Abstract
On the search for sparingly available nutrients, plants may alter their root architecture to improve soil exploration. So far, the examples for root system modifications induced by a heterogeneous availability of nutrients have been reported for the macronutrients nitrogen (N) and phosphorous (P). In an attempt to extend this type of knowledge to other nutrients, we recently provided evidence that Arabidopsis roots are able to sense a local availability of the micronutrient iron (Fe) and to respond with lateral root elongation into the Fe-containing patch. This specific root response was caused by enhanced elongation of cells leaving the root meristem and was dependent on an AUX1-mediated auxin accumulation in the lateral root apices. In this report, we compare mechanisms underlying this response with those known for other nutrients and show that a substantial genotypic variation exists among accessions of A. thaliana in the responsiveness of lateral roots toward localized Fe supplies.
Keywords: AUX1, auxin, iron sensing, lateral root development, natural variation, root architecture
Fe Availability Affects Root System Architecture
To survive in soil environments with patchy or inhomogeneous nutrient distributions, plant roots have evolved strategies allowing them to perceive and respond to a spatially restricted availability of minerals. When grown under a localized supply of ammonium, nitrate or phosphate, roots undergo profound changes, which result from the stimulation of either lateral root initiation or elongation.1-5 We have recently reported that a localized availability of the micronutrient Fe promotes elongation of those lateral roots exposed to the Fe patch.6 Under natural and agricultural growth conditions, such a root system response offers the plant an adaptive advantage to exploit more distantly localized Fe sources, since the diffusion rate of this micronutrient in the soil is generally low.
The effect of localized Fe was mainly restricted to lateral root elongation, since lateral root density was not significantly changed by the mode of Fe delivery to roots.6 Like Fe, a localized supply of nitrate also enhanced lateral elongation,1-4 whereas localized ammonium stimulated lateral root initiation without causing additional changes in lateral root elongation.5 Although Fe and nitrate led to similar changes in lateral root morphology, they apparently target different processes, since localized nitrate primarily stimulated cell division in the apical meristems of lateral roots,1,7 whereas Fe increased the elongation of cells leaving the meristem.6 Thus, distinct nutrients affect root architecture by influencing specific steps in the developmental program of the root.
Localized Fe Alters Auxin Distribution in Lateral Roots
Lateral root development is under tight hormonal control.8 In our study, we found that the increased lateral root length under localized Fe was accompanied by an enhanced accumulation of auxin in lateral root apices.6 Such a response has not yet been reported for other nutrients when supplied locally to plants. Importantly, auxin accumulation was stimulated by the internal Fe pool in the root rather than by externally available Fe.6 In addition, localized Fe supply enhanced the expression of the auxin influx carrier AUX1 specifically in lateral roots exposed to Fe. In the case of nitrate, it was first reported that AXR4, but not AUX1, is necessary for the lateral root response to localized nitrate,1 whereas a further study found that this response was independent of AXR4 and AUX1.3 Thus, although similar root architectural changes are observed when plants are grown under a localized supply of Fe or nitrate, nutrient-specific mechanisms underlie the stimulation of lateral root elongation. Our findings suggest that AUX1 is a Fe-sensitive checkpoint diverting the rootward auxin flow mainly to lateral roots growing into the Fe-containing patch thereby stimulating their elongation.6
Lateral Root Length Under Localized Fe Supply is Subject to Natural Genetic Variation
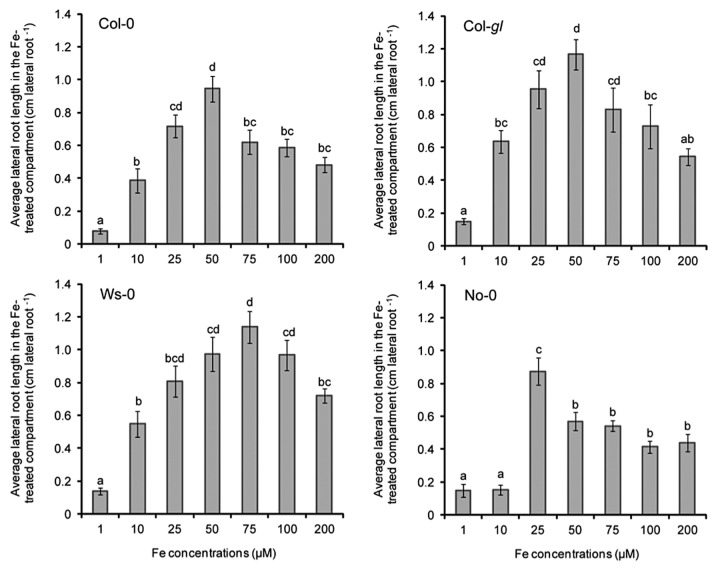
To gain an initial insight into the natural genetic variation of lateral root responsiveness toward a spatially restricted Fe availability, lateral root length was assessed in four accession lines of A. thaliana grown under localized Fe. Although the number of accessions tested was small, significant differences in the response pattern were observed (Fig. 1). Whereas Col-0 and Col-gl plants exhibited greatest average lateral root length under 25 to 50 µM Fe, the accession Ws-0 required Fe concentrations above 50 µM to fully stimulate lateral root length. By contrast, No-0 plants showed the highest lateral root length when 25 µM Fe were supplied (Fig. 1). Lateral roots of No-0 plants were also more sensitive to high Fe, being repressed when ≥ 50 µM Fe were supplied, whereas > 100 µM Fe was required to inhibit lateral root length in Ws-0 plants. Thus, the Fe concentrations necessary to trigger or repress lateral root elongation are dependent on the ecotype, suggesting that genotype-specific factors are involved in the regulation of this response. Significant natural variation has also been reported for primary root sensitivity toward the organic N form L glutamate9 and for root architectural changes under low P availability.10,11 For the latter, the naturally occurring genetic variation was used to map a QTL involved in the regulation of primary root growth under low P,11 allowing the subsequent identification of LPR1 as a major component of the P sensing mechanism in primary root tips of Arabidopsis.12
Figure 1. Average lateral root length in four accessions of A. thaliana grown under localized supply of increasing Fe concentrations. Seeds were germinated on half-strength MS medium without Fe. After 7 d, seedlings were transferred to segmented agar plates, supplied with the indicated Fe concentrations only in the middle segment. After 15 d, plants were scanned for root analysis. Bars indicate means ± SE (n = 7 plates containing three plants). Different letters indicate significant differences among means (p < 0.05 by Tukey’s test). For more details about materials and methods, see Giehl et al.6
The natural variation in the responsiveness of roots to localized Fe might reflect genotype-specific differences in Fe sensing or signaling mechanisms but may also be caused by nutritional factors such as Fe uptake rates or internal Fe allocation. The activity of the ferric-chelate reductase, an important component of the Fe acquisition machinery in roots, also varied in different accession lines of A. thaliana.13,14 Thus, genetic variation in Fe uptake efficiency could determine to which extent the plant invests further resources in elongating lateral roots. Alternatively, because increased lateral root elongation under localized Fe is sustained by enhanced auxin accumulation,6 differences in auxin biosynthesis, sensitivity and/or transport could also affect lateral root elongation. Such differences in auxin sensitivity among accessions of A. thaliana are for instance caused by genotype-specific equilibria of the auxin signaling proteins ARF and Aux/IAA.15 To which extent such differences impact on the responsiveness of plants to localized Fe remains to be tested.
Concluding Remarks and Future Perspectives
Our initial study indicates that Arabidopsis roots perceive a local Fe signal and that AUX1 is a sensitive checkpoint that integrates the Fe nutritional signal into the root developmental program. Furthermore, our data suggest that the Fe sensing event occurs upstream of AUX1 and after Fe is taken up by root cells. The future challenge is to identify additional components involved in sensing Fe and in transmitting the Fe signal to induce lateral root elongation. Further studies may then benefit from the natural genetic variation in the Fe response which paves the way to identify novel regulatory components determining the responsiveness of Arabidopsis roots to internal and external Fe signals.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by research grants from the Deutsche Forschungsgemeinschaft to N.vonW. (WI1728/13-1) and by a Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/Brasília-Brazil PhD Fellowship to R.F.H.G.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20337
References
- 1.Zhang HM, Jennings A, Barlow PW, Forde BG. Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci U S A. 1999;96:6529–34. doi: 10.1073/pnas.96.11.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang HM, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–9. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- 3.Linkohr BI, Williamson LC, Fitter AH, Leyser HMO. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. 2002;29:751–60. doi: 10.1046/j.1365-313X.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- 4.Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E, et al. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc Natl Acad Sci U S A. 2006;103:19206–11. doi: 10.1073/pnas.0605275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lima JE, Kojima S, Takahashi H, von Wirén N. Ammonium triggers lateral root branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-dependent manner. Plant Cell. 2010;22:3621–33. doi: 10.1105/tpc.110.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giehl RFH, Lima JE, von Wirén N. Localized iron supply triggers lateral root elongation in Arabidopsis by altering the AUX1-mediated auxin distribution. Plant Cell. 2012;24:33–49. doi: 10.1105/tpc.111.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang HM, Forde BG. Regulation of Arabidopsis root development by nitrate availability. J Exp Bot. 2000;51:51–9. doi: 10.1093/jexbot/51.342.51. [DOI] [PubMed] [Google Scholar]
- 8.Fukaki H, Tasaka M. Hormone interactions during lateral root formation. Plant Mol Biol. 2009;69:437–49. doi: 10.1007/s11103-008-9417-2. [DOI] [PubMed] [Google Scholar]
- 9.Walch-Liu P, Liu LH, Remans T, Tester M, Forde BG. Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant Cell Physiol. 2006;47:1045–57. doi: 10.1093/pcp/pcj075. [DOI] [PubMed] [Google Scholar]
- 10.Chevalier F, Pata M, Nacry P, Doumas P, Rossignol M. Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions. Plant Cell Environ. 2003;26:1839–50. doi: 10.1046/j.1365-3040.2003.01100.x. [DOI] [Google Scholar]
- 11.Reymond M, Svistoonoff S, Loudet O, Nussaume L, Desnos T. Identification of QTL controlling root growth response to phosphate starvation in Arabidopsis thaliana. Plant Cell Environ. 2006;29:115–25. doi: 10.1111/j.1365-3040.2005.01405.x. [DOI] [PubMed] [Google Scholar]
- 12.Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, et al. Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet. 2007;39:792–6. doi: 10.1038/ng2041. [DOI] [PubMed] [Google Scholar]
- 13.Saleeba JA, Guerinot ML. Induction of ferric reductase activity in response to iron deficiency in Arabidopsis. Biometals. 1995;8:297–300. doi: 10.1007/BF00141602. [DOI] [Google Scholar]
- 14.Stein RJ, Waters BM. Use of natural variation reveals core genes in the transcriptome of iron-deficient Arabidopsis thaliana roots. J Exp Bot. 2012;63:1039–55. doi: 10.1093/jxb/err343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delker C, Pöschl Y, Raschke A, Ullrich K, Ettingshausen S, Hauptmann V, et al. Natural variation of transcriptional auxin response networks in Arabidopsis thaliana. Plant Cell. 2010;22:2184–200. doi: 10.1105/tpc.110.073957. [DOI] [PMC free article] [PubMed] [Google Scholar]