Abstract
We have recently performed the first comprehensive study of the potential immunosuppressive role of soluble CD40L (sCD40L). In addition, we demonstrated that serum sCD40L can potentially be used as an indicator of response to anticancer therapy, and/or to better identify those patients who would have best chances to benefit from tumor-targeting vaccines or other therapeutic modalities.
Keywords: immunosuppression, myeloid derived suppressor cells (MDSC), regulatory T cells (Tregs), sCD40L, vaccine therapy
Tumor cells and the tumor microenvironment can produce immunosuppressive factors such as transforming growth factor β (TGFβ) and vascular endothelial growth factor (VEGF). These can drive the expansion of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSC), both of which can reduce or eliminate antitumor immune responses as generated by vaccines or other forms of immunotherapy.
The CD40-CD40L co-stimulatory pathway has been shown to play a crucial role in the production of cytokines, including interleukin (IL)-10 and IL-12, by monocytes and macrophages. Among various functions, these cytokines also modulate the activity of T lymphocytes and antitumor responses. Many tumor cells also express CD40, and evidence suggests that ligation of CD40 can promote either proliferation or apoptosis, depending on the intensity of CD40L signaling.
Soluble CD40L (sCD40L) is an 18-KDa trimer that is shed by activated T lymphocytes and platelets. It has previously been reported that sCD40L is produced at high levels in lung and nasopharyngeal carcinoma lesions.1 Cancer patients also manifest an elevated propensity for platelet activation.2 We have investigated the role of monomeric sCD40L in the human serum with particular attention to its activity on immune cells and its potential immunosuppressive effects.3
Clinical Correlates
Our investigations have revealed (1) no age-related differences in sCD40L serum levels among healthy donors, and (2) higher levels of circulating sCD40L in patients with metastatic breast and colon cancer, as well as primary and metastatic castration-resistant prostate cancer (mCRPC), compared with age-matched healthy donors.3
A multicenter randomized trial of a viral vaccine developed at the National Cancer Institute against prostate cancer (PROSTVAC®) reported a statistically significant improvement in survival vs. the vector control arm.4 A second phase II study also provided evidence of improved survival in vaccinated mCRPC patients. The Halabi nomogram is used to predict survival in patients with mCRPC following treatment with chemotherapy or hormonal therapy. Patients who survived longer than predicted by the nomogram had lower levels of serum sCD40L prior to vaccination than those surviving shorter than predicted.5 The analysis of a trial enrolling mCRPC patients6 shows that chemotherapy also lowers serum sCD40L levels.
Effect of sCD40L on MDSCs and Tregs
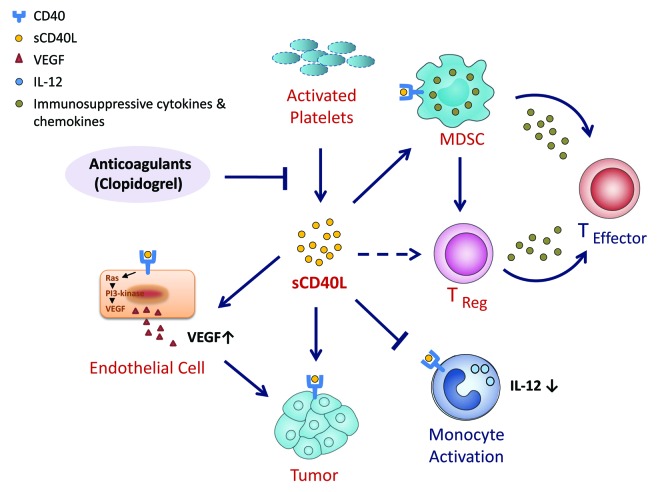
In vitro studies were then performed to investigate possible mechanism(s) by which sCD40L may induce immunosuppression.3 These studies revealed that (1) the frequency of CD40+ MDSCs in cancer patients is much higher than in healthy donors, suggesting that MDSCs from cancer patients are more likely to respond to sCD40L signaling; (2) sCD40L promoted the proliferation of MDSCs in vitro, in a dose-dependent fashion, and (3) sCD40L inhibits the proliferation of autologous T cells co-cultured with MDSCs and their ability to secrete interferon γ (IFNγ). All these effects (Fig. 1) could be reversed by the addition of an anti-CD40 blocking antibody.
Figure 1. The role of sCD40L in immunosuppression. It has been demonstrated that (1) there is a much greater frequency of CD40+ myeloid-derived suppressor cells (MDSCs) in cancer patients than in healthy donors, suggesting that MDSCs from cancer patients are more likely to respond to sCD40L signaling, and that (2) in co-culture models, sCD40L promotes the expansion of MDSCs while inhibiting the proliferation of T cells and their capacity to secrete interferon γ (IFNγ). The administration of sCD40L to cultured peripheral blood mononuclear cells (PBMCs) also resulted in a significant expansion of regulatory T cells (Tregs) and in increased levels of activation markers on T cells from both healthy donors and cancer patients. Only the latter, however, responded to sCD40L by upregulating the inhibitory receptor PD-1. sCD40L has also been shown to block the activation of purified monocytes and the consequent production of interleukin-12 (IL-12).
The addition of sCD40L to cultured peripheral blood mononuclear cells (PBMCs) also resulted in (1) a significant expansion of Tregs and (2) an increase in T-cell activation markers (CD25 and CD70). This was observed in T cells from both healthy donors and cancer patients, but only the latter upregulated the inhibitory receptor PD-1 and produced increased levels of IL-10 and IL-6 in response to sCD40L. sCD40L also blocked the activation of purified monocytes and the consequent production of IL-12 (Fig. 1).
While sCD40L/CD40 interactions have been studied in the context of angiogenesis, carcinogenesis and immune activation, ours were the first studies to comprehensively examine the potential role of serum sCD40L as a negative regulator of anticancer immune responses. These studies highlighted the existence of a fine balance between positive and negative immune responses based on the strength of the signal involved.
Interplay of Immune Therapy with Non-Immune Therapeutics
Our studies reveal yet another example of the potential interplay between chemotherapy (and perhaps other non-immune forms of therapy) and the immune system. Following chemotherapy, mCRPC patients displayed reduced levels of circulating sCD40L. Thus, one could hypothesize that, upon the reduction of tumor burden following surgery, radiation, chemotherapy, and/or small molecule targeted therapies, an immune-based therapy would benefit from reduced levels of sCD40L.
Our findings on sCD40L thus add to the growing body of evidence that demonstrate an interplay between non-immune therapies and immunotherapy, including (1) immunogenic tumor cell death,7 as triggered by some chemotherapeutic agents, (2) the phenotypic modulation of tumor cells induced by specific chemotherapeutic agents and radiation, which renders neoplastic cells more sensitive to T-cell killing,8 and (3) the infiltration of primary tumors by lymphocytes, which constitutes a prognostic indicator of response to subsequent chemotherapy.9
Prognostic Implications
There are also potential prognostic applications in the analysis of circulating sCD40L. For instance, the levels of sCD40L in the serum can potentially be employed to monitor the response of patients to chemotherapy or other forms of therapy. In addition, as demonstrated in the clinical study involving the PROSTVAC® vaccine in mCRPC patients, the pre-treatment level of sCD40L correlated with predicted overall survival as calculated by means of the Halabi nomogram. While additional trials need to be conducted to validate this observation, the levels of sCD40L in patients with metastatic cancer could potentially be employed to identify which patients have the highest chances to favorably respond to anticancer vaccines or other immunotherapeutic regimens.
As mentioned above, sCD40L is produced by activated platelets. Anti-clotting agents such as clopidogrel bisulfate (Plavix®) have been shown to lower sCD40L levels in patients with type II diabetes and coronary artery disease,10 two conditions in which platelet activation constitutes an etiological determinant. These studies thus provide a rationale for identifying agents that may reduce the levels of sCD40L in cancer patients treated with immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22546
References
- 1.Roselli M, Mineo TC, Basili S, Martini F, Mariotti S, Aloe S, et al. Soluble CD40 ligand plasma levels in lung cancer. Clin Cancer Res. 2004;10:610–4. doi: 10.1158/1078-0432.CCR-0348-03. [DOI] [PubMed] [Google Scholar]
- 2.Osada J, Rusak M, Kamocki Z, Dabrowska MI, Kedra B. Platelet activation in patients with advanced gastric cancer. Neoplasma. 2010;57:145–50. doi: 10.4149/neo_2010_02_145. [DOI] [PubMed] [Google Scholar]
- 3.Huang J, Jochems C, Talaie T, Anderson A, Jales A, Tsang KY, et al. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood. 2012;120:3030–8. doi: 10.1182/blood-2012-05-427799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–74. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figg WD, Liu Y, Arlen P, Gulley J, Steinberg SM, Liewehr DJ, et al. A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. J Urol. 2005;173:790–6. doi: 10.1097/01.ju.0000147013.09157.8e. [DOI] [PubMed] [Google Scholar]
- 7.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100–4. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 8.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–44. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10.Azar RR, Kassab R, Zoghbi A, Aboujaoudé S, El-Osta H, Ghorra P, et al. Effects of clopidogrel on soluble CD40 ligand and on high-sensitivity C-reactive protein in patients with stable coronary artery disease. Am Heart J. 2006;151:521.e1–521.e4. doi: 10.1016/j.ahj.2005.10.021. [DOI] [PubMed] [Google Scholar]