Abstract
Water scarcity is a critical limitation for agricultural systems. Two different water management strategies have evolved in plants: an isohydric strategy and an anisohydric strategy. Isohydric plants maintain a constant midday leaf water potential (Ψleaf) when water is abundant, as well as under drought conditions, by reducing stomatal conductance as necessary to limit transpiration. Anisohydric plants have more variable Ψleaf and keep their stomata open and photosynthetic rates high for longer periods, even in the presence of decreasing leaf water potential. This risk-taking behavior of anisohydric plants might be beneficial when water is abundant, as well as under moderately stressful conditions. However, under conditions of intense drought, this behavior might endanger the plant. We will discuss the advantages and disadvantages of these two water-usage strategies and their effects on the plant’s ability to tolerate abiotic and biotic stress. The involvement of plant tonoplast AQPs in this process will also be discussed.
Keywords: abiotic stress, anisohydric, aquaporins, biotic stress, isohydric, leaf water potential, relative water content
Isohydric vs. Anisohydric Plant Behavior
Different regions of the world are characterized by different climatic and environmental conditions, which have led to the development of a wide range of plant adaptation mechanisms and survival strategies. Both anisohydric and isohydric behaviors have been observed in numerous plant groups1 as well as within individual species, such as grapevine (Vitis vinifera2) and poplar (Populus3), suggesting that the availability of water in the natural environment and dynamic plant-environment relations influence these differences in behavior.4-7 A constant midday leaf water potential (Ψleaf), as a characteristic of isohydric plants, is the result of strict and conservative water-balance management, in which the loss of water is limited by the reduction of stomatal conductance. However, our current understanding of the molecular and cellular factors responsible for these two types of plant behaviors is limited. Evidently, differences in the behavior of isohydric and anisohydric plants are due to differences in the sensitivity of their respective guard cells to a critical Ψleaf threshold. As a result, under optimal conditions and mild-to-moderate drought conditions, anisohydric plants maintain higher stomatal conductance (gs) and CO2 assimilation (AN) than isohydric plants and, therefore, are more productive under those conditions.3,6,8-10
Recently, we demonstrated that the constitutive expression of a tonoplast aquaporin (TIP AQP), SlTIP2;2, in an isohydric tomato line led to an increase of the osmotic water permeability of the tonoplast and extended the capacity of the vacuole for osmotic buffering of the cytoplasm under stress conditions.8 This transformation “converted” the isohydric tomato plants, so that they exhibited anisohydric behavior, which led to greater productivity under optimal and mild-to-moderate-drought conditions. A similar effect has been observed for another TIP in Arabidopsis plants.11,12 These observations raise the question of whether anisohydric behavior should be viewed as a valuable agronomic trait.
Effect of Anisohydric Behavior on Abiotic Stress Resistance: A Valuable Agronomic Trait?
From the agronomic point of view, drought resistance is defined as enhanced productivity under the examined conditions. Thus, any innate factor that leads to an increase in crop productivity under stressful conditions may be viewed as a valuable agronomic trait. Due to their higher gs and AN, anisohydric crops will most likely improve their yield under conditions of optimal to moderate water availability.8,10 Nonetheless, there is a need to determine the soil moisture threshold below which anisohydric plants lose their agronomic advantage. To answer this question, one must take in consideration the plant’s ability to recover from the stressful period (i.e., its embolism and desiccation resistance) and return to its pre-stress productivity rate. Several studies have reported that anisohydric plants are resistant to cavitation,13-18 suggesting that these plants may recover rapidly following exposure to drought. These findings support the hypothesis that anisohydric behavior contributes to agronomic drought resistance. Observations of our converted TIP2;2 tomato plants also support this hypothesis.
TIP2;2 plants maintained significantly higher performance (i.e., harvest index) even when received 50% deficit irrigation, as long as they were watered frequently. Interestingly, these plants out-performed those treated with the full irrigation regime, as long as they were not irrigated very frequently (i.e., irrigation once a week, which exposes the plants to longer periods of drought) eliminate their advantage and emphasizing the vacuole “reservoir” role in the short-term.8
A similar trend was reported in a comparison of the drought resistance of isohydric and anisohydric grass species (Miscanthus sinensis and Eragrostis spectabilis respectively), in which better performance of Eragrostis spectabilis was observed under favorable to moderate moisture conditions, but little difference was noted when the plants were subjected to severe drought stress.10 This demonstrates that the anisohydric plant is an “opportunist risk-taker” whose behavior is beneficial under conditions of minimal to moderate stress, but will confer no benefit under conditions of prolonged stress.
This perception can explain the disadvantage of some anisohydric grapevines (Vitis vinifera), such as cv Chardonnay and cv Shiraz, under drought conditions.18,19 In general, anisohydric grapevines were reported to have lower midday water potentials under stressful conditions, but did not have any advantage over isohydric grapevines in terms of gs or water use efficiency.20 This suggests that isohydric grapevines may have an advantage in dry ecosystems. Typically, grapevines are grown without any irrigation, which leaves them exposed to a wide variety of soil moisture levels. This, might explain the contradictory reports of isohydric and anisohydric behavior among plants of the same genotype.21 In addition, recent studies have shown that grapevines could regulate there isohydricy during the growth season and switch from isohydric to anisohydric with varying soil moisture. The authors contribute the changes to hydraulic/hormone signaling.22-24 This new mechanism could be an interesting view of how to examine the isohydric/anisohydric behavior.
Interestingly, the anisohydric strategy has been reported to be beneficial for survival during long periods of drought. In recent studies, about 75% of the examined juniper trees (Juniperus monosperma), which are anisohydric, survived 24 mo of drought, as compared with 5% of the isohydric pinyon trees (Pinus edulis).25,26 The fact that the juniper trees could maintain gas exchange at a significantly lower leaf water potential than the pinyon trees9,13 suggests that, at least for this species under these conditions, the risk of hydraulic failure and desiccation is worthwhile. There are additional examples of tree species that seem to benefit from their anisohydric behavior. The anisohydric white oak (Quercus alba L.) has been shown to survive drought better than the isohydric black walnut (Juglans nigra L.).27 In another study, the anisohydric Eperua falcata was found to be less sensitive to soil drought and atmospheric drought than the isohydric Diplotropis purpurea.28
Nevertheless, we find that defining plant water-balance regulation solely in terms of Ψleaf regulation is incomplete. After all, a plant’s primary necessity is to maintain its water content so as to maintain its biochemical functionality. For this reason, definitions of isohydric vs. anisohydric behavior should include the regulation of plant relative water content (RWC). We suggest that the stable Ψleaf that is typical of isohydric plants is a symptom of the maintenance of high RWC. For this reason, we expect isohydric plants to regulate RWC more strictly than Ψleaf. To test this assumption, we monitored the RWC and Ψleaf of our anisohydric tomato TIP2;2 plants and a corresponding isohydric control as the plants were exposed to drought stress. We observed that the isohydric plants maintained their RWC more strictly than their Ψleaf (Fig. 1). The mechanism regulating this hierarchy is not clear. We suggest that cell-wall elasticity (ε) might play a role. Plants with high ε are more sensitive to water loss and translate minimal RWC loss into maximal Ψleaf change.29 ε may play a central role in the sensing of the water-loss signal and its conversion into a water-potential signal that can be sensed by the stomata, leading to their closure (i.e., cell walls with higher ε will be more sensitive to small changes in water-content differences, resulting in the more rapid closure of the stomata). Furthermore, ε could act to maintain relative water content at the turgor-loss point and prevent cell dehydration.30
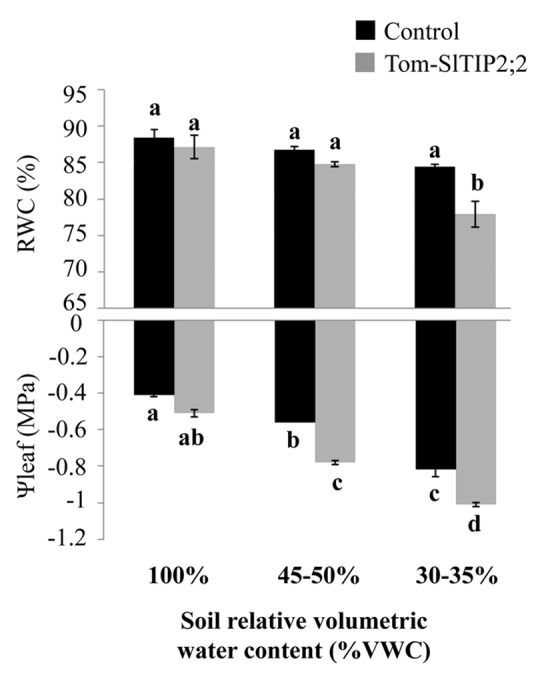
Figure 1. Comparison of midday leaf water potential (Ψleaf) and leaf relative water content (RWC) of Tom-SlTIP2;2 and control plants at different levels of relative soil volumetric water content (VWC). Two independent Tom-SlTIP2;2 lines (Sade et al., 2009)8 and control plants were subjected to drought and the decreasing soil VWC was monitored using a soil moisture probe (10HS, Decagon). (A) Midday leaf Ψleaf and (B) leaf RWC were measured at three different VWC levels (100%,~50%,~30%) using a pressure chamber (ARIMAD3000, MRC). Different letters above the columns represent significant differences (Tukey-Kramer test, p < 0.05). Data points are means ± SE (n = 6).
Isohydric and Anisohydric Effect on Biotic Stress Resistance
The role of anisohydric behavior in the response of plants to biotic stress is not well understood. Similar to responses to abiotic stress, biotic stress response is a complex trait involving multiple mechanisms, including changes in phytohormones and protein interactions.31 Moreover, both types of stress lead to similar physiological responses, such as decreased gs and decreased AN,32,33 suggesting that anisohydric plants might be more tolerant of biotic stress as a result of their greater carbon surpluses.5 Another example of an association between anisohydric behavior and biotic stress resistance may be seen in the ABA-deficient tomato mutant-sitiens (Lycopersicon esculentum Mill. cv Moneymaker34). This mutant could be considered to be the ultimate anisohydric plant as it maintains high stomatal conductance at all times.35 Indeed, these plants were reported to be highly resistant to the necrotrophic fungus Botrytis cinerea.36 Yet, it was suggested that this resistance might not be related to carbohydrate balance, but rather to the relatively high levels of salicylic acid present in these mutants (probably as a feedback response to the low levels of ABA, since salicylic acid is an antagonist of ABA37 and known to play a role in plant resistance to biotic stress38).
When our anisohydricTIP2;2 plants were inoculated with Botrytis cinerea, they exhibited a higher level of disease resistance than the isohydric control (Fig. 2). Moreover, the TIP2;2 plants were also more tolerant to tomato yellow leaf curly virus (TYLCV; unpublished data, D. Sade and N. Sade) than the control. These results support the suggestion that anisohydric plants may be more tolerant of biotic stress, but we still do not understand the mechanism of this tolerance. It has been suggested that isohydric species have higher levels of ABA than anisohydric species.1,27,39,40 This suggests that anisohydric resistance to biotic stress may be related to an ABA-salicylic acid-regulated plant defense mechanism.
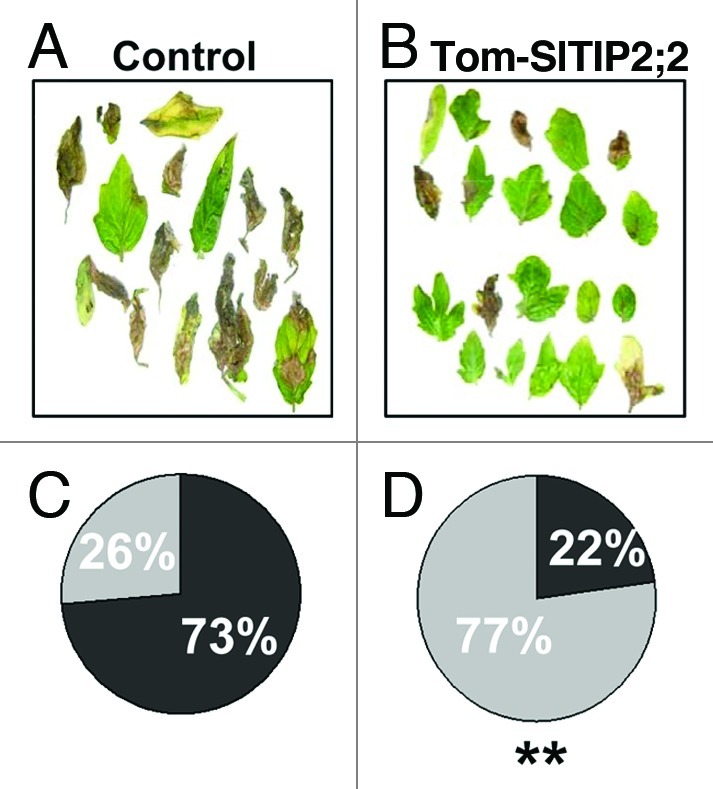
Figure 2. Inoculation of detached leaves with the necrotrophic fungus Botrytis cinerea. (A) Leaves from Control plants (n = 19) and (B) leaves from two independent transgenic Tom-SlTIP2;2 lines (n = 22) were inoculated with 5 µl of a solution containing 1,500 spores/µl. After inoculation, plants were sealed in plastic bags and transferred to a growth chamber for 4 d. Disease was evaluated as > 50% of leaf surface (infected) or < 50% of leaf surface (uninfected) and these data are presented (C and D) as relative incidence of infected (black) and uninfected (gray) leaves among the total number of inoculated leaves. **Significant difference (comparisons of two ratios binomial, p < 0.01).
We would like to suggest an additional explanation for the resistance of anisohydric plants to biotic stress. We suggest that the lower leaf RWC that is characteristic of anisohydric plants8 (and Fig. 1) inhibits the replication and movement of biotic agents such as bacteria and fungus through the apoplast,41 as well as the movement of virions through the plasmodesmata.42 The identification of the origin of this observed resistance to biotic stress is a matter for further research.
Concluding Remarks
Additional research is needed to increase our understanding of the molecular basis for the different strategies that plants use to regulate their water balance. The identification of specific AQP genes with defined roles in the plant’s water-budgeting activities will enhance our understanding of stomatal regulation and provide novel molecular tools for improving plant resistance to many other types of abiotic and perhaps even biotic stress, thereby contributing to our future food, feed and fiber security. It is important that any claim for behavior-related resistance or tolerance of a crop to stress take into consideration the stress level, the duration of the exposure to the stress and the rate at which the plant recovers from this exposure.
Acknowledgments
We thank Dr Maggie Levy for providing the spore and inoculation chambers. This study was partially supported by grant #FE 552/12-1; OR309/1-1 from the German-Israeli Project Cooperation (DIP).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20505
References
- 1.Granier C, Tardieu F. Spatial and temporal analyses of expansion and cell cycle in sunflower leaves. A common pattern of development for all zones of a leaf and different leaves of a plant. Plant Physiol. 1998;116:991–1001. doi: 10.1104/pp.116.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz HR. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003;26:1393–405. doi: 10.1046/j.1365-3040.2003.01064.x. [DOI] [Google Scholar]
- 3.Almeida-Rodriguez AM, Cooke JEK, Yeh F, Zwiazek JJ. Functional characterization of drought-responsive aquaporins in Populus balsamifera and Populus simonii×balsamifera clones with different drought resistance strategies. Physiol Plant. 2010;140:321–33. doi: 10.1111/j.1399-3054.2010.01405.x. [DOI] [PubMed] [Google Scholar]
- 4.West AG, Hultine KR, Sperry JS, Bush SE, Ehleringer JR. Transpiration and hydraulic strategies in a piñon-juniper woodland. Ecol Appl. 2008;18:911–27. doi: 10.1890/06-2094.1. [DOI] [PubMed] [Google Scholar]
- 5.McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 2008;178:719–39. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- 6.Kumagai T, Porporato A. Strategies of a Bornean tropical rainforest water use as a function of rainfall regime: isohydric or anisohydric? Plant Cell Environ. 2012;35:61–71. doi: 10.1111/j.1365-3040.2011.02428.x. [DOI] [PubMed] [Google Scholar]
- 7.Mueller RC, Scudder CM, Porter ME, Trotter RT, Gehring CA, Whitham TG. Differential tree mortality in response to severe drought: evidence for long-term vegetation shifts. J Ecol. 2005;93:1085–93. doi: 10.1111/j.1365-2745.2005.01042.x. [DOI] [Google Scholar]
- 8.Sade N, Vinocur BJ, Diber A, Shatil A, Ronen G, Nissan H, et al. Improving plant stress tolerance and yield production: is the tonoplast aquaporin SlTIP2;2 a key to isohydric to anisohydric conversion? New Phytol. 2009;181:651–61. doi: 10.1111/j.1469-8137.2008.02689.x. [DOI] [PubMed] [Google Scholar]
- 9.McDowell NG. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011;155:1051–9. doi: 10.1104/pp.110.170704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez E, Scheiber SM, Beeson RC, Jr., Sandrock DR. Drought tolerance responses of purple Lovegrass and 'Adagio' maiden grass. HortScience. 2007;42:1695–9. [Google Scholar]
- 11.Peng Y, Lin W, Cai W, Arora R. Overexpression of a Panax ginseng tonoplast aquaporin alters salt tolerance, drought tolerance and cold acclimation ability in transgenic Arabidopsis plants. Planta. 2007;226:729–40. doi: 10.1007/s00425-007-0520-4. [DOI] [PubMed] [Google Scholar]
- 12.Lin WL, Peng YH, Li GW, Arora R, Tang ZC, Su WA, et al. Isolation and functional characterization of PgTIP1, a hormone-autotrophic cells-specific tonoplast aquaporin in ginseng. J Exp Bot. 2007;58:947–56. doi: 10.1093/jxb/erl255. [DOI] [PubMed] [Google Scholar]
- 13.Linton MJ, Sperry JS, Williams DG. Limits to water transport in Juniperus osteosperma and Pinus edulis: implications for drought tolerance and regulation of transpiration. Funct Ecol. 1998;12:906–11. doi: 10.1046/j.1365-2435.1998.00275.x. [DOI] [Google Scholar]
- 14.Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Phillips N, et al. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999;22:1515–26. doi: 10.1046/j.1365-3040.1999.00513.x. [DOI] [Google Scholar]
- 15.Brodribb TJ, Holbrook NM. Stomatal protection against hydraulic failure: a comparison of coexisting ferns and angiosperms. New Phytol. 2004;162:663–70. doi: 10.1111/j.1469-8137.2004.01060.x. [DOI] [PubMed] [Google Scholar]
- 16.Ewers BE, Gower ST, Bond-Lamberty B, Wang CK. Effects of stand age and tree species on canopy transpiration and average stomatal conductance of boreal forests. Plant Cell Environ. 2005;28:660–78. doi: 10.1111/j.1365-3040.2005.01312.x. [DOI] [Google Scholar]
- 17.Clearwater MJ, Clark CJ. In vivo magnetic resonance imaging of xylem vessel contents in woody lianas. Plant Cell Environ. 2003;26:1205–14. doi: 10.1046/j.1365-3040.2003.01042.x. [DOI] [Google Scholar]
- 18.Alsina MM, De Herralde F, Aranda X, Save R, Biel C. Water relations and vulnerability to embolism are not related: Experiments with eight grapevine cultivars. Vitis. 2007;46:1–6. [Google Scholar]
- 19. http://www.gwrdc.com.au/webdata/resources/files/Mod4-VarietalAndRootstockPPT.pdf.
- 20.Lovisolo C, Perrone I, Carra A, Ferrandino A, Flexas J, Medrano H, et al. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: a physiological and molecular update. Funct Plant Biol. 2010;37:98–116. doi: 10.1071/FP09191. [DOI] [Google Scholar]
- 21.Chaves MM, Zarrouk O, Francisco R, Costa JM, Santos T, Regalado AP, et al. Grapevine under deficit irrigation: hints from physiological and molecular data. Ann Bot. 2010;105:661–76. doi: 10.1093/aob/mcq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogiers SY, Greer DH, Hatfield JM, Hutton RJ, Clarke SJ, Hutchinson PA, et al. Stomatal response of an anisohydric grapevine cultivar to evaporative demand, available soil moisture and abscisic acid. Tree Physiol. 2012;32:249–61. doi: 10.1093/treephys/tpr131. [DOI] [PubMed] [Google Scholar]
- 23.Domec JC, Johnson DM. Does homeostasis or disturbance of homeostasis in minimum leaf water potential explain the isohydric versus anisohydric behavior of Vitis vinifera L. cultivars? Tree Physiol. 2012;32:245–8. doi: 10.1093/treephys/tps013. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Oren R, Kang S. Spatiotemporal variation of crown-scale stomatal conductance in an arid Vitis vinifera L. cv. Merlot vineyard: direct effects of hydraulic properties and indirect effects of canopy leaf area. Tree Physiol. 2012;32:262–79. doi: 10.1093/treephys/tpr120. [DOI] [PubMed] [Google Scholar]
- 25.Breshears DD, Cobb NS, Rich PM, Price KP, Allen CD, Balice RG, et al. Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci U S A. 2005;102:15144–8. doi: 10.1073/pnas.0505734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw JD, Steed BE, DeBlander LT. Forest Inventory and Analysis (FIA) annual inventory answers the question: What is happening to pinyon-juniper woodlands? J For. 2005;103:280–5. [Google Scholar]
- 27.Loewenstein NJ, Pallardy SG. Drought tolerance, xylem sap abscisic acid and stomatal conductance during soil drying: a comparison of canopy trees of three temperate deciduous angiosperms. Tree Physiol. 1998;18:431–9. doi: 10.1093/treephys/18.7.431. [DOI] [PubMed] [Google Scholar]
- 28.Bonal D, Guehl JM. Contrasting patterns of leaf water potential and gas exchange responses to drought in seedlings of tropical rainforest species. Funct Ecol. 2001;15:490–6. doi: 10.1046/j.0269-8463.2001.00537.x. [DOI] [Google Scholar]
- 29.Kramer PB. JS, ed. Water Relations of Plants and Soils. New York: Academic Press, 1995. [Google Scholar]
- 30.Bartlett MK, Scoffoni C, Sack L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol Lett. 2012;15:393–405. doi: 10.1111/j.1461-0248.2012.01751.x. [DOI] [PubMed] [Google Scholar]
- 31.Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, et al. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol. 2006;9:436–42. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Kyseláková H, Prokopová J, Nauš J, Novák O, Navrátil M, Safářová D, et al. Photosynthetic alterations of pea leaves infected systemically by pea enation mosaic virus: A coordinated decrease in efficiencies of CO(2) assimilation and photosystem II photochemistry. Plant Physiol Biochem. 2011;49:1279–89. doi: 10.1016/j.plaphy.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Gudesblat GE, Torres PS, Vojnov AA. Stomata and pathogens: Warfare at the gates. Plant Signal Behav. 2009;4:1114–6. doi: 10.4161/psb.4.12.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor IB, Linforth RST, Alnaieb RJ, Bowman WR, Marples BA. The wilty tomato mutants flacca and sitiens are impaired in the oxidation of aba-aldehyde to ABA. Plant Cell Environ. 1988;11:739–45. doi: 10.1111/j.1365-3040.1988.tb01158.x. [DOI] [Google Scholar]
- 35.Tal M. Abnormal stomatal behavior in wilty mutants of tomato. Plant Physiol. 1966;41:1387–91. doi: 10.1104/pp.41.8.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Audenaert K, De Meyer GB, Höfte MM. Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol. 2002;128:491–501. doi: 10.1104/pp.010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Torres Zabala M, Bennett MH, Truman WH, Grant MR, Zabala MdT Antagonism between salicylic and abscisic acid reflects early host-pathogen conflict and moulds plant defence responses. Plant J. 2009;59:375–86. doi: 10.1111/j.1365-313X.2009.03875.x. [DOI] [PubMed] [Google Scholar]
- 38.Hammond-Kosack KE, Jones JD, HammondKosack KE Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773–91. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soar CJ, Speirs J, Maffei SM, Penrose AB, McCarthy MG, Loveys BR. Grape vine varieties Shiraz and Grenache differ in their stomatal response to VPD: apparent links with ABA physiology and gene expression in leaf tissue. Aust J Grape Wine Res. 2006;12:2–12. doi: 10.1111/j.1755-0238.2006.tb00038.x. [DOI] [Google Scholar]
- 40.Loveys BR, During H. Diurnal changes in water relations and abscisic-acid in field-grown vitis-vinifera cultivars. 2. abscisic-acid changes under semi-arid conditions. New Phytol. 1984;97:37–47. doi: 10.1111/j.1469-8137.1984.tb04107.x. [DOI] [Google Scholar]
- 41.Wright CA, Beattie GA. Pseudomonas syringae pv. tomato cells encounter inhibitory levels of water stress during the hypersensitive response of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2004;101:3269–74. doi: 10.1073/pnas.0400461101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oparka KJ, Prior DAM. Direct evidence for pressure-generated closure of plasmodesmata. Plant J. 1992;2:741–50. doi: 10.1111/j.1365-313X.1992.tb00143.x. [DOI] [Google Scholar]


