Abstract
Interplant communication of stress via volatile signals is a well-known phenomenon. It has been shown that plants undergoing stress caused by pathogenic bacteria or insects generate volatile signals that elicit defense response in neighboring naïve plants.1 Similarly, we have recently shown that naïve plants sharing the same gaseous environment with UVC-exposed plants exhibit similar changes in genome instability as UVC-exposed plants.2 We found that methyl salicylate (MeSA) and methyl jasmonate (MeJA) serve as volatile signals communicating genome instability (as measured by an increase in the homologous recombination frequency). UVC-exposed plants produce high levels of MeSA and MeJA, a response that is missing in an npr1 mutant. Concomitantly, npr1 mutants are impaired in communicating the signal leading to genome instability, presumably because this mutant does not develop new necrotic lesion after UVC irradiation as observed in wt plants.2 To analyze the potential biological significance of such plant-plant communication, we have now determined whether bystander plants that receive volatile signals from UVC-irradiated plants, become more resistant to UVC irradiation or infection with oilseed rape mosaic virus (ORMV). Specifically, we analyzed the number of UVC-elicited necrotic lesions, the level of anthocyanin pigments, and the mRNA levels corresponding to ORMV coat protein and the NPR1-regulated pathogenesis-related protein PR1 in the irradiated or virus-infected bystander plants that have been previously exposed to volatiles produced by UVC-irradiated plants. These experiments showed that the bystander plants responded similarly to control plants following UVC irradiation. Interestingly, however, the bystander plants appeared to be more susceptible to ORMV infection, even though PR1 mRNA levels in systemic tissue were significantly higher than in the control plants, which indicates that bystander plants could be primed to strongly respond to bacterial infection.
Keywords: UV-C, abiotic stress, biotic stress, oilseed rape mosaic virus, plant-environment interaction, plant-plant communication, stress tolerance, volatile signaling
Plants are constantly exposed to adverse environmental conditions. Besides conventional responses to stress in the form of signaling between locally exposed and distal tissues (intra-plant communication), plants also employ mechanisms of interplant communication, leading to a higher awareness of the changes in the environment in which they grow. This type of volatile signaling is one of the strategies that plant communities utilize to defend against herbivory.1,3 Less is known about the ability of plants to communicate information about abiotic stress. In a recent study, we showed that exposing plants to UVC results in destabilization of their genome as well as the genome of plants that share the same gaseous environment. We found that UVC-exposed plants release at least two volatile compounds, MeSA and MeJA, which are capable of increasing the homologous recombination frequency (HRF) in non-irradiated bystander (BS) plants.2 We demonstrated that npr1 mutant plants, which are impaired in both salicylic acid (SA) and jasmonic acid (JA) signaling, are also impaired in releasing and perceiving the signal leading to genome destabilization. Our work showed that the volatile signal is not species specific, as Arabidopsis plants were able to communicate the signal to tobacco plants and vice versa. We also discovered that plants infected with viral pathogens release the signal leading to increase genome instability in neighboring non-infected tobacco plants. Surprisingly, however, plants exposed to zebularine or NaCl, two stressors that do not trigger necrosis at the concentrations tested, did not release the signal to bystander plants despite the fact that these compounds cause genome instability in plants directly exposed. This led to the hypothesis that only those types of stress that result in necrotic lesions are able to initiate interplant communication of genome instability. Interestingly, UVC irradiation of npr1 mutants, which fail to communicate genome instability after UVC irradiation, did not trigger an increase in the number of necrotic lesions and concomitantly did not elicit the synthesis of MeSA and MeJA.
Although it is tempting to hypothesize that a coordinated genome instability response may increase the odds of producing new alleles in populations of out-crossing species, the biological significance of communicating genome instability, especially in self-fertilizing species like Arabidopsis, is poorly understood. The perception of volatiles has been shown to play an essential role in increasing resistance to pathogens in the case of a second encounter.1 Therefore, it seems possible that interplant communication of stress caused by UVC exposure makes naïve neighboring plants more tolerant to abiotic or biotic stress. In a previous work we showed a correlation between genome instability and stress tolerance in the progeny of stressed plants.4 Although we could not establish any causative relationship between these two events, we decided to test whether increased genome instability in the somatic tissue of bystander plants also correlates with increased stress tolerance. Here we attempted to test this hypothesis by analyzing the formation of necrotic lesions and by measuring the level of anthocyanin in UVC-exposed, bystander and control plants. We found that after UVC irradiation, bystander plants are more similar to control plants than to previously UVC-irradiated plants. Analysis of response to pathogen infection, however, showed that infected bystander plants exhibit significantly higher levels of PR1 gene expression in systemic non-infected tissue as compared with the same tissue in infected UVC-exposed or control plants.
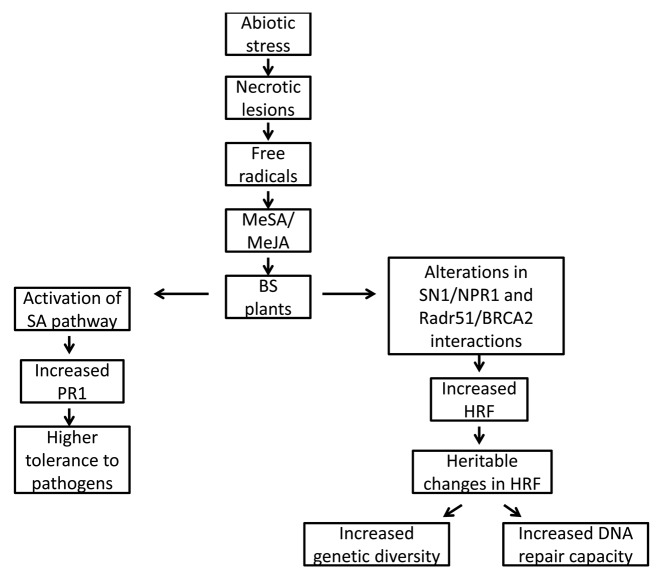
Arabidopsis synthesize high levels of anthocyanins in response to a variety of environmental stresses.5 Unexpectedly, however, we found that anthocyanin levels were lower in UVC-irradiated plants compared with controls, regardless of whether the plants were irradiated with a second dose of UVC or just one as in the case of bystander plants (Fig. 1A). This may indicate that after UV-C irradiation, the precursor of anthocyanins, p-coumarate, is diverted into the production of sinapoyl-malate, the major sunscreen molecule in Arabidopsis. Irradiated bystander plants had slightly lower levels of anthocyanins than either the singly or doubly irradiated plants, although the difference was not significant (Fig. 1A). Similarly, the number of necrotic lesions in UVC-irradiated bystander plants was similar to the number observed in singly irradiated control plants and much less than the doubly irradiated plants (p < 0.05 for both) (Fig. 1B), which suggests that perception of volatiles from UVC-irradiated plants does not protect naïve bystander plants against a direct exposure to UVC light. In the case of ORMV-infected plants, the level of anthocyanin in non-irradiated controls increased upon exposure to ORMV and the response of bystander plants was similar to the response of the non-irradiated control plants (Fig. 2A). In contrast, plants that were both irradiated and infected with ORMV had lower levels of anthocyanins (Fig. 2A) indicating that UVC-mediated depletion of anthocyanin prevent the anthocyanin accumulation triggered by ORMV infection. The number of necrotic lesions after UVC irradiation was similar in the previously irradiated plants, the controls and bystander plants (Fig. 2B), again indicating that perception of volatiles produced by UVC-irradiated plants does not provide naïve bystander plants with protection against UVC irradiation.
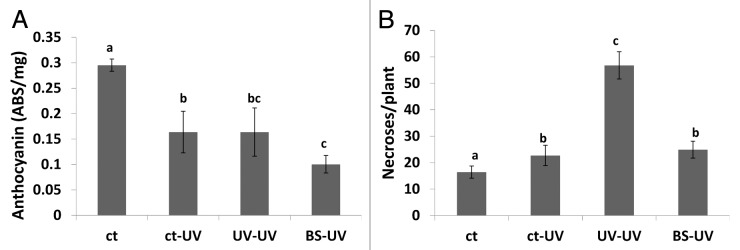
Figure 1. Levels of anthocyanin and number of necrotic lesions in UV irradiated plants. Analysis of anthocyanin levels (A) and the number of necrotic lesions (B) was done as described in methods section. Ct, control non-exposed plants; ct-UV, UV-irradiate control plants; UV-UV, UV-irradiated plants previously exposed to UV; BS-UV, UV-irradiated bystander plants. Different letters indicated significantly different data points (p < 0.05).
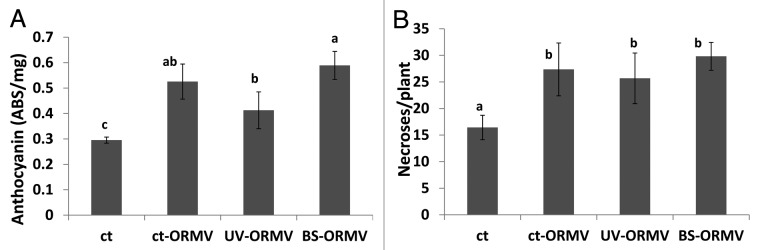
Figure 2. Levels of anthocyanin and number of necrotic lesions in ORMV infection plants. Analysis of anthocyanin levels (A) and the number of necrotic lesions (B) was done as described in methods section. Ct, control non-exposed plants; ct-ORMV, ORMV-infected control plants; UV-ORMV, ORMV-infected plants previously exposed to UV; BS-ORMV, ORMV-infected bystander plants. Different letters indicated significantly different data points (p < 0.05).
Analysis of the steady-state level of ORMV coat protein mRNA, which is a good proxy for the level of infection with the virus, showed that UVC-irradiated plants were dramatically more susceptible than control or bystander plants to ORMV infection. Interestingly, bystander plants were significantly more susceptible than the non-irradiated control plants but much less susceptible than UVC-irradiated plants (Fig. 3A). Analysis of the steady-state mRNA levels corresponding to PR1, a defense-related gene whose expression is completely dependent on SA signaling through NPR1, showed that ORMV-infected bystander plants had higher levels of PR1 expression than ORMV-infected control plants in the ORMV infected tissue (Fig. 3B). In addition, distal non-infected tissue of bystander plants had very high levels of PR1 RNA (p < 0.01) (Fig. 3B), suggesting that bystander plants may be primed to respond to pathogen attack with an aggressive defense response, although in this case, this response did not lead to enhanced resistance against ORMV. In previous work, however, we showed that the progeny of infected plants had higher expression levels of PR1 that was associated with higher tolerance to viral, bacterial and fungal pathogens, as well as a higher HRF.6 Therefore, the advantage for naïve plants in perceiving volatiles from UVC-irradiated plants may only be seen in their progenies. In summary, these data show that bystander plants are similar to non-irradiated control plants in their response to abiotic stress. On the other hand, the enhanced expression of PR1 in bystander plants in both infected and non-infected tissue suggests that bystander plants may be primed for enhanced resistance to pathogen infection.
Figure 3. Quantification of ORMV infection and PR1 gene expression. Steady-state mRNA level of ORMV coat protein (A) and PR1 mRNA (B) was done as described in methods section. Ct, control non-exposed plants; ct-ORMV, ORMV-infected tissue of control plants; ct-distal, non-infected tissue of ORMV-infected control plants; UV-ORMV, ORMV-infected tissues of plants previously exposed to UV; UV-distal, non-infected tissue of ORMV-infected plants previously exposed to UV; BS-ORMV, ORMV-infected tissue of bystander plants; BS-distal, non-infected tissue of ORMV-infected bystander plants. Different letters indicated significantly different data points (p < 0.05).
Combined with our previous data showing that UVC irradiation of plants results in the production of MeSA and MeJA and other uncharacterized volatiles that elicit an increase in HRF in bystander plants,3 the data presented here suggest that although UVC triggers changes in HRF in bystander plants, this response is not necessarily associated with the capacity to resist higher levels of abiotic stress. Indeed, it is possible that increased HRF in bystander plants is part of a secondary response to volatiles, which is not directly linked to stress adaptation. In a previous work we also showed that local UVC irradiation of plants triggers an increase in HRF in non-irradiated distal tissue.7 Moreover, pretreatment of plants with reactive oxygen species (ROS) scavengers substantially blocks the increase in HRF.7 Since ROS production is associated with the formation of necrotic lesions, it is possible that the increase in HRF in distal tissue is due to ROS signaling or ROS-induced signaling through MeJA, MeSA or other volatile molecules. The link between salicylic acid signaling and homologous recombination is well established,8 although it is not clear whether it has any direct biological significance. It is possible that the upregulation of PR1 expression in bystander plants is a consequence of shared signaling pathways in response to abiotic and biotic stresses. As volatile signaling in response to UVC irradiation largely involves MeSA and MeJA, two well-known defense-related signaling molecules, it is not surprising that bystander plants exhibited responses typically observed in plants undergoing an enhanced pathogen resistance response such as high level of PR1 gene expression in distal tissue in response to ORMV infection.
Previous work in several labs showed that the progeny of plants exposed to stress exhibit higher levels of HRF and enhanced resistance to pathogens.9,10 It remains to be seen, however, whether the progeny of naïve bystander plants display similar adaptations to stress. Until we test that, we can only hypothesize that the plant-to-plant communication of genome instability may have evolved as a mechanism to increase genetic diversity that can be passed on to the progeny in response to a stressful environment.
Plants communicate stress from locally exposed tissue to systemic tissue by emitting volatiles. As different parts of a given plant are in closer proximity by air than they are through the vasculature, it is possible that this mechanism evolved to speed up the response to stress in systemic tissue. It is not clear however, whether the mechanism that allows naïve plants to perceive volatiles produced by UV-irradiated plants is the same as the one involved in intra-plant communication or rather a different mechanism that evolved independently. It seems hard to imagine how the plant-plant communication of stress may have evolved in self-pollinating species like Arabidopsis because there is no direct benefit for the emitter of volatiles. In outcrossing species however, plant-plant communication appears to make more sense because it favors the odds of a potential mate to contribute with adaptive new alleles to the progeny of the plant directly exposed to stress. As self-crossing species often evolved from outcrossing ancestors,11 it seems possible that the plant-plant communication of stress may have evolved in outcrossing species and it may have been retained in self-crossing species even though it does not seem to serve any evolutionary advantage for the plant that emits the volatiles. If the volatiles involved in intra-plant communication were the same as the ones involved in plant-plant stress signaling (MeSA and MeJA among others) it seems fair to hypothesize that the plant-plant communication either evolved for the intra-plant communication or it is actually another manifestation of the same signaling mechanism.
We hypothesize that the following chain of events takes place in plant-to-plant communication of genome instability: (1) exposure to stress triggers formation of necrotic lesions that produce MeJA/MeSA (and some other yet unidentified volatiles) and ROS may be involved as intermediate molecules; (2) volatiles released from UVC-irradiated plants are perceived by bystander plants, triggering the activation of SA-dependent pathways, including elevated NPR1-mediated upregulation of PR1 expression, which primes the plant to better tolerate a future encounter with pathogens (Fig. 4) which also correlates with an increase in HRF, probably through the activation of the SNI1/NPR1 and Rad51/BRCA2 complex8; (3) elevated HRF is passed on to the progeny and is probably elevated in gametic cells, leading to an increase in genetic diversity and possibly to better DNA repair capacity. Although seemingly possible, all these predicted events remain to be experimentally tested.
Figure 4. Model explaining changes in bystander plants.
Methods
Plants growth
Wild type Arabidopsis thaliana plants were exposed to 0.7 J/m2 UVC and immediately placed for four days in a plastic bag with non-irradiated bystander (BS) plants.3 Four days later, UVC-irradiated and bystander plants were either irradiated with a second and higher dose of UVC (2.1 J/m2) or infected with 100 ng (50 ng/µl applied to two plant leaves) oilseed rape mosaic virus (ORMV). Infection was performed by rub inoculation with Carborundum powder as abrasive.12
Analysis of anthocyanin level
Anthocyanin extraction was done as described before, with some modification.13 Briefly, the leaf samples were measured for fresh weight then ground into fine powder in liquid nitrogen, and extracted with 80% methanol containing 5% HCl overnight at 4°C. After centrifugation at 14,000 g for 20 min, the supernatants were transferred to new 1.5 ml eppendorf tubes followed by a chloroform purification step and a centrifugation at 14,000 g for 10 min. The clear supernatant was used to quantify the amount of anthocyanins photometrically by FLUROstar Omega (BMG Labtech, Germany).14
Analysis of the level of mRNA of ORMV coat protein and PR1 protein
The RNA was extracted using Trizol by following the provider’s protocol (Invitrogen). The trace gDNA contamination was removed by using DNase I digestion in illustra RNAspin mini column (GE Healthcare). The cDNA was synthesized using the RevertAid M-MuLV first strand cDNA synthesis kit protocol (Fermentas), with random primer for the ORMV coat protein RNA and oligo-dT primer for the PR1 and tubulin genes. The quantitative real time PCR (qRT-PCR) reactions were setup in SsoFast EvaGreen Supermix (Bio-Rad) and the results were processed by Bio-Rad CFX manager software (v2.0), with tubulin as a reference. The gene specific primers are listed in the Table 1. Specificity of Oligos was checked by melting-curve analysis performed by the Bio-Rad CFX96 PCR machine after 45 amplification cycles and by gel-electrophoretic analysis.
Table 1. Sequence of primers used for gene expression analysis.
| Primer | Sequence |
|---|---|
| ORMV_CPF0 |
5′-TCACCCATGGTTTACAACATCACGAGCTCG-3′ |
| ORMV_CPR0 |
5′-CACTTCTAGACTATGTAGCTGGCGCAGTAGCC-3′ |
| ORMV_CPF1 |
5′-CTCGAATCAGTACCAGTATT-3′ |
| ORMV_CPR1 |
5′-CTTCAGTTTCAATGATCCTA-3′ |
| PR1_f |
5′-TTCTTCCCTCGAAAGCTCAA-3′ |
| PR1_r |
5′-CGTTCACATAATTCCCACGA-3′ |
| Tubulin_f_5g62690 |
5′-ACAGAAGCGGAGAGCAACAT-3′ |
| Tubulin_r_5g62690 | 5′-TCCTCATCCTCGTAGTCACCTT-3′ |
Statistical analysis
All analyses were done in three biological replicates. Significance of the differences was calculated by using univariate or multivariate general linear model analysis procedure from SPSS 19.0 (IBM). Multiple comparisons of means were performed only when the model was significant.
Acknowledgments
Financial support was provided by NSERC Discovery, Alberta Agriculture Research Institute and Human Frontiers Scientific Program grants to I.K. and by NIH grant R37 GM48707 and NSF grant MCB-0519898 awarded to F.M.A.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20406
References
- 1.Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science. 2006;311:812–5. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 2.Heil M, Silva Bueno JC. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci U S A. 2007;104:5467–72. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Y, Danna CH, Zemp FJ, Titov V, Ciftci ON, Przybylski R, et al. UV-C-irradiated Arabidopsis and tobacco emit volatiles that trigger genomic instability in neighboring plants. Plant Cell. 2011;23:3842–52. doi: 10.1105/tpc.111.089003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyko A, Blevins T, Yao Y, Golubov A, Bilichak A, Ilnytskyy Y, et al. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS One. 2010;5:e9514. doi: 10.1371/journal.pone.0009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koops P, Pelser S, Ignatz M, Klose C, Marrocco-Selden K, Kretsch T. EDL3 is an F-box protein involved in the regulation of abscisic acid signalling in Arabidopsis thaliana. J Exp Bot. 2011;62:5547–60. doi: 10.1093/jxb/err236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kathiria P, Sidler C, Golubov A, Kalischuk M, Kawchuk LM, Kovalchuk I. Tobacco mosaic virus infection results in an increase in recombination frequency and resistance to viral, bacterial, and fungal pathogens in the progeny of infected tobacco plants. Plant Physiol. 2010;153:1859–70. doi: 10.1104/pp.110.157263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filkowski J, Yeoman A, Kovalchuk O, Kovalchuk I. Systemic plant signal triggers genome instability. Plant J. 2004;38:1–11. doi: 10.1111/j.1365-313X.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Durrant WE, Song J, Spivey NW, Dong X. Arabidopsis BRCA2 and RAD51 proteins are specifically involved in defense gene transcription during plant immune responses. Proc Natl Acad Sci U S A. 2010;107:22716–21. doi: 10.1073/pnas.1005978107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Y, Kovalchuk I. Abiotic stress leads to somatic and heritable changes in homologous recombination frequency, point mutation frequency and microsatellite stability in Arabidopsis plants. Mutat Res. 2011;707:61–6. doi: 10.1016/j.mrfmmm.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Boyko A, Kovalchuk I. Transgenerational response to stress in Arabidopsis thaliana. Plant Signal Behav. 2010;5:995–8. doi: 10.4161/psb.5.8.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlesworth D. Evolution of plant breeding systems. Curr Biol. 2006;16:R726–35. doi: 10.1016/j.cub.2006.07.068. [DOI] [PubMed] [Google Scholar]
- 12.Yao Y, Bilichak A, Golubov A, Kovalchuk I. Local infection with oilseed rape mosaic virus promotes genetic rearrangements in systemic Arabidopsis tissue. Mutat Res. 2011;709-710:7–14. doi: 10.1016/j.mrfmmm.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Gou JY, Felippes FF, Liu CJ, Weigel D, Wang JW. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell. 2011;23:1512–22. doi: 10.1105/tpc.111.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neff MM, Chory J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998;118:27–35. doi: 10.1104/pp.118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]