Abstract
Metastatic melanoma lesions sometimes host ectopic lymphoid structures exhibiting ongoing adaptive immune responses. Here, we discuss how this finding fits in our current view of antitumor immunity.
Keywords: ectopic lymphoid structures, germinal center, humoral response, IgA, lymphoid neogenesis, melanoma, metastasis
We have recently reported in Cancer Research the presence of ectopic lymphoid structures, also called tertiary lymphoid organs, in a subset of metastatic melanoma lesions. These structures are organized in B-cell follicles, adjacent T-cell areas and neighboring high endothelial venules, and thus contain all major components that are required to support local adaptive B- and T-cell responses. The presence of germinal centers and evidence of immunoglobulin affinity maturation in some follicles point to the existence of ongoing B-cell responses. The intimate association of mature dendritic cells and T lymphocytes in the T-cell areas suggests that T-cell responses also take place inside these structures (Fig. 1). Altogether, these findings indicate that adaptive immune responses can develop within the metastatic tumor environment.1 At this stage, we ignore whether these responses are specific for melanoma-associated antigens. This interpretation is supported by the fact that local lymphocyte responses have previously been shown to target antigens expressed in situ in several mouse models of lymphoid neogenesis and in human autoimmune diseases.2
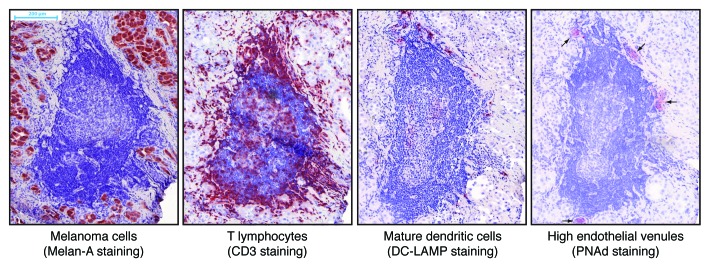
Figure 1. Microscopic images of an ectopic lymphoid structure in a metastatic melanoma lesion. Sequential cryosections from the tumor were immunostained against the indicated antigens and counterstained with hematoxylin. Positively stained antigens appear in red. The central structure with dense blue nuclei in the center of the images is a B-cell follicle, and the central pale structure is a germinal center. A T-cell area associating mature dendritic cells, T cells aggregates and high endothelial venules can be observed on top of the follicle.
The neogenesis of lymphoid centers in melanoma lesions is not a real surprise, as ectopic lymphoid structures have previously been described in other types of cancer, including lung, breast, colorectal and germline tumors. Most presumably, this applies to an even broader range of tumor types. Still, melanoma represents by far the tumor most extensively studied by immunologists of all. In part, this stems from the fact that cultured melanoma cells can easily give rise to immortalized cell lines. In our hands, these melanoma cell lines have been instrumental for the identification of several tumor-specific antigens, the first being members of the MAGE family.3 Moreover, the availability of a broad panel of melanoma-derived cell lines has allowed us to study antitumor T-cell responses in melanoma patients in great detail.4 These cell lines, together with the knowledge that they generated, should also be considered unique tools to investigate whether B- and T-cell responses occurring in ectopic lymphoid structures de facto target tumor-associated antigens, to determine if the genesis of these structures is controlled by tumor-derived factors and, if so, to understand why lymphoid neogenesis occurs in some, but not all, tumors.
Our findings may influence the current view about the origin of melanoma-infiltrating lymphocytes. Indeed, it is widely believed that these cells derive from lymphocyte precursors that have been activated at extratumoral sites, presumably within secondary lymphoid organs. Following activation, lymphocytes express chemokine receptors and cell adhesion molecules, migrate into circulation and reach distant tumor sites, which attract them by expressing corresponding inflammatory chemokines, chemotactic signals and binding partners for adhesion molecules. This phenomenon may involve antitumor lymphocytes as well as lymphocytes that have recently been activated against other antigens. An alternative mechanism of lymphocyte infiltration can be proposed for tumors that host ectopic lymphoid structures. In these tumors, tumor-infiltrating lymphocytes can derive from naïve or memory lymphocytes that have abandoned circulation to reach the tumor bed via high endothelial venules. Consistent with this, lymphocytes with a naïve phenotype have been observed in lung cancer-associated ectopic lymphoid structures.5 Such lymphocytes may encounter their cognate antigen in situ, become activated and proliferate either in the follicle or in the T-cell area, from where they can reach the bulk of the tumor. The contribution of this pathway to lymphocyte infiltration in metastatic melanoma is probably modest, as in this setting activation seems rather unlikely, considering the very low frequency of circulating tumor-specific lymphocytes, and lymphoid neogenesis is observed in only 10–30% of melanoma metastases.
Our findings also relate to the current concept of tumor resistance against destruction by the immune system, which conceives the tumor environment as strongly immunosuppressive, hosting mainly anergic lymphocytes. This view has been challenged by recent publications that correlated the extent of tumor infiltration by lymphocytes to improved disease outcome.6 Lymphoid neogenesis arises as a consequence of sustained immune activation, is associated with chronic inflammatory diseases in which immune-mediated tissue destruction occurs, and generates newly activated B and T lymphocytes. Therefore, the presence of ectopic lymphoid structures in tumors argues against the fact that the tumor microenvironment would be characterized by a profound and generalized immunosuppression.
An important question that remains unanswered is whether the presence of lymphoid neogenesis influences the clinical course of the neoplastic disease. In some auto-immune diseases, the presence of lymphoid neogenesis has been correlated with a worsened prognosis.2 Conversely, in lung cancer patients, the density of mature dendritic cells in ectopic lymphoid structures has been associated with a better clinical outcome.7 Another open question is whether the presence of lymphoid neogenesis in tumors can predict the clinical response to immunotherapeutic approaches such as cancer vaccines and monoclonal antibodies targeting T-cell co-stimulation.
By studying ectopic lymphoid structures in melanoma, we have made two unexpected observations. First, antibody responses that take place in follicles comprise IgA responses. IgA class switching is expected to occur in secondary lymphoid organs that drain mucosal tissues, but not in cutaneous metastases of melanoma. The reason underlying this observations and its consequences are unknown. Second, we observed lymphoid follicles in melanoma metastases, but never in primary melanoma lesions, in spite of the fact that the latter frequently contain high endothelial venules.1,8 This sharp contrast suggests that the development of follicles and high endothelial venules is controlled by distinct factors, and that signals required for lymphoid neogenesis are missing in primary melanomas. These findings offer an opportunity to investigate some unknown aspects of both lymphoid neogenesis and antitumor immunity. Melanoma once again stands out as a unique model, given that in non-melanoma malignancies lymphoid neogenesis has been reported exclusively in primary tumor lesions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22505
References
- 1.Cipponi A, Mercier M, Seremet T, Baurain JF, Théate I, van den Oord J, et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 2.Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33:297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 4.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 5.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–9. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 6.Fridman W-H, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 7.Dieu-Nosjean M-C, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–7. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 8.Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–87. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]


