Abstract
To understand the physiological functions of exosomes, we have recently used the inhibition of Rab27a, which prevents exosome release but also alters other secretion pathways. Our work demonstrates that the secretion of exosomes by some tumors in vivo can influence the immune microenvironment to promote tumor progression, but also that this phenomenon cannot be generalized to all tumors and all exosomes.
Keywords: exosomes, extracellular vesicles, Rab27a, tumor immunity
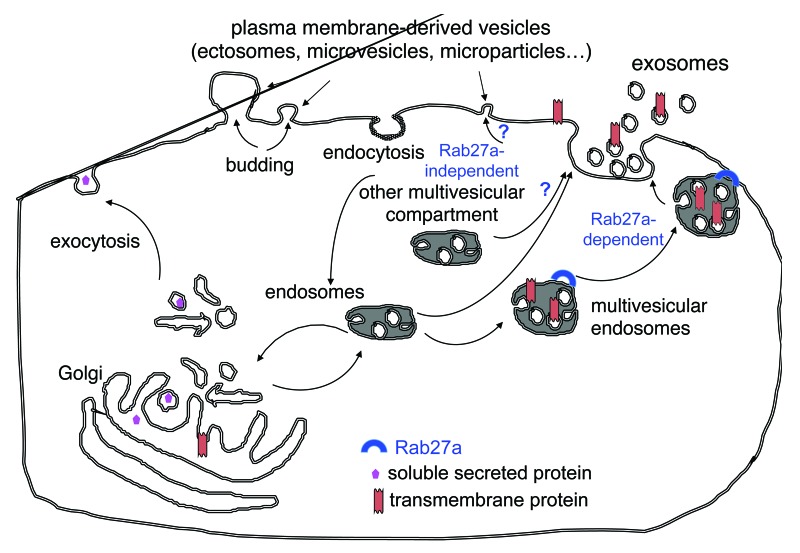
Exosomes are a particular type of membrane vesicles that most cells secrete in their extracellular environment.1 Whereas other vesicles are released by direct budding from the plasma membrane (Fig. 1), exosomes are secreted in a two-step process whereby vesicles form inside the lumen of endosomes, which then fuse with the plasma membrane to release in the extracellular environment such inner vesicles.2
Figure 1. Secretion of multiple types of extracellular vesicles. Cells simultaneously release membrane-enclosed vesicles formed either by direct budding of the plasma membrane (microvesicles, ectosomes, microparticles…) or by initial formation inside multivesicular endosomal compartments followed by fusion with the plasma membrane (exosomes). We have shown that Rab27a is required for the secretion of exosomes, but that other vesicles that are co-purified with exosomes by the classical ultracentrifugation protocol (and thus exhibit a comparable size) are secreted in a Rab27a-independent manner. Whether these latter vesicles are formed in other types of multivesicular compartments, or directly bud off the plasma membrane remains elusive.
In the 1980s, exosomes were only suggested to serve as means to eliminate unwanted components, e.g., plasma membrane receptors during the maturation of reticulocytes.2 A more complex function of exosomes emerged in the late 1990s, when exosomes secreted by antigen-presenting cells were shown to activate T lymphocytes, hence inducing immune responses.3,4 Indeed, once released in the extracellular environment, exosomes can bind to cell surface receptors and/or be internalized by/fuse with a target cell. The cargo of exosomes, including specific proteins, lipids and nucleic acids, can then promote various forms of intracellular signaling. Exosomes are nowadays considered as conveyors of messages from one cell to another.
In particular, the function of exosome release in the context of tumor development has been the subject of intensive research during the past 14 years. Tumor cells can secrete exosomes, which, initially, were shown to constitute a prominent source of tumor antigens for dendritic cells (DCs) to induce antitumor immune responses.5 Although many groups confirmed these observations, especially for exosomes secreted by stressed tumor cells, subsequent studies were published demonstrating that tumor exosomes also can inhibit antitumor immune responses, notably by preventing efficient T-cell, natural killer (NK)-cell, or DC activation, and/or by promoting the development of immunosuppressive cells such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (reviewed in Ref.6). More recently, additional pro-tumoral effects of exosomes have been demonstrated, including their ability to promote angiogenesis and metastasis. Of note, all these studies analyzed the in vitro or in vivo functions of exosomes that had been purified and concentrated in vitro. Hence, the actual outcome of the secretion of exosomes by tumor cells in a truly physiological setting remained unknown.
To bypass this artifactual situation, we have recently started to manipulate tumor cells in vitro, to affect in a stable manner exosome release, and then to analyze in vivo their behavior, in terms of growth and interaction with the immune system. By performing a mid-scale screen of a library of short hairpin (sh)RNA, we demonstrated that the depletion of RAB27A, a protein involved in intracellular trafficking, leads to a drastic reduction in the release of exosomes, but not of soluble proteins, by human cervical carcinoma HeLa cells.7 We thus used this tool to limit exosome secretion in two mouse mammary carcinoma cell lines, stably transfectiong them with a Rab27a-specific shRNA.8,9 These malignant cell lines were then subcutaneously injected in immunocompetent mice and tumor progression was monitored.8 Interestingly, 4T1 cell-derived control tumors grew progressively and formed metastases, whereas those in which Rab27a expression was inhibited developed poorly. By combining the in vitro depletion of exosomes (by the ultracentrifugation of conditioned medium) with their in vivo injection into growing Rab27a-depleted tumors, we could demonstrate that a combination of tumor exosomes and tumor-derived soluble factors promoted the differentiation, recruitment into the tumor and/or activation of neutrophils that were required for tumor growth. Of note, no role of adaptive immunity and NK cells was evidenced in our model of Rab27a-dependent 4T1 tumor progression. In the TS/A model, however, Rab27a inhibition did not prevent tumor growth, nor did it affect the immune microenvironment. Accordingly, we did not observe a positive role for TS/A exosomes in tumor progression. We showed that TS/A cells secrete a different array of soluble factors and functionally different exosomes as compared with 4T1 cells, and that they do not rely on neutrophils for in vivo progression. In summary, our work conclusively demonstrates for the first time that a physiological secretion of exosomes in vivo can promote tumor progression, but it also highlights the variability of exosome functions, which depend on the intrinsic microenvironment of each tumor.
Importantly, by analyzing the general secretome of Rab27a-depleted cells, we showed that the secretion of a subset of exosome-independent soluble proteins was also modified.8 In particular, the absence of Rab27a abolished the secretion of the metalloproteinase 9 (MMP9), which is required for efficient metastatic colonization of 4T1 cells. Therefore, Rab27a is not a truly specific target to modulate the secretion of exosomes, and complementary experiments must be performed, as we did, to unravel the relative contributions of exosomes and soluble factors to the phenotype observed upon Rab27a depletion.
Finally, it is important to note that cells simultaneously secrete different types of vesicles, originating from different intracellular locations (Fig. 1). In our opinion, because of the specific intracellular site of exosome formation, their constitutive components differ from those found in vesicles budding from the plasma membrane, and the functions of exosomes and of the other secreted vesicles must be different. We and others have recently evidenced that vesicles obtained with the classical differential ultracentrifugation method are heterogeneous in their size, density, composition and biogenesis (i.e. some depend on Rab27a for their secretion while others do not).9,10 We are now trying to understand if all these vesicles are different kinds of exosomes coming from different types of intracellular multivesicular compartments, or if some are instead released directly from the plasma membrane (Fig. 1).
The next challenge for the field will be to develop new protocols/tools to separate to purity the different types of extracellular vesicles released by a single type of tumor cell, and to compare side-by-side their physiological functions. Refining our knowledge on the nature, composition and function of extracellular vesicles will help us to improve their use as diagnostic markers and perhaps antitumor agents.
Acknowledgments
I want to thank my collaborators, without whom the experiments described in this viewpoint would not have been possible, especially Angélique Bobrie, Marina Colombo and Matias Ostrowski. Work performed in my group is supported by INSERM, Institut Curie, Agence Nationale de la Recherche, Association pour la Recherche contre le Cancer, Fondation de France, Institut National du Cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22565
References
- 1.Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–68. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–20. [PubMed] [Google Scholar]
- 3.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 5.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 6.Zhang HG, Zhuang X, Sun D, Liu Y, Xiang X, Grizzle WE. Exosomes and immune surveillance of neoplastic lesions: a review. Biotech Histochem. 2012;87:161–8. doi: 10.3109/10520291003659042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30, 1-13. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 8.Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–30. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 9.Bobrie A, Colombo M, Krumeich S, Raposo G, Thery C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. Journal of Extracellular Vesicles. 2012;1:18397. doi: 10.3402/jev.v1i0.18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aalberts M, van Dissel-Emiliani FM, van Adrichem NP, van Wijnen M, Wauben MH, Stout TA, et al. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol Reprod. 2012;86:82. doi: 10.1095/biolreprod.111.095760. [DOI] [PubMed] [Google Scholar]