Abstract
The destruction of tumor cells by the immune system is under the control of positive and negative receptors that tightly regulate T-cell effector functions. The T-cell receptor (TCR) inhibitory molecule CD5 critically contributes to the regulation of antitumor immune responses. Indeed, the modulation of CD5 within the tumor microenvironment corresponds to a strategy adopted by tumor-specific cytotoxic T lymphocytes (CTLs) to optimize their cytotoxic and cytokine secretion functions. In this review, we provide insights into the immunobiology of CD5 and its role in regulating antitumor CD8 T-cell responses, and suggest the possibility of targeting CD5 to improve the efficacy of current immunotherapeutic approaches against cancer.
Keywords: antitumor immune responses, CD5, T cells, tumor-infiltrating T lymphocytes
Introduction
It is now widely accepted that the immune system is able to recognize and destroy transformed cells before they become clinically detectable, a process known as tumor immunosurveillance. The development of effective antitumor immune responses relies on coordinated interactions of host immunocompetent cells as well as on the generation of tumor antigen (Ag)-specific cytotoxic T lymphocytes (CTLs). CTLs play a major role in the defense against cancer as they recognize, via T-cell receptors (TCRs), specific antigenic peptides presented on the surface of transformed cells by major histocompatibility complex (MHC) class I molecules. To become competent killer cells, naive circulating CD8+ T lymphocytes require an efficient priming by professional antigen-presenting cells (APC), namely dendritic cells (DCs), as well as an adequate CD4+ T-cell help, mostly mediated by cytokines and chemokines.1-3 Primed T cells then clonally expand and leave lymph nodes (LNs), infiltrate tumor tissues before and eventually get activated to mediate effector functions (Fig. 1). The killing of target cells occurs either directly, upon the release of cytolytic granule contents, or indirectly, following the secretion of effector cytokines.4-7
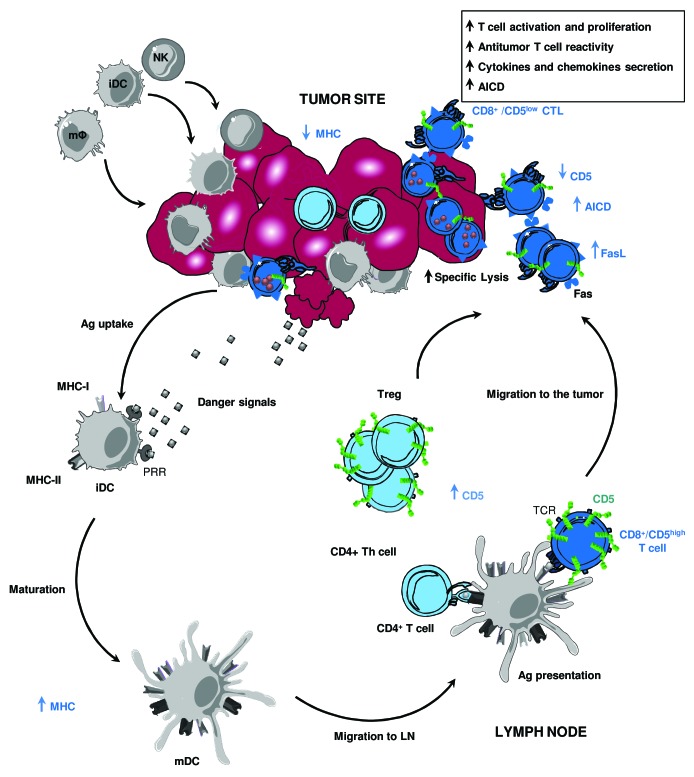
Figure 1. Intratumoral CD5 downregulation potentiates antitumor T-cell responses. Malignant cells express pathogen-associated molecular patterns (PAMPs) that can be recognized by pattern recognition receptors (PRRs) on dendritic cell (DC) precursors, triggering the local release of cytokines and chemokines. This results in the recruitment and activation of innate immunity effector cells, including macrophages (mφ), natural killer (NK) and natural killer T (NKT) cells, all of which are able to kill cancer cells. DCs engulf apoptotic tumor cells, undergo a maturation process and then migrate to regional lymph nodes, where they present processed tumor-derived peptides to CD8+ and CD4+ T cells upon the upregulation of MHC class I and II molecules. Activated cytotoxic T lymphocytes (CTLs) leave lymphoid organs to infiltrate tumor tissues and exert effector functions. Depending on the strength of the TCR/peptide-MHC Class I molecule interactions, tumor-specific CTLs can undergo an intratumoral adaptation process by downregulating the expression of CD5, as a means to enhance TCR signaling and to overcome tumor escape deriving from an altered expression of peptide/MHC (pMHC) complexes. This results in increased T-cell reactivity and optimized cytotoxic activity toward tumors that express low levels of pMHC complexes. Nevertheless, the downregulation of CD5 in situ sensitizes tumor-specific T lymphocytes to activation-induced cell death (AICD) through the upregulation of FAS ligand (FASL).
The identification of tumor-associated antigens (TAAs) and the presence of TAA-specific CD8+ tumor-infiltrating T lymphocytes (TILs) within spontaneously regressing tumors provided evidence in support of the existence of CTL-mediated antitumor immune responses.8 Moreover, tumor infiltration by CD8+ T lymphocytes has often been associated with an improved prognosis.9 However, it is currently acknowledged that this favorable prognostic trend strongly depends on the environmental context of the immune infiltrate, and better outcomes are correlated with the absence of immunosuppressive factors impairing CTL effector functions. Indeed, antitumor immune responses are often inapt to control tumor growth in the immunosuppressive microenvironment that is frequently encountered within tumors, leading to insufficient recruitment and/or altered activation of effector T cells.10
The activation of Ag-specific T cells via the TCR is a complex signaling process leading to their proliferation and differentiation into effector cells. An improved knowledge of the mechanisms controlling T-cell activation and proliferation has led to the identification of regulatory molecules that either activate or inhibit T-cell functions. Indeed, beside activating molecules, T cells also express several inhibitory receptors, such as CTLA-4, TIM-3 and PD-1, which—upon interaction with the respective ligands (i.e., B-7-1/B7-2, galectin-9 and PD-L1/PD-L2)—impair T-cell activation.11,12 CTLA-4, TIM-3 and PD-1 play a key role in T-cell unresponsiveness and dysfunction, and have been involved in the frequently inadequate control of tumor progression.13,14 Blocking these inhibitory receptors has been associated with a beneficial therapeutic effect in experimental tumor models as well as in melanoma patients.15-17 Other TCR inhibitory molecules exist, including CD5, and hence may represent alternative therapeutic targets to treat human neoplasms. In this review, we present a brief overview on the biology of CD5 in health and diseases, focusing on the role of CD5 in antitumor T-cell responses and its potential therapeutic interest for the optimization of current immunotherapeutic strategies against cancer.
CD5 Structure and Cellular Distribution
CD5, also known as Leu-1 in humans and Lyt-1 in mice, is a 67-kDa type I transmembrane glycoprotein that belongs to the highly conserved scavenger-receptor cysteine-rich superfamily (SRCR).18 It is characterized by a cysteine-rich extracellular domain of approximately 100 amino acids19,20 and a cytoplasmic tail containing a pseudo-ITAM-like motif.21 CD5 is constitutively expressed on lymphocyte precursors, mature T cells and on a subset of mature B cells (B1a cells).18,20,22 CD5 is associated both physically and functionally with the TCR/CD3 complex as well as with the B-cell receptor (BCR).23-25
Distinct potential ligands for CD5 including CD5L,26,27 CD72 (Lyb-2 in mice),28 and CD5 itself have been described,26-31 but the actual identity of the physiologically relevant CD5 ligand remains to be determined. More recently, it has been reported that conserved fungal components also interact with membrane-bound CD5 and that a soluble CD5 ectodomain protects mice from zymosan-induced septic-shock-like syndrome.31
CD5 Immunobiology and Function
The precise role of CD5 in the interactions of immune cells has remained unclear for a long time. Initial studies with anti-CD5 monoclonal antibodies (mAbs) pointed to a positive role for CD5 in enhancing TCR-mediated signal transduction,32,33 but more recent studies with Cd5 knockout mice revealed that CD5 negatively regulates Ag receptor-mediated signaling in thymocytes and mature T cells.34 Indeed, based on data from Cd5-deficient mice, it has been demonstrated that CD5 exerts a negative effect on TCR and BCR signaling.34,35 Thus, immature T cells from Cd5−/− mice are hyperresponsive to TCR stimulation and exhibit an altered positive and negative thymic selection. CD5 also inhibits peripheral blood T-cell signaling and mature Cd5−/− T cells exhibit an enhanced proliferation upon TCR stimulation.34,36 CD5 has also been described to play an inhibitory role in the suppressive function of murine CD4+/CD25+ regulatory T cells (Tregs),37 and Cd5−/− mice show increased numbers of CD4+/CD25+/FOXP3+ thymocytes and peripheral natural (n)Tregs as compared with their wild-type counterparts.38 Moreover, a few studies have suggested a role for CD5 in TH17 differentiation. In particular, Cd5-Ck2 double-deficient mice, which are resistance to experimental autoimmune encephalomyelitis (EAE), exhibit a reduced TH17 cell compartment.39 Of note, CD5 co-stimulation can also induce stable TH17 development by promoting the expression of the interleukin (IL)-23 receptor and sustained STAT3 activation.40,41
CD5 is an important physiological regulator of T-cell immune responses. The regulation of CD5 corresponds to a key event in the maintenance of immune homeostasis and tolerance. Studies based on experimental mouse models indicates that CD5 plays a key role in generation and maintenance of immune tolerance and that alterations of its activity can promote autoreactivity.42 In addition to its function as an inhibitory receptor and modulator of autoimmunity, CD5 has recently been documented to regulate activation-induced cell death (AICD) and antitumor immune responses (see below).
CD5 Signaling Mechanisms
Accumulating evidence indicates that CD5 is recruited at the immune synapse formed between T cells and APCs.43,44 It has been demonstrated that CD5 co-localizes with TCR/CD3 complexes at the immune synapse and reduces TCR-conveyed signals, such as the Ca2+ response induced by Ag presentation and the extent of tyrosine phosphorylation, without affecting the formation and stability of conjugates.44 It has also been shown that the CD5-mediated TCR inhibition does not require the extracellular domain of the molecule,45 but only its cytoplasmic tail,36,46 where the pseudo-ITAM domain is likely to play an essential role.44,47 However, a role for the extracellular domain of CD5 cannot be totally excluded, since neutralizing mAbs against this domain48 as well as soluble CD5-Fc molecules49 can block the inhibitory effect of CD5 in some experimental models or specific microenvironment, such as within tumors, also suggesting the participation of a putative ligand to these effects.
The cytoplasmic tail of CD5 contains several tyrosine residues. Among them, the first one, Tyr429, is included in a canonical SRC autophosphorylation site (DNEY). Moreover, Tyr429 and Tyr441 are in a YSQP-(x8)-YPAL pseudo-ITAM motif. The clustering of CD5 with the TCR is probably critical to trigger the phosphorylation of these residues. However, it is not yet clear how these phosphorylation events lead to the inhibition of TCR signaling. Several effector molecules positively or negatively involved in TCR-induced responses, such as SHP-1, rasGAP, CBL, CK2, ZAP70 and PI3K, have been reported to associate with tyrosine phosphorylated CD5.21,47,50 CD5 phosphorylation could therefore be necessary to recruit inhibitory signaling molecules in the proximity of the TCR and/or to sequester activation kinases away from the TCR complex, thereby reducing the strength of Ag-receptor signaling. More recently, it has also been reported that CD5-mediated T-cell inhibition is dependent on phosphorylation of the negative regulatory tyrosine (Tyr531) of the SRC kinase member FYN, resulting in a reduction of its kinase activity and inhibition of ZAP70.51
Regulation of CD5 Expression during Thymocyte Development
The expression of CD5 is tightly regulated during T-cell development.52 It has been shown that thymic selection is sensitive to variations in the levels of CD5 on T-cell surface. During normal thymocyte development, low levels of CD5 are expressed on immature double negative (DN) CD4−/CD8− thymocytes. CD5 surface expression then increases at both the double positive CD4+/CD8+ and single positive (SP) CD4+ or CD8+ stage, and relatively high levels of CD5 are maintained on circulating SP T cells.52,53 The mechanisms by which CD5 expression levels change during thymocyte development involve the pre-TCR first and then the TCR signal intensity for specific peptide-MHC (pMHC) complexes.52
The regulation of CD5 during T-cell development is critical for setting TCR response thresholds to pMHC complexes encountered during thymic selection as well as for the establishment of tolerance, by adjusting thymocyte responsiveness to self pMHC complexes.54 Studies from Cd5-deficient mice have shown that CD5 determines thymic outcomes through the modulation of TCR signaling.35 Indeed, in the absence of CD5, thymocytes are hyperresponsive to TCR stimulation, and the efficiency of thymocyte selection in TCR-transgenic Cd5-deficient mice is altered in a manner consistent with enhanced TCR signaling. The impact of Cd5 deletion on thymocyte selection depends on the avidity of the TCR for its selecting ligand, which in turn is reflected by the level of endogenous CD5 surface expression.46 Analyses of TCR-transgenic T cells under conditions in which the endogenous peptide repertoire is altered have shown that self pMHC complexes regulate T-cell activation thresholds through changes in the expression level of CD5 on DP thymocytes.55 Therefore, the regulation of CD5 in the thymus plays a critical role in tuning the threshold of TCR-mediated responses and in selecting the mature TCR repertoire during thymocyte development.46,52
Peripheral Regulation of CD5 Expression
The precise mechanisms that govern the regulation of CD5 expression by mature T cells are not very well understood. Previous reports have shown that CD5 expression on mature T cells directly parallels the avidity or signaling intensity stemming from the TCR/pMHC interaction.52 As TCR/pMHC interactions maintain the homeostasis of peripheral naive T lymphocytes,56 CD5 expression is continuously tuned at the periphery according to the avidity of the TCR interactions with self pMHC complexes.54 Indeed, pMHC complexes continually modulate the expression levels of CD5 in naive CD4+ T cells, and reduced expression of CD5 in T cells deprived from TCR/pMHC interactions are associated with increased responses to TCR engagement. Moreover, it has been reported that CD5 levels reflect the avidity of T cells for self-pMHC complexes and potentially influence the homeostatic behavior of naive and memory T cells.57
A key role of CD5 in controlling aberrant immune responses by augmenting the threshold needed for TCR activation following Ag recognition has been documented.58 Indeed, the generation of a peripheral T-cell population with elevated levels of CD5, induced in vivo by DCs, can lead to Ag-specific unresponsiveness. An increase in CD5 expression levels has also been observed in peripheral anergic CD8+ T cells chronically exposed to Ags.59 Conversely, the priming of naive CD8+ T cells by IL-7 and IL-21 has been described to increase Ag responsiveness associated with a downmodulation of CD5.60 A decrease in CD5 expression levels has been detected in CD3+/CD8+ T-cell populations from the peripheral blood of HIV-infected patients.61 Moreover, a downregulation of CD5 on TILs can occur at the tumor site. Notably, CD5 levels on tumor-specific T cells parallel the signaling intensity of TCR/pMHC interactions.62 Thus, CD5 levels appear to be adapted to signals received at the periphery and are adjusted in a manner to reflect the intensity of the interactions between the TCR and pMHC complexes.
Role of CD5 in Antitumor T-Cell Responses
As discussed above, CD5 plays a major role in regulating antitumor immune responses, and the downregulation of CD5 expression on TILs potentiates tumor-specific T-cell reactivity. We have previously reported that the cytotoxic activity of human T-cell clones toward specific lung cancer cells is inversely proportional to CD5 expression levels. The downregulation of CD5 on TILs occurs within the tumor microenvironment and presumably corresponds to a strategy used by T cells to adjust their sensitivity to the strength of the TCR/pMHC interaction.62 A decrease in MHC class I molecule expression is often observed in human tumors and reflects a mechanism frequently used by cancer cells to escape from CD8+ T-cell immunity.63 The modulation of CD5 by T lymphocytes infiltrating tumors expressing low levels of pMHC complexes and the subsequent increase in T-cell reactivity might therefore constitute a strategy used by the immune system to overcome tumor evasion. In line with this hypothesis, we have reported that tumor-specific CTLs undergo an intratumoral adaptation process depending on the strength of the TCR/pMHC interaction, as a means to enhance TCR signaling and to overcome tumor escape resulting from altered pMHC expression via the regulation of CD5 (Fig. 1).
Our in vivo experiments based on the B16 melanoma model revealed a delayed tumor growth in Cd5-deficient mice as compared with their wild-type counterparts.64 In the absence of Cd5, mice displayed strong antitumor immune responses that were associated with tumor infiltration by hyperactivated tumor-reactive CD8+ T cells that protected animals from tumor burden. The absence of Cd5 lowered the T-cell activation threshold, resulting in enhanced tumor-specific T-cell responses. Conversely, CD5 expression rendered wild-type murine TILs unresponsive to specific Ag stimulation. The quiescent status of CD5+ tumor-specific CTLs may, at least in part, elucidate the paradoxical lack of correlation between the frequency of pMHC-tetramer+ circulating T cells induced in vaccination trials and tumor regression.
CD5 and AICD Regulation
CD5 is a negative regulator of T-cell activation and thus plays a critical role in preventing AICD.39,49 AICD, an apoptotic pathway triggered at least in part by the death receptor CD95 (APO-1, FAS) and its natural ligand (CD95L, FASL) following T-cell hyperactivation, controls the expansion of activated T lymphocytes after TCR engagement and induces T-cell tolerance.65 Mice lacking Cd5 exhibited a significant delayed onset and decreased severity of EAE.49 EAE resistance was associated with a decreased survival of effector T cells and an attenuated generation of TH cells. In this disease model, convincing results pointing to a direct role for CD5 in protecting T cells from apoptosis were obtained upon the in vivo blockade of CD5 with soluble CD5-Fc molecules.49
CD5 also protects tumor-specific T lymphocytes from AICD as triggered by the recognition of specific targets by preventing T-cell overactivation through the downregulation of FASL expression and inhibition of caspase-8 activation.48 Consequently, Cd5−/− T cells are more susceptible to tumor-mediated AICD than Cd5+/+ T cells. Our in vivo experiments indicate that the potent antitumor immune response elicited in Cd5-deficient mice is transient and that tumor flare-ups correlate with an increased AICD of CD8+ TILs.64 This suggests that tumor-mediated T-cell AICD is likely to be involved in tumor immune escape. Accordingly, the protection of T cells from TCR-mediated apoptosis with soluble FAS-Fc molecules resulted in a dramatic reduction of tumor growth. These results point to a role for CD5 in the fate of tumor-specific T-cells and further substantiate its contribution to the regulation of antitumor CTL responses (Fig. 1).
Conclusions and Perspectives
One of the major challenges in cancer immunotherapy is the induction of strong and durable antitumor immune responses. The discovery of immune inhibitor checkpoints, such as those mediated by CTLA-4 and PD1, offered new immunotherapeutic perspectives to cancer treatment. Clinical trials based on the inhibition of CTLA-4 in patients with metastatic melanoma generated promising results. Indeed, attenuation of the CTLA-4-mediated immune checkpoint using ipilimumab (an anti-CTLA-4 neutralizing mAb) consistently improved patient survival.66 Interference with PD1 or its ligand PD-L1 also promotes antitumor immunity, and human anti-PD1 and anti-PD-L1 mAbs are currently under clinical evaluation.67 Our recent studies support a critical role for CD5 in antitumor immune responses and suggest that CD5 may constitute an interesting target for optimizing immunotherapeutic approaches against cancer. The use of neutralizing anti-CD5 mAbs or soluble CD5-Fc molecules, alone or combined with other immunomodulatory treatments, appears therefore as a forthcoming strategy for cancer immunotherapy. The developers of these strategies, nevertheless, will have to take into account the fact that most peripheral T cells express CD5, implying that therapies that are specifically targeted to the tumor site may be optimal for improving the effector functions of TILs.
Adoptive immunotherapy is currently one of the most promising therapeutic approaches against cancer, and has already been successfully used in clinical trials.68 According to our current knowledge on CD5 functions in T cells, the modulation of CD5 expression and/or activity in tumor-specific T lymphocytes before their transfer to cancer patients may represent a valuable strategy to improve the clinical outcome of adoptive cancer immunotherapies. However, together with CD5 manipulation, the control of T-cell AICD is an additional parameter to be considered for designing optimal cancer therapies. Indeed, the control of AIDC in tumor-specific CTLs through regulation of the FASL pathway may further potentiate intratumoral T-cell responses. Thus, immunotherapeutic approaches combining anti-CD5 neutralizing mAb and soluble FAS-Fc molecules may constitute a powerful alternative for the design of anticancer treatments that are capable of inducing effective and prolonged antitumor responses. This said, it should be noted that the downmodulation of CD5 signaling and the consequent enhanced reactivity of T cells may induce autoimmune reactions, limiting the benefits of this intervention for cancer patients. Despite this obstacle, we believe that intensified studies of the role of CD5 in antitumor immune responses may permit to potentiate current T cell-based immunotherapeutic strategies and offer novel therapeutic approaches against cancer.
Acknowledgments
This work was supported by grants from Inserm, the Association pour la Recherche sur le Cancer (ARC), the Ligue Nationale contre le Cancer and the Institut National contre le Cancer (INCa). MT was supported by a fellowship from the INCa.
Glossary
Abbreviations:
- Ag
antigen
- APC
antigen-presenting cell
- AICD
activation-induced cell death
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- MHC
major histocompatibility complex
- pMHC
peptide-MHC
- mAb
monoclonal antibody
- TAA
tumor-associated antigen
- TCR
T-cell receptor
- TIL
tumor-infiltrating T lymphocyte
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22841
References
- 1.Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–8. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 2.Terheyden P, Straten P, Bröcker EB, Kämpgen E, Becker JC. CD40-ligated dendritic cells effectively expand melanoma-specific CD8+ CTLs and CD4+ IFN-gamma-producing T cells from tumor-infiltrating lymphocytes. J Immunol. 2000;164:6633–9. doi: 10.4049/jimmunol.164.12.6633. [DOI] [PubMed] [Google Scholar]
- 3.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boissonnas A, Combadiere C, Lavergne E, Maho M, Blanc C, Debré P, et al. Antigen distribution drives programmed antitumor CD8 cell migration and determines its efficiency. J Immunol. 2004;173:222–9. doi: 10.4049/jimmunol.173.1.222. [DOI] [PubMed] [Google Scholar]
- 5.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–56. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollenbaugh JA, Reome J, Dobrzanski M, Dutton RW. The rate of the CD8-dependent initial reduction in tumor volume is not limited by contact-dependent perforin, Fas ligand, or TNF-mediated cytolysis. J Immunol. 2004;173:1738–43. doi: 10.4049/jimmunol.173.3.1738. [DOI] [PubMed] [Google Scholar]
- 7.Schüler T, Blankenstein T. Cutting edge: CD8+ effector T cells reject tumors by direct antigen recognition but indirect action on host cells. J Immunol. 2003;170:4427–31. doi: 10.4049/jimmunol.170.9.4427. [DOI] [PubMed] [Google Scholar]
- 8.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Ann rev immunol. 2006; 24:175-208. [DOI] [PubMed]
- 9.Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 10.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 11.Vazquez-Cintron EJ, Monu NR, Frey AB. Tumor-induced disruption of proximal TCR-mediated signal transduction in tumor-infiltrating CD8+ lymphocytes inactivates antitumor effector phase. J Immunol. 2010;185:7133–40. doi: 10.4049/jimmunol.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Tötterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother. 2010;33:225–35. doi: 10.1097/CJI.0b013e3181c01fcb. [DOI] [PubMed] [Google Scholar]
- 14.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–51. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 15.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egen JGKM, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–8. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 18.Jones NH, Clabby ML, Dialynas DP, Huang HJ, Herzenberg LA, Strominger JL. Isolation of complementary DNA clones encoding the human lymphocyte glycoprotein T1/Leu-1. Nature. 1986;323:346–9. doi: 10.1038/323346a0. [DOI] [PubMed] [Google Scholar]
- 19.Freeman M, Ashkenas J, Rees DJ, Kingsley DM, Copeland NG, Jenkins NA, et al. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc Natl Acad Sci U S A. 1990;87:8810–4. doi: 10.1073/pnas.87.22.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang HJ, Jones NH, Strominger JL, Herzenberg LA. Molecular cloning of Ly-1, a membrane glycoprotein of mouse T lymphocytes and a subset of B cells: molecular homology to its human counterpart Leu-1/T1 (CD5) Proc Natl Acad Sci U S A. 1987;84:204–8. doi: 10.1073/pnas.84.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Villar JJ, Whitney GS, Bowen MA, Hewgill DH, Aruffo AA, Kanner SB. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol. 1999;19:2903–12. doi: 10.1128/mcb.19.4.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Ann rev of immunol. 2002; 20:253-300. [DOI] [PubMed]
- 23.Osman N, Ley SC, Crumpton MJ. Evidence for an association between the T cell receptor/CD3 antigen complex and the CD5 antigen in human T lymphocytes. Eur J Immunol. 1992;22:2995–3000. doi: 10.1002/eji.1830221135. [DOI] [PubMed] [Google Scholar]
- 24.Beyers AD, Spruyt LL, Williams AF. Molecular associations between the T-lymphocyte antigen receptor complex and the surface antigens CD2, CD4, or CD8 and CD5. Proc Natl Acad Sci U S A. 1992;89:2945–9. doi: 10.1073/pnas.89.7.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmo AM, Castro MA, Arosa FA. CD2 and CD3 associate independently with CD5 and differentially regulate signaling through CD5 in Jurkat T cells. J Immunol. 1999;163:4238–45. [PubMed] [Google Scholar]
- 26.Biancone L, Bowen MA, Lim A, Aruffo A, Andres G, Stamenkovic I. Identification of a novel inducible cell-surface ligand of CD5 on activated lymphocytes. J Exp Med. 1996;184:811–9. doi: 10.1084/jem.184.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bikah G, Lynd FM, Aruffo AA, Ledbetter JA, Bondada S. A role for CD5 in cognate interactions between T cells and B cells, and identification of a novel ligand for CD5. Int Immunol. 1998;10:1185–96. doi: 10.1093/intimm/10.8.1185. [DOI] [PubMed] [Google Scholar]
- 28.Van de Velde H, von Hoegen I, Luo W, Parnes JR, Thielemans K. The B-cell surface protein CD72/Lyb-2 is the ligand for CD5. Nature. 1991;351:662–5. doi: 10.1038/351662a0. [DOI] [PubMed] [Google Scholar]
- 29.Calvo J, Padilla O, Places L, Vigorito E, Vilà JM, Vilella R, et al. Relevance of individual CD5 extracellular domains on antibody recognition, glycosylation and co-mitogenic signalling. Tissue Antigens. 1999;54:16–26. doi: 10.1034/j.1399-0039.1999.540102.x. [DOI] [PubMed] [Google Scholar]
- 30.Brown MH, Lacey E. A ligand for CD5 is CD5. J Immunol. 2010;185:6068–74. doi: 10.4049/jimmunol.0903823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vera J, Fenutría R, Cañadas O, Figueras M, Mota R, Sarrias MR, et al. The CD5 ectodomain interacts with conserved fungal cell wall components and protects from zymosan-induced septic shock-like syndrome. Proc Natl Acad Sci U S A. 2009;106:1506–11. doi: 10.1073/pnas.0805846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imboden JB, June CH, McCutcheon MA, Ledbetter JA. Stimulation of CD5 enhances signal transduction by the T cell antigen receptor. J Clin Invest. 1990;85:130–4. doi: 10.1172/JCI114402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alberola-Ila J, Places L, Cantrell DA, Vives J, Lozano F. Intracellular events involved in CD5-induced human T cell activation and proliferation. J Immunol. 1992;148:1287–93. [PubMed] [Google Scholar]
- 34.Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Müller W, Killeen N, et al. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–7. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 35.Tarakhovsky A, Müller W, Rajewsky K. Lymphocyte populations and immune responses in CD5-deficient mice. Eur J Immunol. 1994;24:1678–84. doi: 10.1002/eji.1830240733. [DOI] [PubMed] [Google Scholar]
- 36.Peña-Rossi C, Zuckerman LA, Strong J, Kwan J, Ferris W, Chan S, et al. Negative regulation of CD4 lineage development and responses by CD5. J Immunol. 1999;163:6494–501. [PubMed] [Google Scholar]
- 37.Dasu T, Qualls JE, Tuna H, Raman C, Cohen DA, Bondada S. CD5 plays an inhibitory role in the suppressive function of murine CD4(+) CD25(+) T(reg) cells. Immunol Lett. 2008;119:103–13. doi: 10.1016/j.imlet.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ordoñez-Rueda D, Lozano F, Sarukhan A, Raman C, Garcia-Zepeda EA, Soldevila G. Increased numbers of thymic and peripheral CD4+ CD25+Foxp3+ cells in the absence of CD5 signaling. Eur J Immunol. 2009;39:2233–47. doi: 10.1002/eji.200839053. [DOI] [PubMed] [Google Scholar]
- 39.Axtell RC, Xu L, Barnum SR, Raman C. CD5-CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J Immunol. 2006;177:8542–9. doi: 10.4049/jimmunol.177.12.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Wit J, Souwer Y, van Beelen AJ, de Groot R, Muller FJ, Klaasse Bos H, et al. CD5 costimulation induces stable Th17 development by promoting IL-23R expression and sustained STAT3 activation. Blood. 2011;118:6107–14. doi: 10.1182/blood-2011-05-352682. [DOI] [PubMed] [Google Scholar]
- 41.Ayyoub M, Raffin C, Valmori D. Generation of Th17 from human naive CD4+ T cells preferentially occurs from FOXP3+ Tregs upon costimulation via CD28 or CD5. Blood. 2012;119:4810–2, author reply 4812-3. doi: 10.1182/blood-2012-02-409722. [DOI] [PubMed] [Google Scholar]
- 42.Raman C. CD5, an important regulator of lymphocyte selection and immune tolerance. Immunol Res. 2002;26:255–63. doi: 10.1385/IR:26:1-3:255. [DOI] [PubMed] [Google Scholar]
- 43.Gimferrer I, Farnós M, Calvo M, Mittelbrunn M, Enrich C, Sánchez-Madrid F, et al. The accessory molecules CD5 and CD6 associate on the membrane of lymphoid T cells. J Biol Chem. 2003;278:8564–71. doi: 10.1074/jbc.M209591200. [DOI] [PubMed] [Google Scholar]
- 44.Brossard C, Semichon M, Trautmann A, Bismuth G. CD5 inhibits signaling at the immunological synapse without impairing its formation. J Immunol. 2003;170:4623–9. doi: 10.4049/jimmunol.170.9.4623. [DOI] [PubMed] [Google Scholar]
- 45.Bhandoola A, Bosselut R, Yu Q, Cowan ML, Feigenbaum L, Love PE, et al. CD5-mediated inhibition of TCR signaling during intrathymic selection and development does not require the CD5 extracellular domain. Eur J Immunol. 2002;32:1811–7. doi: 10.1002/1521-4141(200206)32:6<1811::AID-IMMU1811>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 46.Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, et al. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166:5464–72. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 47.Gary-Gouy H, Harriague J, Dalloul A, Donnadieu E, Bismuth G. CD5-negative regulation of B cell receptor signaling pathways originates from tyrosine residue Y429 outside an immunoreceptor tyrosine-based inhibitory motif. J Immunol. 2002;168:232–9. doi: 10.4049/jimmunol.168.1.232. [DOI] [PubMed] [Google Scholar]
- 48.Friedlein G, El Hage F, Vergnon I, Richon C, Saulnier P, Lécluse Y, et al. Human CD5 protects circulating tumor antigen-specific CTL from tumor-mediated activation-induced cell death. J Immunol. 2007;178:6821–7. doi: 10.4049/jimmunol.178.11.6821. [DOI] [PubMed] [Google Scholar]
- 49.Axtell RC, Webb MS, Barnum SR, Raman C. Cutting edge: critical role for CD5 in experimental autoimmune encephalomyelitis: inhibition of engagement reverses disease in mice. J Immunol. 2004;173:2928–32. doi: 10.4049/jimmunol.173.5.2928. [DOI] [PubMed] [Google Scholar]
- 50.Dennehy KM, Broszeit R, Garnett D, Durrheim GA, Spruyt LL, Beyers AD. Thymocyte activation induces the association of phosphatidylinositol 3-kinase and pp120 with CD5. Eur J Immunol. 1997;27:679–86. doi: 10.1002/eji.1830270316. [DOI] [PubMed] [Google Scholar]
- 51.Bamberger MAM, Santos AM, Gonçalves CM, Oliveira MI, James JR, Moreira A, et al. A new pathway of CD5 glycoprotein-mediated T cell inhibition dependent on inhibitory phosphorylation of Fyn kinase. J Biol Chem. 2011;286:30324–36. doi: 10.1074/jbc.M111.230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–11. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fowlkes BJ, Edison L, Mathieson BJ, Chused TM. Early T lymphocytes. Differentiation in vivo of adult intrathymic precursor cells. J Exp Med. 1985;162:802–22. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. Sensory adaptation in naive peripheral CD4 T cells. J Exp Med. 2001;194:1253–61. doi: 10.1084/jem.194.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong P, Barton GM, Forbush KA, Rudensky AY. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J Exp Med. 2001;193:1179–87. doi: 10.1084/jem.193.10.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–81. doi: 10.1016/S1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 57.Kassiotis G, Zamoyska R, Stockinger B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J Exp Med. 2003;197:1007–16. doi: 10.1084/jem.20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Stamou P, de Jersey J, Carmignac D, Mamalaki C, Kioussis D, Stockinger B. Chronic exposure to low levels of antigen in the periphery causes reversible functional impairment correlating with changes in CD5 levels in monoclonal CD8 T cells. J Immunol. 2003;171:1278–84. doi: 10.4049/jimmunol.171.3.1278. [DOI] [PubMed] [Google Scholar]
- 60.Gagnon J, Chen XL, Forand-Boulerice M, Leblanc C, Raman C, Ramanathan S, et al. Increased antigen responsiveness of naive CD8 T cells exposed to IL-7 and IL-21 is associated with decreased CD5 expression. Immunol Cell Biol. 2010;88:451–60. doi: 10.1038/icb.2009.109. [DOI] [PubMed] [Google Scholar]
- 61.Indraccolo SM, Mion M, Zamarchi R, Coppola V, Calderazzo F, Amadori A, et al. A CD3+CD8+ T cell population lacking CD5 antigen expression is expanded in peripheral blood of human immunodeficiency virus-infected patients. Clin Immunol Immunopathol. 1995;77:253–61. doi: 10.1006/clin.1995.1151. [DOI] [PubMed] [Google Scholar]
- 62.Dorothée G, Vergnon I, El Hage F, Le Maux Chansac B, Ferrand V, Lécluse Y, et al. In situ sensory adaptation of tumor-infiltrating T lymphocytes to peptide-MHC levels elicits strong antitumor reactivity. J Immunol. 2005;174:6888–97. doi: 10.4049/jimmunol.174.11.6888. [DOI] [PubMed] [Google Scholar]
- 63.Algarra I, Cabrera T, Garrido F. The HLA crossroad in tumor immunology. Hum Immunol. 2000;61:65–73. doi: 10.1016/S0198-8859(99)00156-1. [DOI] [PubMed] [Google Scholar]
- 64.Tabbekh M, Franciszkiewicz K, Haouas H, Lécluse Y, Benihoud K, Raman C, et al. Rescue of tumor-infiltrating lymphocytes from activation-induced cell death enhances the antitumor CTL response in CD5-deficient mice. J Immunol. 2011;187:102–9. doi: 10.4049/jimmunol.1004145. [DOI] [PubMed] [Google Scholar]
- 65.Budd RC. Activation-induced cell death. Curr Opin Immunol. 2001;13:356–62. doi: 10.1016/S0952-7915(00)00227-2. [DOI] [PubMed] [Google Scholar]
- 66.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simeone E, Ascierto PA. Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti-CTLA-4, anti-CD137, and anti-PD1. J Immunotoxicol. 2012;9:241–7. doi: 10.3109/1547691X.2012.678021. [DOI] [PubMed] [Google Scholar]
- 68.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]