Abstract
The dissection of the molecular mechanisms underlying tumor-cell recognition by γδ T-cells is crucial to improve their performance in cancer immunotherapy. Here, we discuss the controversy around the relative contributions of the γδ T-cell receptor (TCR) and natural killer receptors (NKRs) to tumor-cell targeting by γδ T cells.
Keywords: NK cell, NK receptor, NKG2D, T-cell receptor, γδ T cell
γδ cells: T cells with an NK character
γδ cells constitute the prototype of unconventional T lymphocytes that are not restricted by classical MHC-mediated antigen presentation. Instead, these innate-like lymphocytes are thought to directly recognize molecular “indicators of stress” in dysfunctional cells (such as tumor cells), against which they exert potent cytolytic activity.1,2 This establishes a clear functional parallel between γδ cells and their lymphoid relatives, natural killer (NK) cells.3 This parallel was also evident upon genome-wide expression analyses that highlighted the similarities between the transcriptional profiles of activated human Vγ9Vδ2 γδ T-cell lines and NK cells.4 An additional key characteristic shared by these two lymphocyte subsets is the expression of a wide set of germline-encoded receptors that were initially described in NK cells and hence are collectively known as “NK receptors” (NKRs). These include killer-activating receptors (KARs), such as NKG2D, CD94/NKG2C, DNAX accessory molecule-1 (DNAM-1), NKp30 and NKp445-7; and killer-inhibitory receptors (KIRs), like CD94/NKG2A, ILT2, CD161 or KIR2DL 1–3.5,8
This said, there is a key difference between γδ T and NK cells, i.e., the expression of a somatically rearranged TCR (which in this case is a heterodimer of γ and δ chains) by the former but not the latter. Alike its αβ counterpart, the γδ TCR was considered as the dominant receptor for tumor-antigen recognition by γδ cells.5 This view has been challenged by a consistent set of reports highlighting the importance of activating NKRs in cancer-cell recognition by human and mouse γδ cells. Here, we discuss this controversy and propose that γδ cells rely on the TCRγδ for the perception of “danger” (often pathogen)-associated molecular patterns and on NKRs for the actual discrimination between (“stressed”) tumor cells and their (“healthy”) non-transformed counterparts.
γδ TCR-mediated tumor cell recognition?
In the early 1990s, the distinct patterns of cytotoxicity of human γδ T and NK-cell clones from the same individuals was interpreted as suggestive of TCR-dependent tumor cell recognition by γδ T cells.9 However, the global lack of success in identifying γδ TCR ligands (including in the tumor context) has severely limited the credit given to this hypothesis.
It is known since the mid-1990s that the major γδ T-cell subset in the human peripheral blood, Vγ9Vδ2 cells, are uniquely activated by non-peptidic prenyl-pyrophosphate antigens (phosphoantigens) such as isopenthenyl pyrophosphate, (IPP) (reviewed in ref. 10). In fact, this constitutes the basis of current γδ T cell-based immunotherapeutic strategies against cancer.11,12 Phosphoantigen-activated Vγ9Vδ2 T cells can recognize and kill a large variety of tumor cell lines in vitro and in vivo (in xenograft models), including breast, colon and nasopharyngeal carcinoma, melanoma, pancreatic adenocarcinomas and a particularly large panel of hematological tumors.12-15
As IPP constitutes an intermediate of the mevalonate pathway of isoprenoid biosynthesis that significantly increases upon malignant transformation, it might act as a “tumor-associated antigen” for Vγ9Vδ2 cells.16 Furthermore, since the reactivity of Vγ9Vδ2 T cells to phosphoantigens can be abolished with TCR-blocking antibodies and conferred by the transfer of Vγ9Vδ2 TCR-coding genes,17 phosphoantigens were postulated to act as Vγ9Vδ2 TCR ligands. However, no direct binding could ever be demonstrated in vitro between the Vγ9Vδ2 TCR and phophoantigens, including those of microbial origin, such as (E)-4-hydroxy-3-methyl-but-enyl pyrophosphate (HMBPP), which possess a much higher bioactivity (in the picomolar rather than in the micromolar range) than IPP.5
These important limitations raised the possibility that phosphoantigens trigger the Vγ9Vδ2 TCR by indirect means. In 2005, Scotet et al. proposed that the ecto-F1 ATPase, a form of the mitochondrial ATP synthase ectopically expressed at the cell membrane, would form a complex with the serum protein apolipoprotein A1 (ApoA1) that would be recognized by the Vγ9Vδ2 TCR and would underpin the presentation of endogenous phosphoantigens.5,18 Consistent with this, F1 ATPase-coated beads were later shown to stably bind an adenylated derivative of IPP, and to promote Vγ9Vδ2 TCR aggregation, cytokine secretion and cytotoxicity.19
More recently, Scotet and collaborators have identified a new potential link between phosphoantigens and Vγ9Vδ2 TCR activation.20 This seems to require the conformational modification and/or clustering of CD277 (butyrophilin 3) molecules in target cells, which in turn depends on intracellular accumulation of phosphoantigens. The effects of both agonistic and blocking anti-CD277 monoclonal antibodies on Vγ9Vδ2 TCR transductants indicate that the TCR is necessary for such a CD277-dependent activation process.20 How Vγ9Vδ2 T cells detect phosphoantigen-induced changes of CD277 remains to be determined, since the authors have not detected cognate interactions between recombinant Vγ9Vδ2 TCR and CD277.
In sum, the currently available data places phosphoantigens as clear Vγ9Vδ2 TCR agonists, even if most likely they do no operate as direct ligands. In the context of tumor-cell recognition, this raises the key question whether any putative TCR ligand, somehow linked to phosphoantigens, might indeed account for the discrimination between tumor and healthy cells.
An important study has recently identified an unexpected MHC-like protein as a cognate ligand for a Vγ4Vδ5 clone isolated from a cytomegaovirus (CMV)-infected patient.21 This new TCR ligand is the endothelial protein C receptor (EPCR), a key component of the protein C pathway, which has been linked to endothelial barrier protection during inflammation and hypoxia. Human EPCR expression is restricted to endothelial cells (which are key targets for CMV infection in vivo), and is upregulated after cellular transformation, in particular by carcinomas. The TCR/EPCR interaction allowed γδ T cells to recognize both endothelial cells targeted by CMV and epithelial tumors.21 However, the recognition of target cells by γδ T cells required a multimolecular stress signature composed of EPCR and co-stimulatory ligand(s). The presence of EPCR protein could thus “flag” target endothelial cells, but additional factors (such as NKG2D, ILT2 and others) are likely used by Vδ2 T cells to distinguish CMV-infected and tumor cells from healthy cells.
The involvement of molecules of the MHC family in tumor-cell recognition by γδ T cells is ambiguous, as they also participate in T-cell activation through NKRs. For example, the interaction of human intestinal epithelial Vδ1 T cells with MHC Class I polypeptide-related sequence A (MICA)+ and MHC Class I polypeptide-related sequence B (MICB)+ target cells occurs through MIC recognition, which drives both TCR-dependent and NKG2D-dependent stimulatory signals. Both receptors compete for binding to MIC ligands, and interaction analyses showed that MIC binding by the two receptors is mutually exclusive.22 However, surface plasmon resonance-based interaction analyses revealed strikingly different binding kinetics and affinities for TCR/MIC vs. NKG2D/MIC interactions. MIC proteins bind to NKG2D receptors with affinities (submicromolar KD) that are nearly 1,000-fold lower than those characterizing the TCR/MIC interaction (hundreds of micromolar KD). These data suggest a temporally ordered model for the formation of hypothetical T cell/target cell synapses, with implications for signaling mechanisms. In this setting, initial interactions at the point of contact may be dominated by NKG2D/MICA binding, which may then give way to longer-lived γδ TCR/MICA complexes.22
From a more global standpoint, if we consider most of the molecules claimed to constitute γδ TCR ligands (Table 1), many do not comply with the expression pattern of tumor-associated antigens. This has led us and other researchers to consider in more detail the potential role of NKRs as determinants of tumor-cell recognition by γδ T cells.
Table 1. Molecules described as γδ TCR ligands. List of molecules (with respective biochemical nature) proposed to bind directly to γδ TCRs in a particular human γδ T-cell subset or clone. The published evidence (PMID provided) was based on anti-γδ TCR blocking antibodies, γδ TCRtransfection experiments, surface plasmon resonance or co-crystallization studies.
| Molecule(s) | Biochemistry | γδcell subset | PubMed ID (references) |
|---|---|---|---|
| DRw53 + C. tetani peptide |
MHC-II + peptide |
Vγ9Vδ2 |
2469770, 1345917, 2524009 |
| Staphylococcal enterotoxin A |
superantigen |
Vδ2 |
8144918, 2377230 |
| Staphylococcal enterotoxin B |
superantigen |
Vδ1 |
8696008 |
| Oxidative stress response regulatory protein (OXYS) |
Bacillus Calmette-Guerin protein |
|
21526117 |
| Apolipoprotein A-I |
Lipoprotein |
Vγ9Vδ2 |
15664160 |
| Ecto-F1-ATPase (+ ApppI) |
Mitochondrial ATPase domain |
Vγ9Vδ2 |
15664160, 20483757 |
| Phosphoantigens (+ undefined presenting molecule) |
Prenyl pyrophosphates (bacterial/ mammalian) |
Vγ9Vδ2 |
7584140, 7529807, 18802083 |
| MutS homolog 2 (hMSH2) |
DNA repair protein |
|
18321859, 22433851 |
| Histidyl-tRNA synthetase |
tRNA synthetases (bacterial/ mammalian) |
Vγ1.3Vδ2 |
22549773 |
| Heat shock protein 60 (HSP-60) |
Heat shock protein (bacterial/ mammalian) |
Vγ9Vδ2 |
2473405, 1978758, 8094731, 18321859 |
| MICA |
MHC-Ib protein |
Vδ1+ |
12133944, 16297874, 9497295 |
| ULBP-4 |
MHC-Ib protein |
Vδ2+ |
19436053 |
| CD1c |
MHC-Ib protein |
Vδ1+ |
10727456, 2477705, 1690662, 12486100 |
| CD1d + sulfatide |
MHC-Ib protein + lipid |
Vδ1+ |
22829134 |
| Endothelial protein C receptor (EPCR) | MHC-Ib protein | Vγ4Vδ5 | 22885985 |
NKR-mediated tumor targeting by γδ cells?
Our studies with hematological tumors have highlighted a major role for activating NKRs in tumor-cell recognition by human γδ T cells. This was observed for both Vγ9Vδ2+ and Vδ1+NKp30+ T-cell subsets, in which NKG2D and/or NKp30, but not the respective TCRs, mediated leukemia/lymphoma-cell recognition.7,23
NKG2D is a C-type lectin receptor shared by NK, γδ T and CD8+ αβ T lymphocytes, which recognizes MICA, MICB and UL16-binding proteins (ULBP1–6) in humans as well as Rae, Mult and H-60 in mice.24 NKG2D ligands are not expressed by most normal tissues but are upregulated by many tumor-cell types, as well as by virus- or bacteria-infected cells.2,24,25
In line with this notion, various studies have emphasized the unique contribution of NKG2D for γδ T cell-mediated tumor immunosurveillance both in humans26 and in mice.27 Mouse γδ T cells respond rapidly in vivo to the self stress antigen Rae-1 that engages their activating receptor NKG2D.27 Mice lacking γδ T cells are highly susceptible to cutaneous carcinogenesis and this susceptibility appears to be regulated by NKG2D expressed on γδ cells.28 Moreover, Nkg2d−/− mice exhibit an impaired immunosurveillance of epithelial and lymphoid malignancies in two transgenic models of de novo tumorigenesis.29 Another study has shown that the sustained localized expression of the murine NKG2D ligand Rae-1 impairs T-cell cytotoxicity in vivo and reduce tumor immunosurveillance.30 In our studies with human γδ T cells, the NKG2D ligand ULBP1, which is abundant in γδ T cell-susceptible hematological tumors,13 was both required and sufficient for leukemia/lymphoma cell-recognition by Vγ9Vδ2 T cells.23 These findings feed the general concept of NK receptors, particularly NKG2D, being key molecular determinants for the immune recognition of “oncogenic stress.”24
Although nearly all Vγ9Vδ2 T cells express NKG2D on their surface, the hierarchy between NKG2D and Vγ9Vδ2 TCR signals remains highly controversial. Some studies reported the ability of Vγ9Vδ2 T cells to trigger effector responses through NKG2D stimulation alone (i.e., similarly to NK cells).15,25,31 However, other authors have failed to evidence an NKG2D-induced Vγ9Vδ2 T-cell activation without coincident TCR stimulation.32 In this case, NKG2D would function in γδ T cells like in CD8+ αβ T cells, i.e., as an accessory (“co-stimulatory”) receptor to the TCR (reviewed in ref. 5). Future research should clarify this issue, together with the signaling pathways that are activated in γδ T cells when TCR and NKG2D are triggered individually or simultaneously.
Another NKR implicated in tumor cell recognition by Vγ9Vδ2 T-cells is DNAM-1.6,15 DNAM-1 ligands Nectin-like-5 and Nectin-2 are expressed on most hepatocellular carcinoma (HCC) cell lines, and antibody-based masking experiments demonstrated that the cytotoxic response of, as well as the production of IFNγ by, γδ T cells exposed to HCC cells involve interactions between DNAM-1 and Nectin-like-5.6
We have recently characterized a Vδ1+ T-cell population capable of targeting hematological tumors that are highly resistant to fully activated Vγ9Vδ2 peripheral blood lymphocytes (PBLs).7 We have shown that this Vδ1+ subset owes its specialized killer function to an increased expression of the natural cytotoxicity receptors (NCRs) NKp30, NKp44 and NKp46, which had been previously regarded as NK-specific markers. Importantly, NCRs are also clearly implicated in tumor-cell recognition by human NK cells.33,34 Although neither Vδ1+ nor Vδ2+ cells express NCRs constitutively, these can be selectively upregulated in Vδ1+ cells by AKT-dependent signals provided synergistically by γc cytokines (IL-2 or IL-15) and TCR stimulation.7 Thus, NCR induction in Vδ1+ T cells occurs downstream of TCR activation, which provides a link between the two types of receptor signals.
We have also demonstrated that NKp30 and NKp44 are both functional in NCR+ Vδ1+ T cells, and non-redundantly contribute to the targeting of lymphocytic leukemia cells, with NKp30 playing the most prominent role in this process.7 Of note, we tested three different γδ TCR-blocking monoclonal antibodies and could not detect any reduction in tumor-cell killing by NCR+ Vδ1+ T cells. Thus, we suggest that while TCR signals are critically required for the differentiation and activation of NCR+ Vδ1+ T cells, these cells recognize tumor-cell targets through activating NK receptors, namely NKp30, NKp44 and NKG2D.
Other groups have suggested that γδ T cells could recognize tumor targets through the interaction of their TCR with self-ligands that are overexpressed by tumor cells, and use NKR signals to fine-tune the cell-activation threshold (reviewed in Refs. 5,10,14,35). In this scenario, the TCR-mediated activity would be tightly regulated by an interplay between activating and inhibitory NKRs.5 Of note, MHC Class I expression did not consistently segregate between γδ T cell-susceptible and resistant tumor cell lines, thus excluding a direct “missing self” mechanism as the basis for γδ T-cell recognition of hematological tumors.13 This is also consistent with the observation that MHC Class I knockdown does not enhance the Vγ9Vδ2-mediated lysis of γδ T cell-resistant Raji and B-cell chronic lymphocytic leukemia cells.18 Nevertheless, the contribution of inhibitory NKRs to γδ T-cell activation and tumor targeting8,36 should be further evaluated in studies that mostly focus on activating NKRs.
Concluding Remarks
The current understanding of the role of γδ TCRs in tumor-cell recognition is hampered by the limited number of tumor-associated antigens that are known to bind directly these unconventional TCRs. The fact that many of the identified ligands for human γδ TCRs are molecules expressed by microbes (Table 1) suggests that γδ TCRs were evolutionary selected to detect microbial molecules or metabolites. In this scenario, the sensing/recognition of self and “stressed self” components would be mainly mediated by NKRs. Building on these considerations, our current working model propose two stages of γδ T-cell activation/differentiation and tumor-cell recognition. First, γδ cells are potently activated by (mostly unknown) γδ TCR ligands in the presence of IL-2. This, which can be achieved for Vγ9Vδ2 cells using (microbial or synthetic) phosphoagonists (plus IL-2), endows them with potent cytolytic (and cytokine-secreting) functions, but requires a subsequent phase of target discrimination between tumor and healthy cells. We propose this is mainly determined by the binding of activating NKRs to stress-inducible proteins that selectively accumulate on the surface of tumor cells. Of note, the segregation of these two processes (activation vs. tumor cell recognition) in experimental systems requires the pre-activation of γδ T cells (via their TCR) before their use against tumor targets. More importantly, we believe the integrated exploitation of these two phases will be the key for the clinical success of future γδ cell-based therapeutic protocols (Fig. 1).
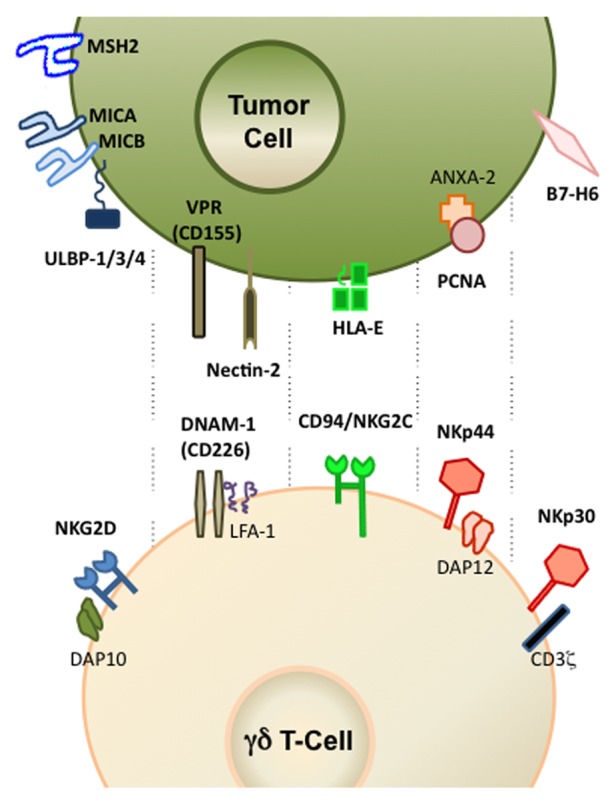
Figure 1. Activating NK receptors and corresponding ligands implicated in tumor cell recognition by human γδ T cells. Depicted are also key signaling molecules associated with natural killer receptors (NKRs). Additional details are provided in the main text.
Acknowledgments
The authors thank Adrian Hayday, Domenico Mavilio, Kelly Hudspeth, Telma Lança, Anita Gomes and Haakan Norell for helpful discussions on this topic; and the support from the European Research Council (StG_260352) and European Molecular Biology Organization (Young Investigator Programme).
Glossary
Abbreviations:
- NK
natural killer
- IFNγ
interferonγ
- IL
interleukin
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22892
References
- 1.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–96. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Gomes AQ, Correia DV, Silva-Santos B. Non-classical major histocompatibility complex proteins as determinants of tumour immunosurveillance. EMBO Rep. 2007;8:1024–30. doi: 10.1038/sj.embor.7401090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pont F, Familiades J, Déjean S, Fruchon S, Cendron D, Poupot M, et al. The gene expression profile of phosphoantigen-specific human γδ T lymphocytes is a blend of αβ T-cell and NK-cell signatures. Eur J Immunol. 2012;42:228–40. doi: 10.1002/eji.201141870. [DOI] [PubMed] [Google Scholar]
- 5.Nedellec S, Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: from signals to functions. Semin Immunol. 2010;22:199–206. doi: 10.1016/j.smim.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Toutirais O, Cabillic F, Le Friec G, Salot S, Loyer P, Le Gallo M, et al. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T cells. Eur J Immunol. 2009;39:1361–8. doi: 10.1002/eji.200838409. [DOI] [PubMed] [Google Scholar]
- 7.Correia DV, Fogli M, Hudspeth K, da Silva MG, Mavilio D, Silva-Santos B. Differentiation of human peripheral blood Vδ1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood. 2011;118:992–1001. doi: 10.1182/blood-2011-02-339135. [DOI] [PubMed] [Google Scholar]
- 8.Carena I, Shamshiev A, Donda A, Colonna M, Libero GD. Major histocompatibility complex class I molecules modulate activation threshold and early signaling of T cell antigen receptor-gamma/delta stimulated by nonpeptidic ligands. J Exp Med. 1997;186:1769–74. doi: 10.1084/jem.186.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisch P, Malkovsky M, Braakman E, Sturm E, Bolhuis RL, Prieve A, et al. Gamma/delta T cell clones and natural killer cell clones mediate distinct patterns of non-major histocompatibility complex-restricted cytolysis. J Exp Med. 1990;171:1567–79. doi: 10.1084/jem.171.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 11.Gomes AQ, Martins DS, Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 2010;70:10024–7. doi: 10.1158/0008-5472.CAN-10-3236. [DOI] [PubMed] [Google Scholar]
- 12.Hannani D, Ma Y, Yamazaki T, Déchanet-Merville J, Kroemer G, Zitvogel L. Harnessing γδ T cells in anticancer immunotherapy. Trends Immunol. 2012;33:199–206. doi: 10.1016/j.it.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Gomes AQ, Correia DV, Grosso AR, Lança T, Ferreira C, Lacerda JF, et al. Identification of a panel of ten cell surface protein antigens associated with immunotargeting of leukemias and lymphomas by peripheral blood gammadelta T cells. Haematologica. 2010;95:1397–404. doi: 10.3324/haematol.2009.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–78. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 15.Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, et al. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol. 2007;66:320–8. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 16.Gober HJ, Kistowska M, Angman L, Jenö P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. V gamma 2V delta 2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 18.Scotet E, Martinez LO, Grant E, Barbaras R, Jenö P, Guiraud M, et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Mookerjee-Basu J, Vantourout P, Martinez LO, Perret B, Collet X, Périgaud C, et al. F1-adenosine triphosphatase displays properties characteristic of an antigen presentation molecule for Vgamma9Vdelta2 T cells. J Immunol. 2010;184:6920–8. doi: 10.4049/jimmunol.0904024. [DOI] [PubMed] [Google Scholar]
- 20.Harly C, Guillaume Y, Nedellec S, Peigné CM, Mönkkönen H, Mönkkönen J, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood. 2012;120:2269–79. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol. 2012;13:872–9. doi: 10.1038/ni.2394. [DOI] [PubMed] [Google Scholar]
- 22.Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, et al. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci U S A. 2011;108:2414–9. doi: 10.1073/pnas.1015433108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lança T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C, et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood. 2010;115:2407–11. doi: 10.1182/blood-2009-08-237123. [DOI] [PubMed] [Google Scholar]
- 24.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol. 2009;9:568–80. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, et al. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/S1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 26.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 27.Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–54. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 28.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–9. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 29.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(5):571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–37. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 31.Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144–51. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- 32.Nedellec S, Sabourin C, Bonneville M, Scotet E. NKG2D costimulates human V gamma 9V delta 2 T cell antitumor cytotoxicity through protein kinase C theta-dependent modulation of early TCR-induced calcium and transduction signals. J Immunol. 2010;185:55–63. doi: 10.4049/jimmunol.1000373. [DOI] [PubMed] [Google Scholar]
- 33.Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251–8. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 34.Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest. 2009;119:1251–63. doi: 10.1172/JCI36022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thedrez A, Sabourin C, Gertner J, Devilder MC, Allain-Maillet S, Fournié JJ, et al. Self/non-self discrimination by human gammadelta T cells: simple solutions for a complex issue? Immunol Rev. 2007;215:123–35. doi: 10.1111/j.1600-065X.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 36.Halary F, Peyrat MA, Champagne E, Lopez-Botet M, Moretta A, Moretta L, et al. Control of self-reactive cytotoxic T lymphocytes expressing gamma delta T cell receptors by natural killer inhibitory receptors. Eur J Immunol. 1997;27:2812–21. doi: 10.1002/eji.1830271111. [DOI] [PubMed] [Google Scholar]


