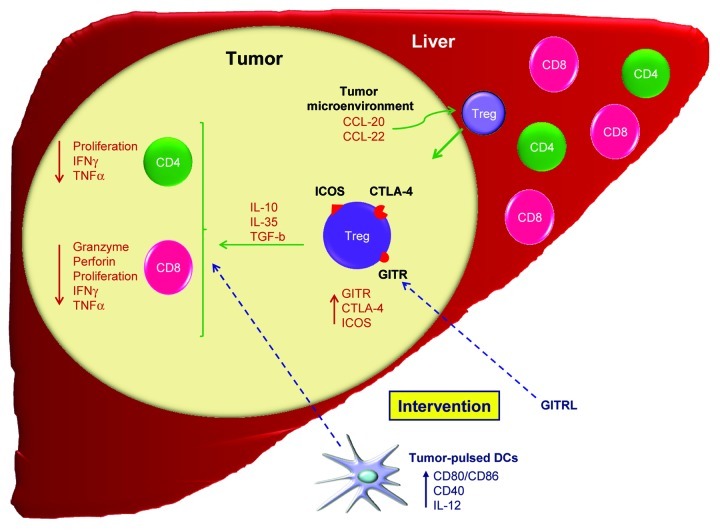
The mechanisms responsible for Tregs accumulation in hepatic tumors are not well elucidated, but a role for specific chemokines like CCL-20 and 22 has been reported.6,8 In our study, we showed that TiTreg, as found within LM-CRC, actively proliferate,7 indicating that local expansion contributes to the accumulation of TiTreg observed in this setting. In contrast, Tregs infiltrating HCC lesions seem not to proliferate, and it has been proposed that tumor-derived CCL-20 plays a major role in promoting the accumulation of Tregs in HCC lesions.9 Therefore, chemokine-mediated migration and local expansion are two important mechanisms involved in the accumulation of Tregs in hepatic tumors (Fig. 1). These findings point to a tumor-specific, rather than to an organ-specific, Treg accumulation in primary and secondary liver neoplasms.
Figure 1. Immunoregulation in hepatic cancer. The liver contains significant numbers of lymphocytes with a dominant presence of CD8+ T cells. In contrast, liver neoplasms are characterized by a dominant population of CD4+ T cells, which include an important proportion of activated CD4+FOXP3+ regulatory T cells (Tregs). Both conventional CD4+ and CD8+ T cells found within the tumor lesion are functionally impaired. Tregs can either be recruited to the tumor tissue by specific chemokines, or expand locally. Tumor-infiltrating Tregs (TiTreg) potently suppress tumor-specific T cells, for instance by expressing CTLA-4 as well as by producing immunosuppressive molecules like interleukin (IL)-10, IL-35 and transforming growth factor β (TGFβ). Tregs infiltrating hepatic tumors express high levels of glucocorticoid-induced tumor necrosis factor receptor (GITR) and inducible T-cell costimulator (ICOS), and the administration of a soluble GITR ligand (GITRL) partially abrogates the immunosuppression mediated by TiTreg. Targeting the immunosuppressive functions of TiTreg may augment the efficacy of immunotherapeutic approaches like dendritic cell (DC)-based vaccination.
High frequencies of TiTreg and their potent immunosuppressive activity may have interfered with the immunotherapeutic efforts to induce efficient antitumor immunity in liver cancer patients. Manipulation of Treg function may therefore be an attractive option to improve the outcome of immunotherapy in patients with hepatic cancer. In our study, we found that Tregs infiltrating both HCC and LM-CRC lesions are characterized by an elevated expression of the glucocorticoid-induced tumor necrosis factor receptor (GITR) and the inducible T-cell costimulator (ICOS) as compared with Tregs purified from tumor-free liver tissue and the blood. Similar findings have been reported for breast tumors.8 GITR and ICOS are regulators of the immunosuppressive function of Tregs and can be targeted for immunotherapeutic interventions. In our study, we showed that a soluble GITR ligand (GITRL) partially prevents the immunosuppressive activity of TiTreg derived from both HCC and LM-CRC lesions. Even though the mechanism by which GITRL exerts immunostimulatory effects is still elusive, it has been suggested that it can act either by abrogating the immunosuppressive functions of Tregs or by rendering effector T cells resistant to Treg-mediated suppression.10 Of note, GITR expressed on activated conventional T cells acts as a costimulatory molecule.10 These observations suggest that modulating GITR may serve as an adjuvant for the immunotherapy of hepatic tumors, restoring the effector functions of CD4+ and CD8+ T cells and enhancing tumor-specific T-cell activity.
Our data not only provide new insights into the pathogenesis of hepatic malignancies, but also identify a potentially novel therapeutic target. Based on these findings, we propose that reversing the immunosuppressive tumor microenvironment with GITRL or other molecules that may affect the suppressive capacity of TiTreg, like ICOS and CTLA-4 blocking antibodies, is critical to restore local antitumor immune responses. Therefore, immunotherapeutic approaches, such as dendritic cell (DC)-based vaccination or tumor-specific T cell transfer, may benefit from the targeting of TiTreg to prevent the local suppression of antitumor T-cell responses (Fig. 1).
Glossary
Abbreviations:
- GITR
glucocorticoid-induced tumor necrosis factor receptor
- HCC
hepatocellular carcinoma
- ICOS
inducible T-cell costimulator
- LM-CRC
liver metastases from colorectal cancer
- Tregs
regulatory T cells
- Ti-Tregs
tumor-infiltrating Tregs
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22450
References
- 1.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–63. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 2.Kemeny N. The management of resectable and unresectable liver metastases from colorectal cancer. Curr Opin Oncol. 2010;22:364–73. doi: 10.1097/CCO.0b013e32833a6c8a. [DOI] [PubMed] [Google Scholar]
- 3.Pardee AD, Butterfield LH. Immunotherapy of hepatocellular carcinoma: Unique challenges and clinical opportunities. Oncoimmunology. 2012;1:48–55. doi: 10.4161/onci.1.1.18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208:479–90. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 7.Pedroza-Gonzalez A, Verhoef C, Ijzermans JN, Peppelenbosch MP, Kwekkeboom J, Verheij J, et al. Activated tumor-infiltrating CD4+ regulatory T cells restrain antitumor immunity in patients with primary or metastatic liver cancer. Hepatology. 2012 doi: 10.1002/hep.26013. In Press. [DOI] [PubMed] [Google Scholar]
- 8.Faget J, Biota C, Bachelot T, Gobert M, Treilleux I, Goutagny N, et al. Early detection of tumor cells by innate immune cells leads to T(reg) recruitment through CCL22 production by tumor cells. Cancer Res. 2011;71:6143–52. doi: 10.1158/0008-5472.CAN-11-0573. [DOI] [PubMed] [Google Scholar]
- 9.Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One. 2011;6:e24671. doi: 10.1371/journal.pone.0024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nocentini G, Riccardi C. GITR: a modulator of immune response and inflammation. Adv Exp Med Biol. 2009;647:156–73. doi: 10.1007/978-0-387-89520-8_11. [DOI] [PubMed] [Google Scholar]