Abstract
Regulation of cell elongation is important for plant morphogenesis. Many studies have shown that cortical microtubules play crucial roles during cell elongation and that microtubule stability, organization, and dynamics are regulated by microtubule regulatory proteins.1 Recently, we reported that a novel protein from Arabidopsis, termed microtubule-destabilizing protein 25 (MDP25), functions as a negative regulator of hypocotyl cell elongation. MDP25 destabilizes microtubules and exerts its effect on microtubules as a result of transient elevation of cytosolic calcium levels.2
Keywords: MAP65, MDP25, calcium, microtubule destabilization
Although micromolar levels of calcium can inhibit tubulin assembly and depolymerize microtubules in vitro, microtubules in plant cells have been shown to be less sensitive to short-term treatment with calcium, even in the presence of high concentrations of Mg2+ or high ionic strengths.3-5 Therefore, in cells, calcium likely exerts its effect on microtubules via indirect mechanisms. However, it is important to understand the underlying mechanisms by which calcium affects microtubule dynamics. Assessing the function of calcium-binding microtubule regulatory proteins represents a reasonable approach to explore this issue. Previous studies have indicated that the MDP25 protein directly binds calcium in vitro and that calcium regulates MDP25 translocation from the plasma membrane to the cytosol.2,6 However, the effects on microtubules of calcium binding to MDP25 remain unknown. In this addendum, we tested the possibility that calcium affects the ability of MDP25 to destabilize microtubules.
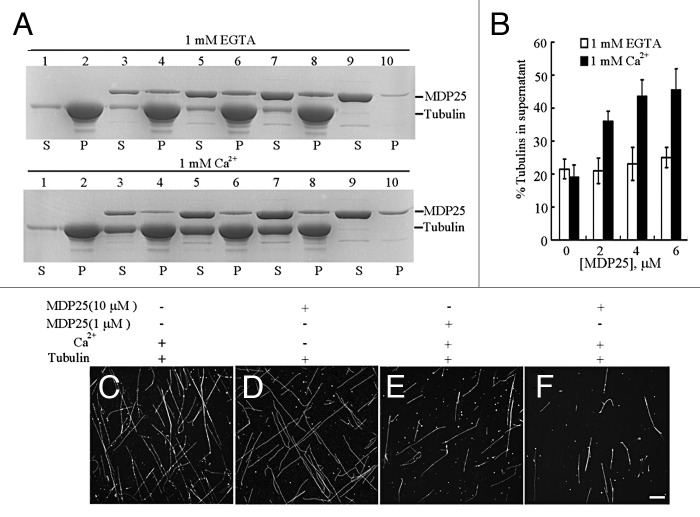
Various concentrations (0, 2, 4 and 6 μΜ) of MDP25 protein were incubated with 5 μΜ paclitaxel-stabilized microtubules in the presence or absence of calcium, followed by a high-speed co-sedimentation assay. The amount of tubulin in the supernatant was estimated by gel density scanning analysis. Results from this experiment revealed that increasing concentrations of MDP25 did not affect the amount of depolymerized tubulin in the absence of calcium (Fig. 1A, upper gel, and B). However, in the presence of calcium, MDP25 increased the amount of tubulin in the supernatant in a concentration-dependent manner (Fig. 1A, lower gel, and B). Rhodamine-labeled, paclitaxel-stabilized microtubules were used to further confirm this result via confocal microscopy. Results from this analysis confirmed that fewer microtubule filaments were detected in the presence of 1 μM (Fig. 1E) or 10 μM (Fig. 1F) MDP25 in the presence of calcium, in comparison to microtubules treated with calcium (Fig. 1C) or 10 μM MDP25 protein alone (Fig. 1D), respectively. These results indicate that calcium dramatically enhances the microtubule-destabilizing activity of MDP25. Notably, no small pieces of microtubules were observed under the microscope in the presence of MDP25 and calcium. Considering the fact that our previous study showed that MDP25 depolymerized microtubules directly,2 we hypothesize that the binding of calcium to MDP25 may affect its activity in microtubule depolymerization, but not severing.
Figure 1. MDP25 depolymerizes paclitaxel-stabilized microtubules in the presence of calcium in vitro. (A) MDP25 protein co-sedimented with paclitaxel-stabilized microtubules in the presence of calcium or EGTA, respectively. S, supernatant; P, pellet. (B) The amount of disassembled tubulin is presented as a percentage of total tubulin determined by gel density scanning. (C–F) Rhodamine-labeled and paclitaxel-stabilized microtubules were co-incubated with MDP25 proteins with or without calcium and examined by confocal microscopy. Representative images show microtubules in the presence of calcium (C), 10 μM MDP25 (D), or 1 μM and 10 μM MDP25 plus calcium (E and F), respectively. Scale bar in (F) = 20 μm.
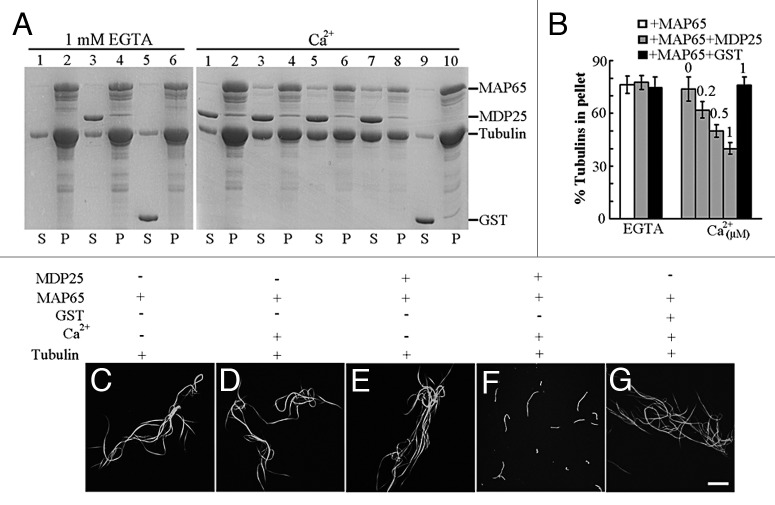
Next, we further investigated whether enhanced depolymerization also occurred in the presence of bundled microtubules, a common state for cortical microtubules in plant cells. The Arabidopsis MAP65-1 protein, a microtubule bundling and stabilizing protein that is ubiquitously expressed during the cell cycle in most plant organs and tissues,7,8 was employed for the test. We analyzed whether, in the presence of calcium, MDP25 could disrupt microtubule bundles induced by MAP65-1 in vitro. Microtubule-MAP65 bundles were obtained by incubating 20 μM tubulin with 8 μM GST-MAP65-1, followed by low-speed centrifugation at 5,900 × g. Co-sedimentation of MDP25 and microtubule-MAP65 bundles was performed in the presence of various concentrations of calcium or the absence of calcium, respectively. Results from this experiment revealed little difference in the amount of tubulin in the pellets for samples incubated with 0 or 6 μM MDP25 or 8 μM GST protein in the absence of calcium, indicating that MDP25 alone did not disrupt the formation of microtubule bundles induced by MAP65-1 (Fig. 2A, left gel, and B). However, when various concentrations of free calcium (0, 0.2, 0.5 and 1 μM) were added in the same co-sedimentation analysis, the amount of tubulin in the pellets was reduced dramatically (Fig. 2A, right gel, and Fig. 2B). Rhodamine-labeled microtubule-bundles induced by the addition of MAP65-1 were used to confirm these results by confocal microscopy. Microtubule bundles induced by MAP65-1 (Fig. 2C) showed little change in the presence of calcium (Fig. 2D), 8 μM MDP25 (Fig. 2E), or 10 μM GST protein and calcium, respectively (Fig. 2G). However, microtubule bundles were greatly disrupted in the presence of 8 μM MDP25 and 0.5 μM free calcium (Fig. 2F). These results demonstrate that calcium enhances MDP25-induced disruption of microtubule bundles.
Figure 2. MDP25 disrupts microtubule bundles induced by the addition of MAP65-1 in vitro. MDP25 protein co-sedimented with microtubule bundles induced by MAP65-1 in the presence of various concentrations of free calcium or 1 mM EGTA. (A) The gel on the left shows that MDP25 does not depolymerize microtubule bundles in the presence of EGTA. Lanes contain microtubule bundles formed by MAP65 (lanes 1 and 2), with the addition of MDP25 (lanes 3 and 4) or GST (lanes 5 and 6), respectively. The gel on the right shows that MDP25 depolymerizes microtubule bundles in a calcium-dependent manner. Lanes contain microtubule-MAP65 bundles incubated with MDP25 in the presence of 0 μM (lanes 1 and 2), 0.2 μM (lanes 3 and 4), 0.5 μM (lanes 5 and 6), and 1 μM free calcium (lanes 7 and 8), respectively, or microtubule bundles in the presence of GST protein plus 1 μM free calcium (lanes 9 and 10). (B) The amount of tubulin was estimated by gel density scanning, and the amounts of microtubule bundles in the pellets are presented as percentages of total tubulin. (C–G) Preformed rhodamine-labeled microtubule bundles induced by the addition of MAP65-1 were incubated with MDP25 in the presence of 0 or 0.5 μM calcium and observed by confocal microscopy. Representative images reveal microtubule bundles alone (C) or in the presence of calcium (D), MDP25 (E), MDP25 plus calcium (F), or GST plus calcium (G). Scale bar in (G) = 20 μm.
Protein function is frequently modulated by covalent modifications, such as phosphorylation. For example, the microtubule-associated protein NtMAP65-1 is phosphorylated, and its microtubule-bundling activity is regulated by the tobacco mitogen-activated protein kinase (MAPK) cascade, specifically by NRK1/NTF6.9 The activity of many actin-binding proteins (ABPs) has been reported to be calcium-dependent, and these proteins often play a regulatory role in actin dynamics. For example, F-actin bundles of uniform polarity induced by plant villin are formed in a calcium-dependent fashion in vitro.10-12 This report is the first to demonstrate that the function of a microtubule regulatory protein can also be regulated by calcium. The diversity of microtubule array structures underlies the distinct patterns of cell morphogenesis in plant cells, and regulation of these arrays, therefore, must require the diverse functions of many microtubule regulatory proteins. Based on our in vitro experimental analysis and our previous model,2 we propose that MDP25 is involved in disrupting cortical microtubule bundles by facilitating microtubule reorganization in a calcium-dependent manner, resulting in inhibition of hypocotyl cell elongation.
Conclusions and Perspectives
MDP25 plays a crucial role in the negative regulation of hypocotyl cell elongation by destabilizing microtubules. This study demonstrates that, in addition to regulating MDP25 cellular localization, calcium also enhances the microtubule-depolymerizing activity of MDP25. The precise mechanism by which calcium regulates the microtubule-depolymerizing activity of MDP25 is of interest and will be dissected in future studies. In addition, investigation of the interactions of calcium with microtubule regulatory proteins will give a new insight into understanding how the functions of microtubule regulatory proteins are regulated in plant cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported by grants from the National Basic Research Program of China (2012CB114200 to M.Y.), the Natural Science Foundation of China (31070258 to T.M.; 30830058 and 30721062 to M.Y.), and the Chinese Universities Scientific Fund (2011JS108 to T.M.).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20336
References
- 1.Buschmann H, Lloyd CW. Arabidopsis mutants and the network of microtubule-associated functions. Mol Plant. 2008;1:888–98. doi: 10.1093/mp/ssn060. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Wang X, Qin T, Zhang Y. Liu Xi, Sun J, Zhou Y, Zhu L, Zhang Z, Yuan M, Mao T. MDP25, a novel calcium regulatory protein, mediates hypocotyl cell eElongation by destabilizing cortical microtubules in Arabidopsis. Plant Cell. 2011;23:4411–27. doi: 10.1105/tpc.111.092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisenberg RC. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972;177:1104–5. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- 4.White E, Tolbert EM, Katz ER. Identification of tubulin in Dictyostelium discoideum: characterization of some unique properties. J Cell Biol. 1983;97:1011–9. doi: 10.1083/jcb.97.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakimoto T, Shibaoka H. Calcium-sensitivity of cortical microtubules in the green alga Mougeotia. Plant Cell Physiol. 1986;27:91–101. [Google Scholar]
- 6.Ide Y, Nagasaki N, Tomioka R, Suito M, Kamiya T, Maeshima M. Molecular properties of a novel, hydrophilic cation-binding protein associated with the plasma membrane. J Exp Bot. 2007;58:1173–83. doi: 10.1093/jxb/erl284. [DOI] [PubMed] [Google Scholar]
- 7.Smertenko AP, Chang HY, Wagner V, Kaloriti D, Fenyk S, Sonobe S, et al. The Arabidopsis microtubule-associated protein AtMAP65-1: molecular analysis of its microtubule bundling activity. Plant Cell. 2004;16:2035–47. doi: 10.1105/tpc.104.023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao T, Jin L, Li H, Liu B, Yuan M. Two microtubule-associated proteins of the Arabidopsis MAP65 family function differently on microtubules. Plant Physiol. 2005;138:654–62. doi: 10.1104/pp.104.052456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasabe M, Soyano T, Takahashi Y, Sonobe S, Igarashi H, Itoh TJ, et al. Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev. 2006;20:1004–14. doi: 10.1101/gad.1408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokota E, Shimmen T. The 135-kDa actin-bundling protein from lily pollen tubes arranges F-actin into bundles with uniform polarity. Planta. 1999;209:264–6. doi: 10.1007/s004250050631. [DOI] [PubMed] [Google Scholar]
- 11.Yokota E, Shimmen KiTT. Actin-bundling protein isolated from pollen tubes of lily. Biochemical and immunocytochemical characterization. Plant Physiol. 1998;116:1421–9. doi: 10.1104/pp.116.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokota E, Tominaga M, Mabuchi I, Tsuji Y, Staiger CJ, Oiwa K, et al. Plant villin, lily P-135-ABP, possesses G-actin binding activity and accelerates the polymerization and depolymerization of actin in a Ca2+-sensitive manner. Plant Cell Physiol. 2005;46:1690–703. doi: 10.1093/pcp/pci185. [DOI] [PubMed] [Google Scholar]