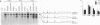Abstract
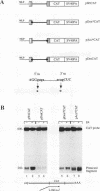
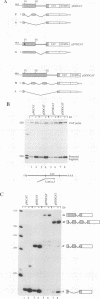
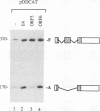
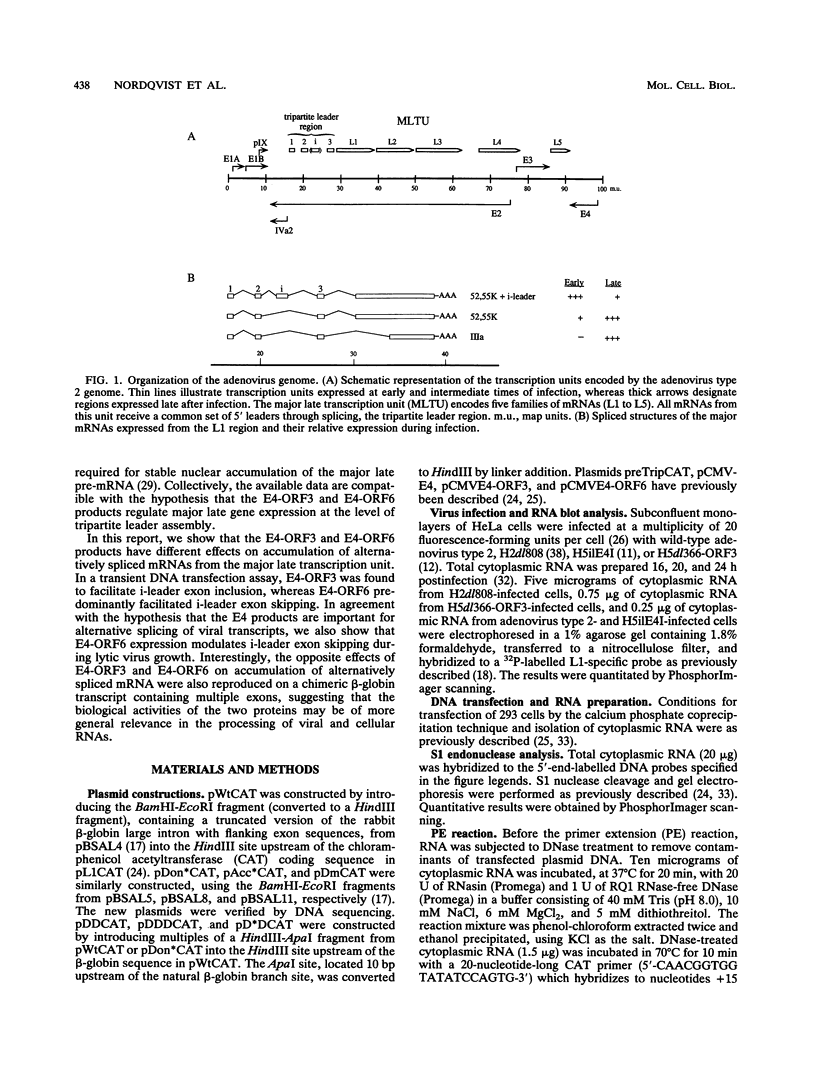
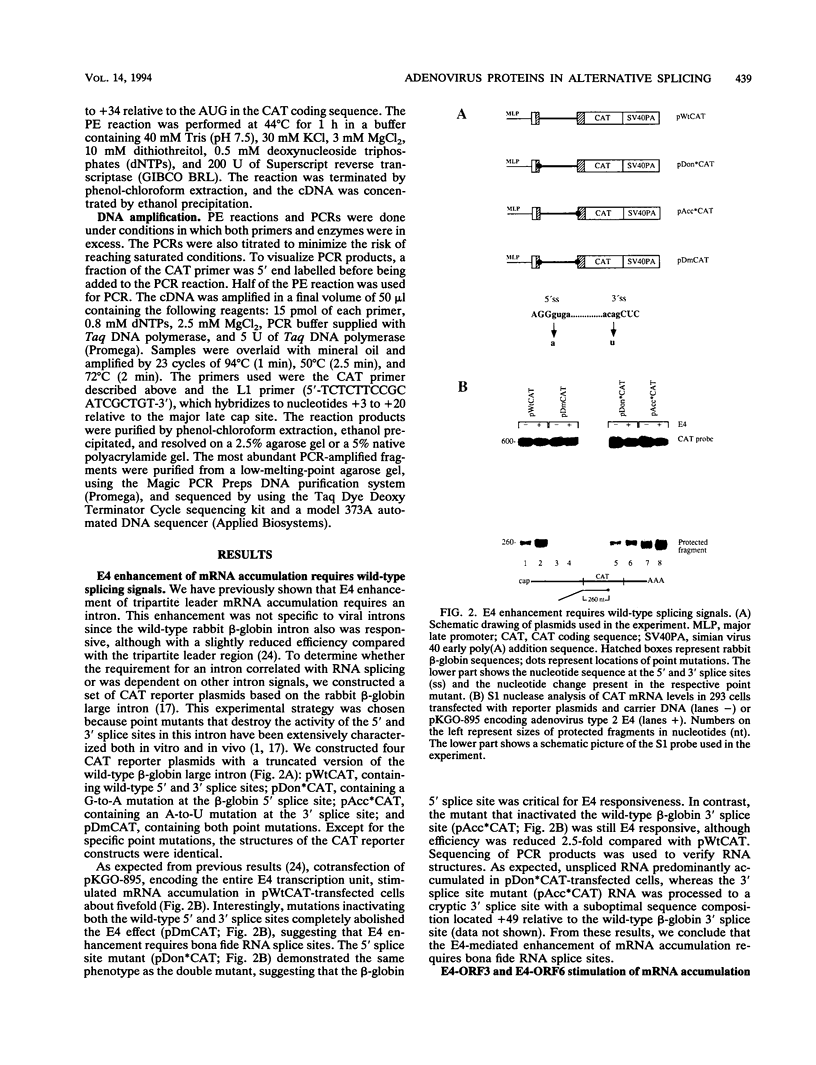
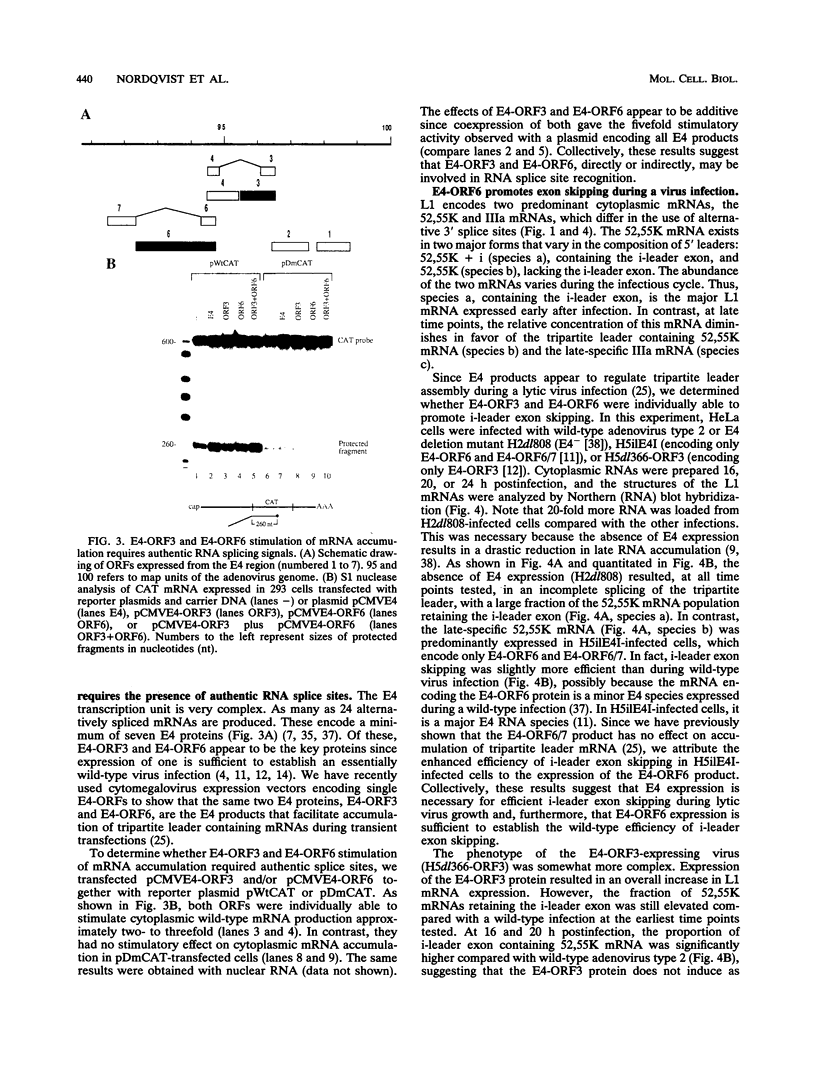
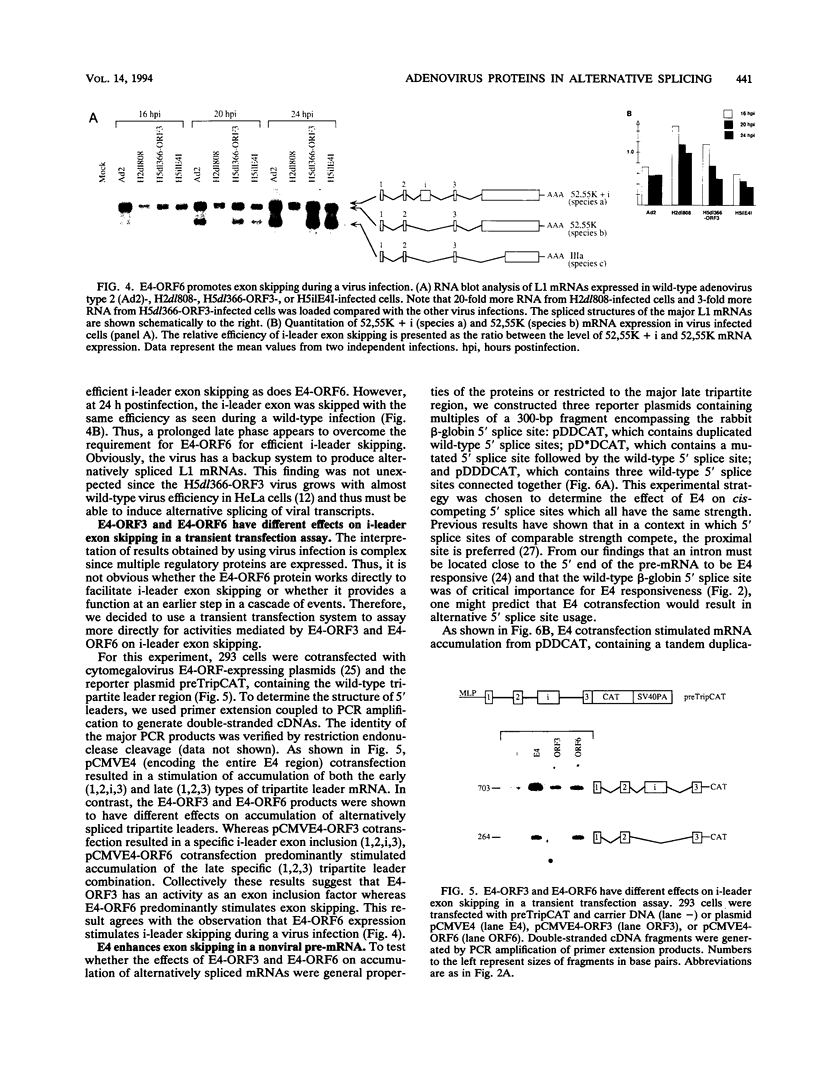
All mRNAs expressed from the adenovirus major late transcription unit have a common, 201-nucleotide-long 5' leader sequence, which consists of three short exons (the tripartite leader). This leader has two variants, either with or without the i-leader exon, which, when present, is spliced between the second and the third exons of the tripartite leader. Previous studies have shown that adenovirus early region 4 (E4) encodes two proteins, E4 open reading frame 3 (E4-ORF3) and E4-ORF6, which are required for efficient expression of mRNAs from the major late transcription unit. These two E4 proteins appear to have redundant activities, and expression of one has been shown to be sufficient for efficient major late mRNA accumulation during a lytic virus infection. In this report, we provide evidence that E4-ORF3 and E4-ORF6 both regulate major late mRNA accumulation by stimulating constitutive splicing. Moreover, we show that the two proteins have different effects on accumulation of alternatively spliced tripartite leader exons. In a DNA transfection assay, E4-ORF3 was shown to facilitate i-leader exon inclusion, while E4-ORF6 preferentially favored i-leader exon skipping. In addition, E4-ORF3 and E4-ORF6 had the same effects on accumulation of alternatively spliced chimeric beta-globin transcripts. This finding suggests that the activities of the two proteins may be of more general relevance and not restricted to splicing of major late tripartite leader-containing pre-mRNAs. Interestingly, E4-ORF6 expression was also shown to stimulate i-leader exon skipping during a lytic virus infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Akusjärvi G., Persson H. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature. 1981 Jul 30;292(5822):420–426. doi: 10.1038/292420a0. [DOI] [PubMed] [Google Scholar]
- Bridge E., Ketner G. Redundant control of adenovirus late gene expression by early region 4. J Virol. 1989 Feb;63(2):631–638. doi: 10.1128/jvi.63.2.631-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin C., Bank A. Reversibility of IVS 2 missplicing in a mutant human beta-globin gene. J Biol Chem. 1985 Dec 25;260(30):16332–16337. [PubMed] [Google Scholar]
- Dominski Z., Kole R. Cooperation of pre-mRNA sequence elements in splice site selection. Mol Cell Biol. 1992 May;12(5):2108–2114. doi: 10.1128/mcb.12.5.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer G. A., Katoh Y., Roberts R. J. Characterization of the major mRNAs from adenovirus 2 early region 4 by cDNA cloning and sequencing. Nucleic Acids Res. 1984 Apr 25;12(8):3503–3519. doi: 10.1093/nar/12.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H., Zuo P., Manley J. L. Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell. 1991 Jul 26;66(2):373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- Halbert D. N., Cutt J. R., Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985 Oct;56(1):250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. E., Manley J. L. A novel protein factor is required for use of distal alternative 5' splice sites in vitro. Mol Cell Biol. 1991 Dec;11(12):5945–5953. doi: 10.1128/mcb.11.12.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemström C., Nordqvist K., Pettersson U., Virtanen A. Gene product of region E4 of adenovirus type 5 modulates accumulation of certain viral polypeptides. J Virol. 1988 Sep;62(9):3258–3264. doi: 10.1128/jvi.62.9.3258-3264.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. M., Hearing P. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J Virol. 1989 Jun;63(6):2605–2615. doi: 10.1128/jvi.63.6.2605-2615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Ketner G., Bridge E., Virtanen A., Hemström C., Pettersson U. Complementation of adenovirus E4 mutants by transient expression of E4 cDNA and deletion plasmids. Nucleic Acids Res. 1989 Apr 25;17(8):3037–3048. doi: 10.1093/nar/17.8.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainer A. R., Mayeda A., Kozak D., Binns G. Functional expression of cloned human splicing factor SF2: homology to RNA-binding proteins, U1 70K, and Drosophila splicing regulators. Cell. 1991 Jul 26;66(2):383–394. doi: 10.1016/0092-8674(91)90627-b. [DOI] [PubMed] [Google Scholar]
- Kuo H. C., Nasim F. H., Grabowski P. J. Control of alternative splicing by the differential binding of U1 small nuclear ribonucleoprotein particle. Science. 1991 Mar 1;251(4997):1045–1050. doi: 10.1126/science.1825520. [DOI] [PubMed] [Google Scholar]
- Lamond A. I., Konarska M. M., Sharp P. A. A mutational analysis of spliceosome assembly: evidence for splice site collaboration during spliceosome formation. Genes Dev. 1987 Aug;1(6):532–543. doi: 10.1101/gad.1.6.532. [DOI] [PubMed] [Google Scholar]
- Larsson S., Svensson C., Akusjärvi G. Control of adenovirus major late gene expression at multiple levels. J Mol Biol. 1992 May 20;225(2):287–298. doi: 10.1016/0022-2836(92)90922-7. [DOI] [PubMed] [Google Scholar]
- Legrain P., Choulika A. The molecular characterization of PRP6 and PRP9 yeast genes reveals a new cysteine/histidine motif common to several splicing factors. EMBO J. 1990 Sep;9(9):2775–2781. doi: 10.1002/j.1460-2075.1990.tb07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W. RNA recognition: a family matter? Cell. 1993 Jun 4;73(5):837–840. doi: 10.1016/0092-8674(93)90265-r. [DOI] [PubMed] [Google Scholar]
- Mayeda A., Helfman D. M., Krainer A. R. Modulation of exon skipping and inclusion by heterogeneous nuclear ribonucleoprotein A1 and pre-mRNA splicing factor SF2/ASF. Mol Cell Biol. 1993 May;13(5):2993–3001. doi: 10.1128/mcb.13.5.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda A., Krainer A. R. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell. 1992 Jan 24;68(2):365–375. doi: 10.1016/0092-8674(92)90477-t. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Wilson M. C. Regulation of adenovirus-2 gene expression at the level of transcriptional termination and RNA processing. Nature. 1981 Mar 12;290(5802):113–118. doi: 10.1038/290113a0. [DOI] [PubMed] [Google Scholar]
- Nordqvist K., Akusjärvi G. Adenovirus early region 4 stimulates mRNA accumulation via 5' introns. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9543–9547. doi: 10.1073/pnas.87.24.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman K., Nordqvist K., Akusjärvi G. Two adenovirus proteins with redundant activities in virus growth facilitates tripartite leader mRNA accumulation. Virology. 1993 May;194(1):50–58. doi: 10.1006/viro.1993.1234. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L. Adenovirus assay by the fluorescent cell-counting procedure. Virology. 1961 Nov;15:263–268. doi: 10.1016/0042-6822(61)90357-9. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. A role for exon sequences and splice-site proximity in splice-site selection. Cell. 1986 Aug 29;46(5):681–690. doi: 10.1016/0092-8674(86)90343-0. [DOI] [PubMed] [Google Scholar]
- Robberson B. L., Cote G. J., Berget S. M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol. 1990 Jan;10(1):84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler A. B., Ketner G. Adenovirus early region 4 is essential for normal stability of late nuclear RNAs. J Virol. 1989 Feb;63(2):624–630. doi: 10.1128/jvi.63.2.624-630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Soloway P. D., Shenk T. The adenovirus type 5 i-leader open reading frame functions in cis to reduce the half-life of L1 mRNAs. J Virol. 1990 Feb;64(2):551–558. doi: 10.1128/jvi.64.2.551-558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C., Akusjärvi G. Adenovirus VA RNAI: a positive regulator of mRNA translation. Mol Cell Biol. 1984 Apr;4(4):736–742. doi: 10.1128/mcb.4.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C., Pettersson U., Akusjärvi G. Splicing of adenovirus 2 early region 1A mRNAs is non-sequential. J Mol Biol. 1983 Apr 15;165(3):475–495. doi: 10.1016/s0022-2836(83)80214-9. [DOI] [PubMed] [Google Scholar]
- Symington J. S., Lucher L. A., Brackmann K. H., Virtanen A., Pettersson U., Green M. Biosynthesis of adenovirus type 2 i-leader protein. J Virol. 1986 Mar;57(3):848–856. doi: 10.1128/jvi.57.3.848-856.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges M. A., Raskas H. J. Splice junctions in adenovirus 2 early region 4 mRNAs: multiple splice sites produce 18 to 24 RNAs. J Virol. 1984 Apr;50(1):106–117. doi: 10.1128/jvi.50.1.106-117.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Virtanen A., Gilardi P., Näslund A., LeMoullec J. M., Pettersson U., Perricaudet M. mRNAs from human adenovirus 2 early region 4. J Virol. 1984 Sep;51(3):822–831. doi: 10.1128/jvi.51.3.822-831.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D. H., Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J Virol. 1986 Mar;57(3):833–838. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Patton J. G., Green M. R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992 Feb 13;355(6361):609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]