Abstract
Mechanosensing and its downstream responses are speculated to involve sensory complexes containing Ca2+-permeable mechanosensitive channels. On recognizing hypo-osmotic stress, plant cells initiate activation of a widespread signal transduction network involving second messengers such as Ca2+ to trigger inducible defense responses including the induction of transcriptional factors.1 However, most of the components involved in these signaling networks still remain to be identified. Recently we identified and investigated OsMCA1, the sole homolog of the MCA family putative Ca2+-permeable mechanosensitive channels in rice. Functional characterization of the OsMCA1-suppressed cells as well as the overexpressing cells indicated that OsMCA1 is involved in the regulation of plasma membrane Ca2+ influx and NADPH oxidase-mediated generation of reactive oxygen species (ROS) induced by hypo-osmotic stress. Here we will discuss possible molecular mechanisms and physiological functions of the MCA protein in hypo-osmotic signaling.
Keywords: calcium signaling, hypo-osmotic stress, mechanosensitive Ca2+ channel, reactive oxygen species (ROS), rice
Introduction
Plants respond to mechanical stimuli, such as touch, wind, gravity, pathogen attack and cellular deformation during development.2 Mechanical stimuli often trigger an increase in cytosolic free Ca2+ concentration [(Ca2+)cyt] mediated by mechanosensitive Ca2+ channels located at the plasma membrane and endomembranes.3-6 However, molecular identity, structure and physiological functions of these mechanosensitive channels are still largely unknown.
Arabidopsis MSL9 and MSL10, homologs of the bacterial mechanosensitive channel MscS, are reported to be required for mechanosensitive channel activity at the plasma membrane of root cells, which are more permeable to Cl– than Ca2+.7,8 We recently identified the MCA family proteins including MCA1 (At4g35920), MCA2 (At2g17780), NtMCA1 (AB622811) and NtMCA2 (AB622812) in Arabidopsis and tobacco as putative Ca2+-permeable mechanosensitive channels.9-11 Ectopic overexpression of MCAs enhances Ca2+ uptake in roots9 and cultured cells,11 as well as [Ca2+]cyt elevation and hypo-osmotic stress-induced gene expression.9-11 However, components involved in the MCA-mediated signaling pathways have been still mostly unknown.
Recognition of osmotic stress initiates activation of a widespread signal transduction network that induces second messengers and triggers inducible defense responses.1,12-15 Characteristic early signaling events other than Ca2+ influx include protein phosphorylation and ROS generation, most of which are often prevented when Ca2+ influx is compromised by either Ca2+ chelators or Ca2+-channel blockers, such as La3+.16,17 These results suggest that regulation of these osmotic signaling events including ROS generation requires Ca2+ influx. However, their molecular basis and regulatory mechanisms have remained poorly elucidated.
Intracellular Localization of OsMCA1
The GFP-OsMCA1 protein was localized at the plasma membrane in tobacco BY-2 cells.18 We also transiently expressed in onion epidermal cells and analyzed the intracellular localization of the GFP-OsMCA1 protein. When GFP alone was expressed, it localized to the nucleus and the cytoplasm (Fig. 1g--l). Interestingly, the GFP-OsMCA1 fusion protein was localized specifically targeted to the plasma membrane in patches and at punctuated structures on the cell surface (Fig. 1a--f). These fluorescence signals were also observed in tobacco BY-2 cells expressing the NtMCA1-GFP fusion protein.11 The patchy localization of GFP-OsMCA1 at the plasma membrane appears similar to abscisic acid-binding sites in guard cells19 and GFP-OsTPC120 and may be related to plasma membrane microdomains. The inward rectifying K+ channel, KST1, also forms clusters in plasma membranes, and its GFP-tagged derivatives were observed as patches in both endomembranes and plasma membranes.21,22 Arabidopsis MCA1 and MCA2 form a homo-oligomer in yeast cells.23 Plant MCA family proteins may form clusters or complexes with other signaling molecules such as other ion channels in planta.
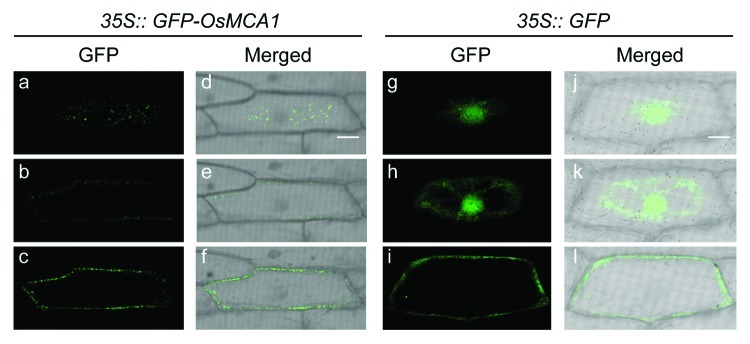
Figure 1. Intracellular localization of OsMCA1. A GFP-OsMCA1 plasmid was introduced into onion epidermal cells by bombardment. (a--f) 35S::GFP-OsMCA1, (g--l) 35S::GFP. (a--l) Optical sections of an onion epidermal cell, from the surface (a and d, g and j) to the equatorial plane (c and f, i and l), were obtained by confocal laser scanning microscopy. Fluorescence of GFP (a--c and g--i) and merged with bright field (d--f and j--l). Scale bar: 20 μm.
Roles of OsMCA1 in Ca2+- and ROS-Mediated Hypo-osmotic Signaling
Plasma membrane Ca2+ influx induced by various stresses or extracellular stimuli are mediated by at least several types of Ca2+-permeable channels, whose molecular identity are still mostly elusive in plants. Recent studies revealed that temporal patterns of [Ca2+]cyt changes vary among stimuli and molecular nature of Ca2+ channels involved.6,24
Hypo-osmotic shock as well as treatment with trinitrophenol, an activator of mechanosensitive channels, induce a rapid and transient rise in [Ca2+]cyt predominantly due to plasma membrane Ca2+ influx. Both of them are partially impaired in the OsMCA1-suppressed cells.18 In contrast, a major microbe-associated molecular pattern, N-acetylchitooligosaccharides, also triggers a [Ca2+]cyt increase with a similar temporal pattern, which is not affected by suppression of OsMCA1.18 These results suggest possible involvement of OsMCA1 as a putative mechanosensitive Ca2+-permeable channel component in the regulation of mechanical stress-triggered plasma membrane Ca2+ influx.
Hypo-osmotic shock has also been shown to trigger ROS generation following a [Ca2+]cyt increase in various plant cells.13,14,17 In rice cultured cells, hypo-osmotic shock-triggered ROS generation is predominantly attributed to NADPH oxidases.18 Respiratory burst oxidase homologs (Rbohs) possess ROS-producing activity synergistically activated by binding of Ca2+ to the EF-hand motifs in the N-terminal cytosolic domain and phosphorylation in rice and Arabidopsis.25-28 A functional NADPH oxidase AtRbohC/RHD2 affects mechanical stress-induced ROS generation in a Ca2+-dependent manner.29 Both Arabidopsis MCA1 and ROS generated by AtRbohD and/or AtRbohF have recently been suggested to be involved in the regulation of osmo-sensitive metabolic changes.30 These findings suggest the following initial plasma membrane responses in response to osmo-stimulation: Activation of the plasma membrane mechanosensitive Ca2+-permeable channels such as MCA family induces the influx of Ca2+, leading to activation of Rboh(s) to generate ROS (Fig. 2). The Ca2+-ROS signaling network27 may play a crucial role in the regulation of downstream events.
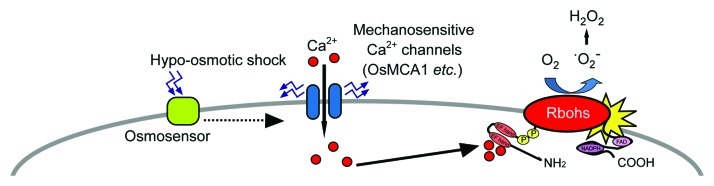
Figure 2. A proposed model for the early plasma membrane responses of rice cells exposed to hypo-osmotic stress. Following the recognition of hypo-osmotic shock by osmosensors or mechanosensitive channels, cells initiate early signaling events including the influx of Ca2+. Respiratory burst oxidase homologs (Rbohs) are synergistically activated by binding of Ca2+ to the EF hand motifs and phosphorylation to activate ROS generation.25-28 Signaling network involving Ca2+ and ROS may play a crucial role in induction of stress adaptation. Solid and dotted arrows indicate established and hypothetical links, respectively.
In conclusion, OsMCA1 has been shown to be involved in the regulation of plasma membrane Ca2+ influx and NADPH oxidase-mediated ROS generation induced by hypo-osmotic stress in cultured rice cells. These findings shed light on our understanding of mechanical sensing pathways. It should be an important future subject whether OsMCA1 itself also plays a role as a plasma membrane mechanical sensor and/or whether a signal is transduced from unknown mechanical sensor(s) to OsMCA1/Ca2+-permeable channel(s) (Fig. 2).
Acknowledgments
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology for Scientific Research on Innovative Areas (21200067) to T.K., for Exploratory Research (21658118) to K.K., for Scientific Research on Priority Area (21026009) to H.I., for Scientific Research B (19370023) to K.K. and (21370017) to H.I., and by grants from Japan Science and Technology Agency for CREST to H.I. and K.K..
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20521
References
- 1.Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res. 2011;124:509–25. doi: 10.1007/s10265-011-0412-3. [DOI] [PubMed] [Google Scholar]
- 2.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14(Suppl):S401–17. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasano JM, Massa GD, Gilroy S. Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul. 2002;21:71–88. doi: 10.1007/s003440010049. [DOI] [PubMed] [Google Scholar]
- 4.Lee D, Polisensky DH, Braam J. Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. New Phytol. 2005;165:429–44. doi: 10.1111/j.1469-8137.2004.01238.x. [DOI] [PubMed] [Google Scholar]
- 5.Toyota M, Furuichi T, Tatsumi H, Sokabe M. Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiol. 2008;146:505–14. doi: 10.1104/pp.107.106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- 7.Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol. 2008;18:730–4. doi: 10.1016/j.cub.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 8.Peyronnet R, Haswell ES, Barbier-Brygoo H, Frachisse JM. AtMSL9 and AtMSL10: Sensors of plasma membrane tension in Arabidopsis roots. Plant Signal Behav. 2008;3:726–9. doi: 10.4161/psb.3.9.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci U S A. 2007;104:3639–44. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamanaka T, Nakagawa Y, Mori K, Nakano M, Imamura T, Kataoka H, et al. MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol. 2010;152:1284–96. doi: 10.1104/pp.109.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurusu T, Yamanaka T, Nakano M, Takiguchi A, Ogasawara Y, Hayashi T, et al. Involvement of the putative Ca2+-permeable mechanosensitive channels, NtMCA1 and NtMCA2, in Ca2+ uptake, Ca2+-dependent cell proliferation and mechanical stress-induced gene expression in tobacco (Nicotiana tabacum) BY-2 cells. J Plant Res. 2012;125:555–68. doi: 10.1007/s10265-011-0462-6. [DOI] [PubMed] [Google Scholar]
- 12.Zingarelli L, Marré MT, Massardi F, Lado P. Effects of hyperosmotic stress on K+ fluxes, H+ extrusion, transmembrane electric potential difference and comparison with the effects of fusicoccin. Physiol Plant. 1999;106:287–95. doi: 10.1034/j.1399-3054.1999.106305.x. [DOI] [Google Scholar]
- 13.Beffagna N, Buffoli B, Busi C. Modulation of reactive oxygen species production during osmotic stress in Arabidopsis thaliana cultured cells: involvement of the plasma membrane Ca2+-ATPase and H+-ATPase. Plant Cell Physiol. 2005;46:1326–39. doi: 10.1093/pcp/pci142. [DOI] [PubMed] [Google Scholar]
- 14.Rouet MA, Mathieu Y, Barbier-Brygoo H, Laurière C. Characterization of active oxygen-producing proteins in response to hypo-osmolarity in tobacco and Arabidopsis cell suspensions: identification of a cell wall peroxidase. J Exp Bot. 2006;57:1323–32. doi: 10.1093/jxb/erj107. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, Harada A, Sakai T, Takagi S. Ca2+ transient induced by extracellular changes in osmotic pressure in Arabidopsis leaves: differential involvement of cell wall-plasma membrane adhesion. Plant Cell Environ. 2006;29:661–72. doi: 10.1111/j.1365-3040.2005.01447.x. [DOI] [PubMed] [Google Scholar]
- 16.Moran N. Osmoregulation of leaf motor cells. FEBS Lett. 2007;581:2337–47. doi: 10.1016/j.febslet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Cazalé AC, Rouet-Mayer MA, Barbier-Brygoo H, Mathieu Y, Laurière C. Oxidative burst and hypoosmotic stress in tobacco cell suspensions. Plant Physiol. 1998;116:659–69. doi: 10.1104/pp.116.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurusu T, Nishikawa D, Yamazaki Y, Gotoh M, Nakano M, Hamada H, et al. Plasma membrane protein OsMCA1 is involved in regulation of hypo-osmotic shock-induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biol. 2012;12:11. doi: 10.1186/1471-2229-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamazaki D, Yoshida S, Asami T, Kuchitsu K. Visualization of abscisic acid-perception sites on the plasma membrane of stomatal guard cells. Plant J. 2003;35:129–39. doi: 10.1046/j.1365-313X.2003.01782.x. [DOI] [PubMed] [Google Scholar]
- 20.Kurusu T, Yagala T, Miyao A, Hirochika H, Kuchitsu K. Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J. 2005;42:798–809. doi: 10.1111/j.1365-313X.2005.02415.x. [DOI] [PubMed] [Google Scholar]
- 21.Ehrhardt T, Zimmermann S, Müller-Röber B. Association of plant K+(in) channels is mediated by conserved C-termini and does not affect subunit assembly. FEBS Lett. 1997;409:166–70. doi: 10.1016/S0014-5793(97)00502-4. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann S, Talke I, Ehrhardt T, Nast G, Müller-Röber B. Characterization of SKT1, an inwardly rectifying potassium channel from potato, by heterologous expression in insect cells. Plant Physiol. 1998;116:879–90. doi: 10.1104/pp.116.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano M, Iida K, Nyunoya H, Iida H. Determination of structural regions important for Ca2+ uptake activity in Arabidopsis MCA1 and MCA2 expressed in yeast. Plant Cell Physiol. 2011;52:1915–30. doi: 10.1093/pcp/pcr131. [DOI] [PubMed] [Google Scholar]
- 24.Hamada H, Kurusu T, Okuma E, Nokajima H, Kiyoduka M, Koyano T, et al. Regulation of a proteinaceous elicitor-induced Ca2+ influx and production of phytoalexins by a putative voltage-gated cation channel, OsTPC1, in cultured rice cells. J Biol Chem. 2012;287:9931–9. doi: 10.1074/jbc.M111.337659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem. 2008;283:8885–92. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- 26.Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–4. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 27.Kimura S, Kaya H, Kawarazaki T, Hiraoka G, Senzaki E, Michikawa M, Kuchitsu K. Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim Biophys Acta 2012; 1823:398-405; 10.1016/j.bbamcr.2011.09.011 [DOI] [PubMed]
- 28.Takahashi S, Kimura S, Kaya H, Iizuka A, Wong HL, Shimamoto K, et al. Reactive oxygen species production and activation mechanism of the rice NADPH oxidase OsRbohB. J Biochem. 2012;152:37–43. doi: 10.1093/jb/mvs044. [DOI] [PubMed] [Google Scholar]
- 29.Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell. 2009;21:2341–56. doi: 10.1105/tpc.109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wormit A, Butt SM, Chairam I, McKenna JF, Nunes-Nesi A, Kjaer L, et al. Osmosensitive changes of carbohydrate metabolism in response to cellulose biosynthesis inhibition. Plant Physiol. 2012;159:105–17. doi: 10.1104/pp.112.195198. [DOI] [PMC free article] [PubMed] [Google Scholar]


