Abstract
Novel strategies for the therapy of recurrent ovarian cancer are warranted. We report a study of a combinatorial approach encompassing dendritic cell (DC)-based autologous whole tumor vaccination and anti-angiogenesis therapy, followed by the adoptive transfer of autologous vaccine-primed CD3/CD28-co-stimulated lymphocytes. Recurrent ovarian cancer patients for whom tumor lysate was available from prior cytoreductive surgery underwent conditioning with intravenous bevacizumab and oral metronomic cyclophosphamide, sequentially followed by (1) bevacizumab plus vaccination with DCs pulsed with autologous tumor cell lysate supernatants, (2) lymphodepletion and (3) transfer of 5 × 109 autologous vaccine-primed T-cells in combination with the vaccine. Feasibility, safety as well as immunological and clinical efficacy were evaluated. Six subjects received this vaccination. Therapy was feasible, well tolerated, and elicited antitumor immune responses in four subjects, who also experienced clinical benefits. Of these, three patients with residual measurable disease received outpatient lymphodepletion and adoptive T-cell transfer, which was well tolerated and resulted in a durable reduction of circulating regulatory T cells and increased CD8+ lymphocyte counts. The vaccine-induced restoration of antitumor immunity was achieved in two subjects, who also demonstrated clinical benefits, including one complete response. Our findings indicate that combinatorial cellular immunotherapy for the treatment of recurrent ovarian cancer is well tolerated and warrants further investigation. Several modifications of this approach can be envisioned to optimize immunological and clinical outcomes.
Keywords: adoptive T-cell transfer, autologous immunotherapy, DC vaccines
Introduction
The overall five-year survival of patients affected by advanced stage epithelial ovarian cancer is < 40%, highlighting the unmet need for alternative therapies.1 Recent studies have demonstrated that ovarian cancer patients exhibit spontaneous antitumor immune responses. Peripheral blood tumor-specific T-cell precursors have been detected in 50% of subjects bearing ovarian cancer.2 Along similar lines, tumor-infiltrating lymphocytes (TILs) have also been found in approximately 50% of ovarian cancer patients, correlating with improved overall survival.3,4 This suggests that immunotherapy constitutes a meaningful approach to improve disease outcome among ovarian cancer patients.
Ovarian cancers express numerous tumor-associated antigens,5-9 although it is unclear which of these can drive tumor rejection. Molecular cancer vaccines have used peptide sequences, full-length proteins, or nucleic sequences, to date exhibiting limited success. An alternative approach is constituted by vaccines based on whole tumor cell lysates, which have generally produced better clinical responses than highly specific tumor vaccines in various types of cancers.10 In principle, whole tumor vaccines encompass all potential antigens of a specific tumor, including both major histocompatibility complex (MHC) Class I and II-restricted epitopes and hence eliciting a multivalent CD8+ and CD4+ antitumor response.11,12 Although there are theoretical concerns that tumor lysates could dampen the immunogenicity of dendritic cells (DCs), it has been shown that DCs pulsed with tumor lysates can expand tumor-reactive autologous T cells in vitro.2,13 However, significant improvements are required to increase the potency of such DC vaccines.
Immunomodulation of the host represents one effective means to augment the efficacy of vaccines. Among various targets of this approach, regulatory T cells (Tregs) are known to accumulate in ovarian tumors, where they promote immune dysfunction.14 Hence, the elimination of Tregs could be an important prerequisite for successful anticancer vaccines. In addition, the tumor vasculature, under the influence of the vascular endothelial growth factor (VEGF) and the endothelin systems, blocks the access of T cells to tumors, implying that an attenuation of this barrier could also enhance vaccine efficacy.15-17 Another approach to augment the activity of anticancer vaccines is represented by the stimulation of effector T cells. Indeed, cancer vaccines normally rely on endogenous low-avidity tumor-reactive T-cell precursors, whose activity could be significantly enhanced by the provision of co-stimulatory signals.18,19
We report a pilot clinical study that tested the feasibility, safety as well as immunological and clinical outcomes of a combinatorial cell-based immunotherapy that translated the concepts described above into the clinic. In a first part of the study, we treated subjects bearing advanced ovarian cancer with commercially available drugs that suppress Treg functions and tumor angiogenesis, then administered them with vaccines based on DCs pulsed with autologous tumor cell lysate supernatants. Subjects who achieved at least stable disease after the vaccine but failed to exhibit a complete remission were enrolled in the second part of the trial, involving lymphodepletion and adoptive transfer of autologous vaccine-primed CD3/CD28-co-stimulated T cells. Our findings indicate that this sequence of treatments is feasible, safe, and can yield clinical benefits to advanced ovarian cancer patients.
Results
UPCC-11807
Seven subjects (age range 46–69, mean 55) were enrolled in the UPCC-11807 trial (Table S1). One subject failed to yield sufficient DCs and was not vaccinated. Vaccine products met release criteria in six subjects (Table S2). The yield of DCs weakly correlated with monocyte counts in the apheresis product (Fig. S1). All 6 subjects completed bevacizumab and metronomic cyclophosphamide courses as well as vaccine doses. A total of 22 intradermal vaccinations were administered. All vaccines were well tolerated and there were no grade > 2 toxicities. The most frequent adverse event was grade 1 or 2 hypertension (3 occurrences), which we attributed to bevacizumab (Table S3).
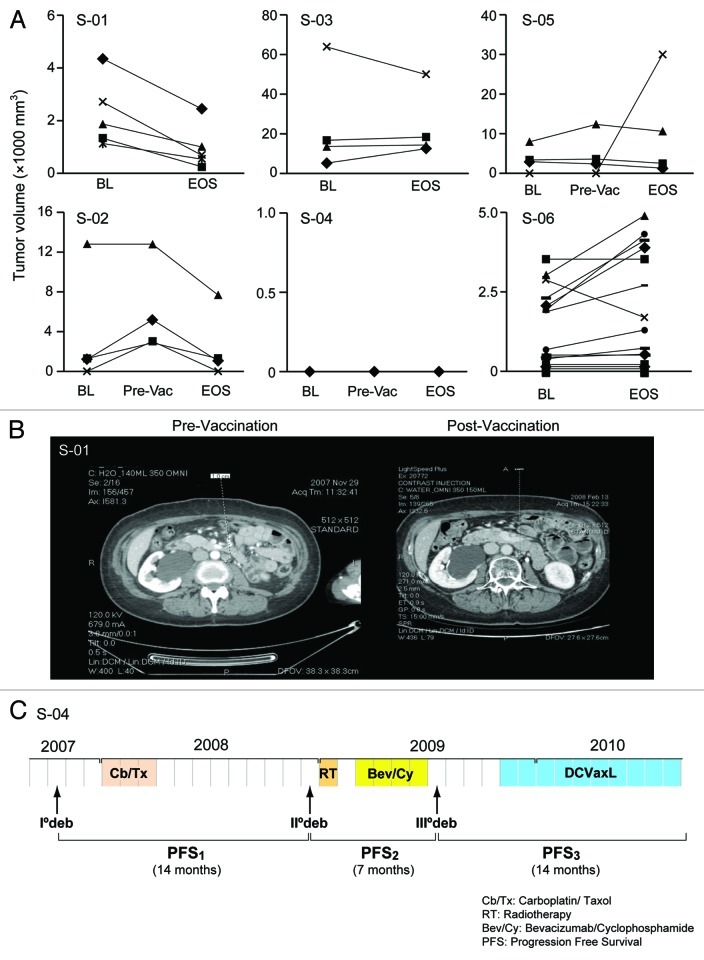
Two subjects exhibited partial responses (PR) according to Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 (Fig. 1A and B). Interestingly, one of these subjects progressed on bevacizumab and metronomic cyclophosphamide prior to receiving the vaccine, but exhibited a PR afterwards. Two other subjects exhibited stable disease (SD). One of these subjects entered the trial with no evidence of disease (NED) after tertiary debulking surgery, remained in remission until end of study (EOS), and continued to receive vaccine boosts every 4 weeks combined with bevacizumab, remaining disease-free for 14 mo, until vaccine was exhausted (Fig. 1C). Interestingly, this subject had previously failed to respond to bevacizumab. Two additional exhibited progressive disease, according to RECIST 1.1.
Figure 1. Radiological response in UPCC-11807. (A) Radiological assessments were performed by CT (CT) at enrollment or baseline (BL), and at the end of study (EOS). Two subjects also had evaluation after completing bevacizumab and metronomic cyclophosphamide and before starting vaccine (pre-vac). Volumes of all individual tumor metastases are shown. Estimated tumor volumes were calculated based on the formula V = ½ S * ½ S * L, where L is the longest and S is the shortest tumor diameter. Tumors were measured on the CT section that had the biggest tumor dimensions. Subject (S)-01 and -02 achieved partial response. S-02 demonstrated disease progression on bevacizumab and metronomic cyclophosphamide, although her lesions regressed after vaccine administration. S-03 had overall bulky disease, while S-04 had stable no evidence of disease. Subjects S-05 and S-06 exhibited disease progression. (B) Example of a left para-aortic lymph node regressing in S-01 post-vaccination CT (right) relative to pre-vaccination CT (left). (C) S-04 experienced remission inversion with progression-free survival (PFS3) being twice as long as PFS2.
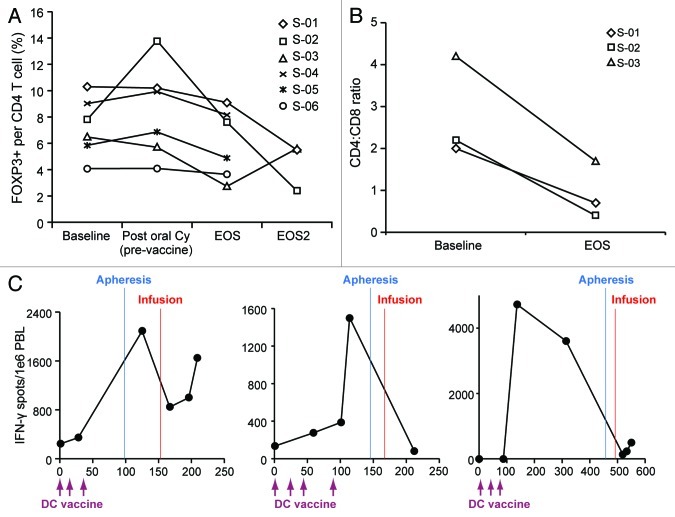
We measured the effect of therapy on the frequency of peripheral blood CD4+CD25+ FOXP3+ Tregs. Subjects had baseline circulating Treg frequencies ranging from 4% to 10%, consistent with previous reports of increased Tregs in cancer patients.20 We did not observe a reduction in circulating Tregs following the administration of bevacizumab and metronomic cyclophosphamide (Figure 2A). However, a modest reduction in Tregs was observed in all subjects post-vaccination (mean = 6.02% at EOS vs. 7.26 at baseline; p = 0.03).
Figure 2. Lymphocyte changes during UPCC-11807 and UPCC-10808. (A) CD4+CD25+FOXP3+ regulatory T cell (Treg) frequency during therapy. No Treg reduction was seen following bevacizumab and metronominc cyclophosphamide. A mild (statistically significant) reduction in Treg frequency was seen after vaccine plus bevacizumab relative to pre-vaccine. Two out of three subjects exhibited marked and sustained reduction in circulating Tregs after T -cell transfer. (B) Circulating CD4:CD8 T-cell ratio as measured at baseline and at the end of the study (EOS). Longitudinal assessment of tumor-reactive T cells by interferon γ (IFNγ) ELISpot in S-01, S-02 and S-03 during UPCC-10808.
We detected T cells specific for the Her2689–697 peptide in subject S-02 following vaccination, but neither at baseline nor between vaccination and bevacizumab and metronomic cyclophosphamide courses (Fig. 3A). We also quantified tumor-specific T cells in baseline, pre-vaccine, and post-vaccine peripheral blood lymphocytes (PBLs) using an interferon γ (IFNγ) ELISpot. We detected a significant increase in tumor-reactive T cells after vaccination (but not after bevacizumab alone) in all four subjects who exhibited clinical benefits (p < 0.05), but found no signs of an immune response in the two subjects who experienced disease progression (Fig. 3B). The frequency of tumor-specific T cells ranged between ~1:500 and ~1:5,000.
Figure 3. Immune responses in UPCC-11807. (A,B) Thawed peripheral blood mononuclear cells (PBMCs) obtained at baseline (BL) and at the end of study (EOS) were co-incubated, in the absence of additional stimulation, with autologous DCs pulsed with autologous tumor-cell lysates or control DCs for ~18 h and evaluated by interferon γ (IFNγ) ELISpot. (A) Representative data of T-cell responses. Tumor-specific responses are not detectable among PBMCs collected prior to therapy or after two doses of bevacizumab and metronomic cyclophosphamide, demonstrating the requirement for vaccine to elicit antitumor immunity. S-02 was HLA-A0201, allowing for the detection of immune responses to HER2 (H2N)- or hTERT-derived epitopes. A response is seen against HER2689–697. (B) Summary of vaccine-induced T cell responses. A positive response was detected in S-01, -02, -03 and -04. (C) Humoral responses induced by the vaccine. Sera from S-01 and S-02 were profiled on ProtoArray® Human Protein Microarrays v. 4.1, containing more than 8,000 human proteins. Increased IgG and IgM seropositivity is detected against human proteins post-vaccine as compared with pre-vaccine. Y-axes indicate the number of proteins recognized by serum antibodies. (D) CD3+ tumor-infiltrating T-cells (top) and MHC Class I expression levels (bottom) in tumors at BL. (E) Table depiciting the scoring system.
We then tested the induction of humoral responses by protein array in the two subjects who exhibited T-cell and clinical responses to the vaccine (S-01 and S-02). We detected IgG as well as IgM seropositivity to a number of proteins that was markedly higher in post-vaccine than in pre-vaccine sera, indicating not only a significant boost in general immunity but also a priming of humoral responses (Fig. 3C). The targets recognized by circulating IgM and IgG obtained from these two patients are listed in Supplemental Tables 4 and 5.
Lastly, we examined the expression of MHC Class I molecules and infiltration by CD3+ cells in tumor tissues harvested prior to vaccination. There was a positive correlation between the expression of MHC-I and the number of tumor-infiltrating lymphocytes (TILs) (Spearman rank correlation = 0.84, p = 0.04). In addition, there was an association between the abundance of TILs and immunological or clinical responses to vaccine, although this did not reach the threshold for statistical significance (p = 0.06) (Fig. 3D).
UPCC-10808
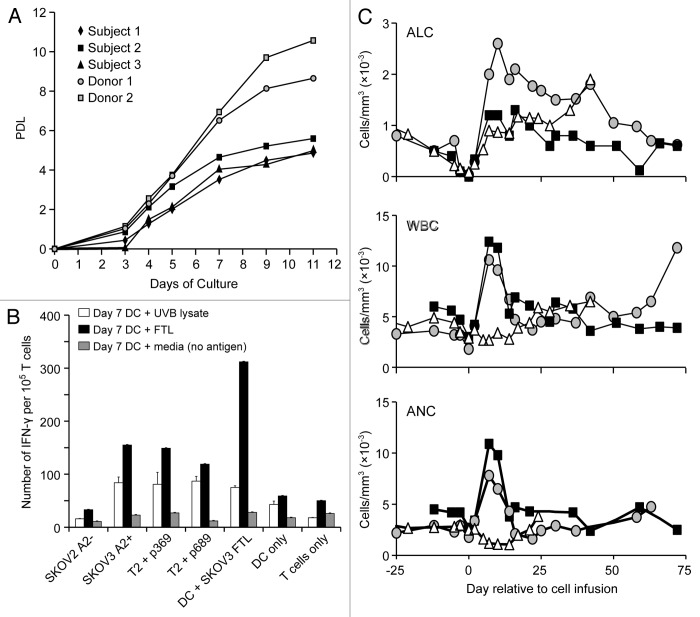
Following the completion of UPCC-11807 trial, three of the four subjects (S-01, -02 and -03) who had at least SD after vaccination but did not achieve complete remission, were eligible for and were enrolled in UPCC-10808. Post-vaccine elutriated T cells expanded efficiently in response to CD3/CD28 beads (Fig. 4A). Lymphocytes from patient S-01, S-02 and S-03 underwent ~29-, ~48 and ~31-fold expansion, respectively, by day 11 of culture, and were harvested for infusion thereafter. Post-expansion T cells retained tumor-reactive clones, as documented by the reactivity to HLA-A2-restricted HER2/neu peptides or to shared epitopes from SKOV3 cells (Fig. 4B).
Figure 4. Hematopoietic reconstitution in UPCC-10808. (A) Lymphocytes purified from peripheral blood mononuclear cells (PBMCs) by elutriation were stimulated and expanded for ~11 d using Dynal microbeads coated with anti-CD3 and anti-CD28 antibodies. Expansion of three subjects and two normal donors is shown. A population doubling level (PDL) average of 5.14 is seen for three subjects with ovarian cancer. (B) Expanded T cells from S-02 were tested by interferon γ (IFNγ) ELISpot for cross-reactivity against non-transduced or HLA-A2-transduced SKOV3 cells, T2 cells pulsed with HER2/neu 369 or 689 peptides or unpulsed T2 cells, autologous dendritic cells (DCs) pulsed with SKOV3-cell lysates or unpulsed DCs, or media (T cells only). Prior to assessments, T cells were incubated for 7 d with autologous DCs pulsed with SKOV3-cell lysates prepared either by freeze-thawing (FTL) or UV B (UVB) irradiation. Results are means of the number of IFNγ spots per 10 T cells ± SEM of triplicates (p < 0.001; paired Student’s t-test) (C) Absolute lymphocyte count (ALC), white blood cells (WBC) and absolute neutrophil count (ANC) in subjects receiving adoptive T-cell transfer. S-02 (black) and S-03 (gray) received pegfilgrastim on the day of T-cell transfer.
The infusion of 5 × 109 T cells after lymphodepleting chemotherapy was well tolerated, the most frequent toxicity being myelosuppression (Table S3). Absolute lymphocyte counts (ALCs) recovered to pre-treatment levels by day 2–7. Absolute neutrophil counts (ANCs) recovered to baseline levels in all subjects by day 7 (Fig. 4C). At the EOS, patient S-01, who exhibited a PR during UPCC-11807, achieved a complete response (CR). Patient S-02, who also exhibited a PR during UPCC-11807, progressed, while patient S-03, achieving a SD in UPCC-11807, remained stable.
We followed T-cell subsets in the peripheral blood upon the T-cell transfer. The frequency of circulating Tregs decreased significantly in patients S-01 and S-02 but not in S-03 (Fig. 5A). In addition, following adoptive T-cell transfer, reconstituted PBLs were skewed toward CD8+ T-cells, the CD4:CD8 ratio being decreased 2–4-fold (Fig. 5B). Finally, we tested whether tumor-reactive T cells would reconstitute in vivo after adoptive transfer. We quantified these cells by IFN-γ ELISpot using autologous DCs pulsed with autologous tumor lysate as the antigen-presenting platform. At the time of apheresis, there were detectable tumor-reactive T-cells in all subjects (0.5 to 2.6 spots per 1,000 PBLs) (Fig. 5C). Tumor-reactive T cells were detectable after hematologic reconstitution in S-01 and S-03, who experienced CR and SD, respectively, but were not detectable in S-02, who experienced disease progression.
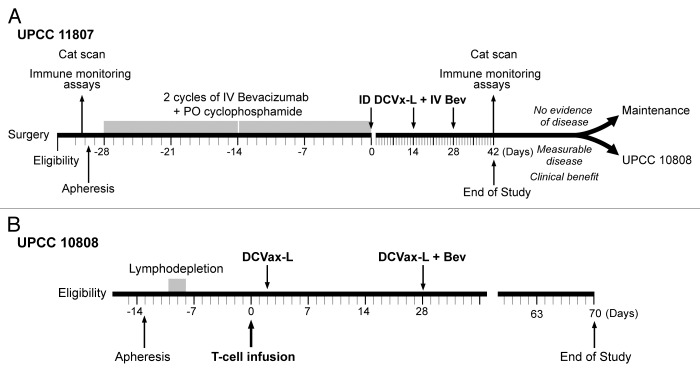
Figure 5. (A,B) Clinical design of UPCC-11807 (A) and UPCC-10808 (B).
Discussion
In this study, we demonstrated that whole tumor cell-based vaccination, using lysate-pulsed autologous DCs in combination with bevacizumab and metronomic cyclophosphamide, followed by autologous adoptive T-cell therapy using vaccine-primed CD3/CD28-co-stimulated whole PBLs, is feasible and well tolerated by advanced recurrent ovarian cancer patients. Four of six patients (66%) achieved clinical benefits with the combination of bevacizumab, metronomic cyclophosphamide and the vaccine, which included two objective PRs and two SDs. One subject experiencing SD had NED at study entry, and her post-vaccine remission lasted for 14 mo in spite of a prior progression-free interval of 7 mo. These observations are suggestive of an effective therapy. Although one cannot dissect the contribution of bevacizumab and metronomic cyclophosphamide to the clinical response in this small pilot study, it is worth noting that one of the subjects exhibiting a PR and the 14-mo remission were observed in subjects who had previously failed this chemotherapy, indicating that the observed clinical benefits could be (at least in part) attributed to the elicitation of an antitumor immune response. In support of this hypothesis, clinical responses closely correlated with the detection of cellular and humoral immune response against tumor antigen upon vaccination. Along similar lines, the absence of immune responses to the vaccine correlated with clinical progression.
All subjects exhibited a dampened T-cell response to the diphtheria carrier protein CRM197, a potent xeno-neoantigen of Prevnar™, which was given along with the first vaccination to monitor immune responsiveness. Interestingly, myeloma patients have previously been shown to exhibit robust T-cell responses to CRM197, suggesting that ovarian cancer patients may be characterized by a profound level of systemic immunosuppression. Nevertheless, DC vaccination induced an immune response against whole tumor-cell lysates and specific immune responses against peptides of known tumor-associated antigens such as HER2. Furthermore, we detected increased IgM seropositivity post-vaccine, indicating effective de novo B-cell priming.
The frequency of tumor-specific T cells elicited by our vaccine was quite low (< 1 tumor reactive T cell per 500 PBLs). Under these conditions, it is quite encouraging to see some clinical benefits. It is possible that the observed benefits were due to a synergistic effect of vaccine with anti-angiogenesis therapy. Tumor vasculature not only directly supports increased tumor growth, but also can also provide a barrier to the extravasation of T cells into the tumor.15,21 Anti-VEGF therapy normalizes tumor vasculature and increases tumor infiltration by T cells, and in the mouse this has been shown to improve the outcome of adoptive immunotherapy.22 Metronomic cyclophosphamide also suppresses tumor angiogenesis and reduces the number of circulating endothelial progenitor cells in subjects with metastatic cancer.23 Cyclophosphamide also has a dose-dependent immunomodulatory effect.24 Metronomic cyclophosphamide has been reported to decrease Tregs in subjects with advanced cancer, restore T-cell and NK-cell effector functions,25 and enhance the efficacy of anticancer vaccines and anti-HER2 targeted therapy.26 Interestingly, we did not observe a decrease in circulating Tregs frequency after bevacizumab and metronomic cyclophosphamide, as previously reported.25 Perhaps this reflects the fact that we employed a comparatively lower dose of cyclophosphamide. Alternatively, the lack of Treg depletion may not necessarily preclude a positive effect of metronomic cyclophosphamide on the immunogenicity of DC vaccines.
We sought to enhance antitumor immune responses generated by the vaccine by lymphodepletion followed by the adoptive transfer of post-vaccine ex vivo co-stimulated T cells. The theoretical advantages of this approach mainly lie in the co-stimulatory signals delivered to T cells during ex vivo CD3/CD28 bead-driven expansion and in the host conditioning through lymphodepletion. We used this approach in patients who developed antitumor immune responses to the vaccine, but did not achieve a CR in the first part of our study. Our pilot study demonstrates the feasibility of ex vivo T-cell expansion even in this heavily pretreated patient population, and the tolerability of outpatient lymphodepletion followed by T-cell transfer. The hematologic recovery was rapid and resulted in two important changes in circulating T cells: (1) it reduced the relative frequency of Tregs over total CD4+ cells, and (2) it decreased the CD4 to CD8 ratio by 2–4-fold. In two subjects, we documented a restoration of antitumor immunity and a durable decline of circulating Tregs, which was associated with clinical benefit (one subject achieved CR, while the other one—with bulky disease—was stable at the EOS). In the third subject, adoptive T-cell transfer did not restore antitumor immunity, and disease progression was observed. Interestingly, Tregs returned to pre-treatment levels in this patient.
In summary, we treated a population of patients affected by recurrent ovarian cancer (who have few effective therapeutic options) and obtained an overall clinical benefit of 50%, including one CR and one remission that lasted 14 mo. The latter patient was subsequently enrolled in a second vaccine study, using improved lysate-pulsed DCs13 without bevacizumab, and remained in remission for one additional year (not shown). Importantly, this patient and another who achieved PR had previously failed bevacizumab and metronomic cyclophosphamide, indicating that therapeutic success cannot be attributed solely to anti-angiogenic therapy, at least in this setting. Although these data are considered encouraging, a number of opportunities may be pursued to optimize the efficacy of this approach. Importantly, we observed a marked systemic immunosuppression, which was also reported in patients with ovarian cancer in remission receiving vaccines.27 Hence, therapeutic maneuvers that effectively reduce Tregs, activate innate immunity and stimulate antigen presentation (e.g., TLR agonists), enhance T-cell activation (e.g., IL-2, IL-7) or block inhibitory checkpoints (e.g., anti-CTLA-4 and anti-PD-1 anitbodies) could markedly enhance immune activation and vaccine efficacy. In addition, we used supernatants from freeze-thawed tumor lysates. Alternative ways to prepare lysates may be preferable. Furthermore, we used DCs that were not matured with any exogenous stimuli. Although lysate pulsing can induce DC maturation, additional stimulation with exogenous cytokines and TLR agonists could significantly enhance vaccine potency. Whether the adoptive transfer of vaccine-primed co-stimulated T-cells is an effective way to boost the efficacy of cancer vaccines still remains an important question. In our study, the only CR was observed in the one patient who had high numbers of reactive T-cells in the T cell preparation and whose ALC was 0 on the day of transfer. Adoptive T-cell therapy could be improved in the future by an intensification of lymphodepleting regimens as well as by an enrichment of the preparation with tumor-reactive T-cells. The depletion of Tregs from the apheresis product and systemic administration of cytokines in support of T cells following transfer could further enhance the efficacy of therapy. Future trials are required to address these issues.
Materials and Methods
Overall design and subjects
We performed two linked open-label pilot studies in subjects with recurrent ovarian cancer. The first (UPCC-11807) assessed the feasibility, safety and immunological as well as clinical effects of a DC-based autologous whole-tumor lysate vaccination in combination with anti-angiogenesis therapy. The second (UPCC-10808) tested the adoptive transfer of autologous vaccine-primed CD3/CD28-co-stimulated lymphocytes. Subjects completing UPCC-11807 had the option of enrolling immediately into UPCC-10808, if study eligibility criteria were met.
All eligible subjects were ≥ 18 y old; had recurrent advanced-stage ovarian cancer; had undergone prior cytoreductive surgery for recurrent disease, with sufficient tumor harvested for obtaining a lysate; had undergone physician’s choice post-operative chemotherapy; had residual tumors at study entry ≤ 4 cm; and had a baseline Eastern Cooperative Oncology Group performance status < 2. Enrollment was allowed ≥ 4 weeks after surgery or ≥ 3 weeks after any post-operative chemotherapy. Both studies (NCT01312376 and NCT00603460) were approved by the Food and Drug Administration and the University of Pennsylvania Institutional Review Board. Both studies are now closed for accrual.
UPCC-11807
Subjects underwent leukapheresis on day -35 to -29, followed by at least two cycles of intravenous bevacizumab (10 mg/kg, on days -28 and -14) and oral metronomic cyclophosphamide (50 mg daily for 7 d after bevacizumab) (Fig. 5A), followed by three doses of intradermal DCs (~5–10 × 106) every two weeks in combination with intravenous bevacizumab. EOS was set to day 42. If subjects demonstrated clinical benefit by RECIST 1.1 but still had measurable disease (i.e., PR or SD), then they were eligible to enroll in protocol UPCC-10808.
UPCC-10808
Subjects underwent apheresis on day -13, followed by outpatient non-myeloablating lymphodepleting chemotherapy with intravenous cyclophosphamide (300 mg/m2/day) and fludarabine (30 mg/m2/day) on days (-3 to -15); infusion of 5 × 109 ex vivo CD3/CD28-co-stimulated T-cells on day 0; and DC vaccine boost on day 2 (Fig. 5B). All subjects were managed as outpatients and received antibiotic and antifungal prophylaxis. EOS was set to day 70.
Cell manufacturing
Vaccine was manufactured at Cognate Bioservices as described in Supplemental Materials and elsewhere.28 DCs were cryopreserved at -140°C and shipped to the University of Pennsylvania on dry ice. T cells were manufactured at the Cell and Vaccine Production Facility, University of Pennsylvania, as detailed in Supplemental Materials and administered fresh.
Evaluation
Subjects were evaluated every two weeks in UPCC-11807; and twice a week until hematologic recovery, and weekly thereafter, in UPCC-10808. Safety was determined using the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Subjects underwent CT at enrollment and at EOS. Clinical response was determined using RECIST 1.1. Clinical benefit was defined as CR, PR or SD.
Immunological assessments
Immunological assessments were performed at the time of enrollment (baseline), after completion of bevacizumab and metronomic cyclophosphamide (pre-vaccine), and at EOS in UPCC-11807; and at enrollment, before lymphodepletion, before T-cell infusion, and at EOS in UPCC-10808. T-cell responses to tumor antigens were assessed by interferon γ (IFNγ) ELISpot assays following incubation of peripheral blood lymphocytes (PBLs) with autologous DCs that were either left untreated or pulsed with autologous tumor cell lysates. CD8+ responses to HER2/neu369–377 were also measured by IFNγ ELISpot in HLA-A*0201 subjects. CD4+CD25+ FOXP3+ Tregs were quantified by flow cytometry. Serum IgG and IgM were assessed using the ProtoArray® Human Protein Microarrays v. 4.1 (Invitrogen). We assessed pre-vaccine tumor-infiltrating CD3+ T cells and tumor MHC Class I expression levels by immunohistochemistry. Hematologic recovery was followed with blood cell analysis every 1–3 d after chemotherapy, until blood populations returned to normal values. Details are reported in Supplemental Materials.
Statistical analysis
Data were analyzed by paired Student's t-test, and p values < 0.05 were considered statistically significant. Pearson's correlation was employed to test the correlation between two interval-scaled variables, while Spearman's rank correlation was employed to test the correlation between two ordinal-scaled variables. The non-parametric Wilcoxon rank sum test was used to compare immunologic outcomes (e.g., CD3+ TILs) between clinical responders and non-responders. These analyses were conducted in SPSS 19 (SPSS, Inc.).
Supplementary Material
Acknowledgments
We thank our patients for participating in this protocol.
Financial support
This study was supported by National Cancer Institute Ovarian SPORE grant P01-CA83638, National Institution of Health R01 grant FD003520–02, and the Ovarian Cancer Immunotherapy Initiative.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22664
References
- 1.Coukos G, Conejo-Garcia JR, Roden RB, Wu TC. Immunotherapy for gynaecological malignancies. Expert Opin Biol Ther. 2005;5:1193–210. doi: 10.1517/14712598.5.9.1193. [DOI] [PubMed] [Google Scholar]
- 2.Schlienger K, Chu CS, Woo EY, Rivers PM, Toll AJ, Hudson B, et al. TRANCE- and CD40 ligand-matured dendritic cells reveal MHC class I-restricted T cells specific for autologous tumor in late-stage ovarian cancer patients. Clin Cancer Res. 2003;9:1517–27. [PubMed] [Google Scholar]
- 3.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 4.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellstrom I, Raycraft J, Kanan S, Sardesai NY, Verch T, Yang Y, et al. Mesothelin variant 1 is released from tumor cells as a diagnostic marker. Cancer Epidemiol Biomarkers Prev. 2006;15:1014–20. doi: 10.1158/1055-9965.EPI-05-0334. [DOI] [PubMed] [Google Scholar]
- 6.Hellström I, Goodman G, Pullman J, Yang Y, Hellström KE. Overexpression of HER-2 in ovarian carcinomas. Cancer Res. 2001;61:2420–3. [PubMed] [Google Scholar]
- 7.Odunsi K, Qian F, Matsuzaki J, Mhawech-Fauceglia P, Andrews C, Hoffman EW, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:12837–42. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vonderheide RH. Telomerase as a universal tumor-associated antigen for cancer immunotherapy. Oncogene. 2002;21:674–9. doi: 10.1038/sj.onc.1205074. [DOI] [PubMed] [Google Scholar]
- 9.Luby TM, Cole G, Baker L, Kornher JS, Ramstedt U, Hedley ML. Repeated immunization with plasmid DNA formulated in poly(lactide-co-glycolide) microparticles is well tolerated and stimulates durable T cell responses to the tumor-associated antigen cytochrome P450 1B1. Clin Immunol. 2004;112:45–53. doi: 10.1016/j.clim.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Neller MA, López JA, Schmidt CW. Antigens for cancer immunotherapy. Semin Immunol. 2008;20:286–95. doi: 10.1016/j.smim.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Toes RE, Schoenberger SP, van der Voort EI, Offringa R, Melief CJ. CD40-CD40Ligand interactions and their role in cytotoxic T lymphocyte priming and anti-tumor immunity. Semin Immunol. 1998;10:443–8. doi: 10.1006/smim.1998.0147. [DOI] [PubMed] [Google Scholar]
- 12.Zajac AJ, Murali-Krishna K, Blattman JN, Ahmed R. Therapeutic vaccination against chronic viral infection: the importance of cooperation between CD4+ and CD8+ T cells. Curr Opin Immunol. 1998;10:444–9. doi: 10.1016/S0952-7915(98)80119-2. [DOI] [PubMed] [Google Scholar]
- 13.Chiang CL, Maier DA, Kandalaft LE, Brennan AL, Lanitis E, Ye Q, et al. Optimizing parameters for clinical-scale production of high IL-12 secreting dendritic cells pulsed with oxidized whole tumor cell lysate. J Transl Med. 2011;9:198. doi: 10.1186/1479-5876-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 15.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 16.Kandalaft LE, Facciabene A, Buckanovich RJ, Coukos G. Endothelin B receptor, a new target in cancer immune therapy. Clin Cancer Res. 2009;15:4521–8. doi: 10.1158/1078-0432.CCR-08-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–9. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 18.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, et al. Redirected Antitumor Activity of Primary Human Lymphocytes Transduced With a Fully Human Anti-mesothelin Chimeric Receptor. Mol Ther. 2012;20:633–43. doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Audia S, Nicolas A, Cathelin D, Larmonier N, Ferrand C, Foucher P, et al. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin Exp Immunol. 2007;150:523–30. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–8. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 22.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–80. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfaro C, Perez-Gracia JL, Suarez N, Rodriguez J, Fernandez de Sanmamed M, Sangro B, et al. Pilot clinical trial of type 1 dendritic cells loaded with autologous tumor lysates combined with GM-CSF, pegylated IFN, and cyclophosphamide for metastatic cancer patients. J Immunol. 2011;187:6130–42. doi: 10.4049/jimmunol.1102209. [DOI] [PubMed] [Google Scholar]
- 24.Greten TF, Ormandy LA, Fikuart A, Höchst B, Henschen S, Hörning M, et al. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33:211–8. doi: 10.1097/CJI.0b013e3181bb499f. [DOI] [PubMed] [Google Scholar]
- 25.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–8. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu CS, Boyer J, Schullery DS, Gimotty PA, Gamerman V, Bender J, et al. Phase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission. Cancer Immunol Immunother. 2012;61:629–41. doi: 10.1007/s00262-011-1081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–25. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.