Abstract
Sucrose synthase (SuSy) catalyzes the reversible conversion of sucrose and NDP into the corresponding nucleotide-sugars and fructose. The Arabidopsis genome possesses six SUS genes (AtSUS1–6) that code for proteins with SuSy activity. As a first step to investigate optimum fructose and UDP-glucose (UDPG) concentrations necessary to measure maximum sucrose-producing SuSy activity in crude extracts of Arabidopsis, in this work we performed kinetic analyses of recombinant AtSUS1 in two steps: (1) SuSy reaction at pH 7.5, and (2) chromatographic measurement of sucrose produced in step 1. These analyses revealed a typical Michaelis-Menten behavior with respect to both UDPG and fructose, with Km values of 50 μM and 25 mM, respectively. Unlike earlier studies showing the occurrence of substrate inhibition of UDP-producing AtSUS1 by fructose and UDP-glucose, these analyses also revealed no substrate inhibition of AtSUS1 at any UDPG and fructose concentration. By including 200 mM fructose and 1 mM UDPG in the SuSy reaction assay mixture, we found that sucrose-producing SuSy activity in leaves and stems of Arabidopsis were exceedingly higher than previously reported activities. Furthermore, we found that SuSy activities in organs of the sus1/sus2/sus3/sus4 mutant were ca. 80–90% of those found in WT plants.
Keywords: ADP-glucose, UDP-glucose, cellulose, starch, sucrose synthase
Sucrose synthase (SuSy) catalyzes the following reversible reaction:
| sucrose + NDP↔NDP-glucose + fructose |
where N stands for uridine, adenosine, guanosine, cytidine, thymidine or inosine. This sucrolytic enzyme is highly regulated both at transcriptional and post-translational levels.1-5 In many heterotrophic organs SuSy activity acts as a major determinant of sink strength that highly controls the conversion of sucrose into ADP-glucose (ADPG) and UDP-glucose (UDPG) linked to the biosynthesis of starch and cell wall polysaccharides, respectively.6-12 Genetic evidence demonstrating the importance of SuSy in starch and cell wall polysaccharide production, and in sink strength determination in heterotrophic organs comes from QTL analyses of maize endosperms and cotton,13,14 from the altered biomass and fiber yield in cotton plants with altered SuSy expression,11,15 from the substantial (ca. 50–70%) reduction of starch levels in the seed endosperms of sh1 maize mutants,6 and from genetically engineered potato tubers and carrot roots exhibiting altered SuSy activity.8,12,16 In addition, SuSy has been suggested to be involved, at least in part, in the direct conversion of sucrose into ADPG linked to starch biosynthesis in autotrophic organs.17,18
The Arabidopsis genome possesses six SUS genes (AtSUS1–6) displaying different developmental expression patterns that code for proteins with SuSy activity.19 Earlier studies20,21 have questioned the involvement of SuSy activity in starch and cellulose biosynthesis in Arabidopsis, showing that (a) leaves, siliques, seeds, stems and roots of the sus1/sus2/sus3/sus4 mutant impaired in SuSy activity accumulate wild type (WT) content of ADPG, UDPG, cellulose and starch, and (b) SuSy activity in WT leaves is too low to account for the rate of starch accumulation in illuminated leaves. Most recently however, we22 have shown that SuSy activity in the cleavage direction in WT Arabidopsis leaves is ca. 10-fold higher than UDP-producing SuSy activities shown in earlier studies,20,21 greatly exceeding the minimum needed to support normal rate of starch accumulation during illumination. Furthermore, we found that SuSy activities in the insoluble and soluble fractions of sus1/sus2/sus3/sus4 stems were ca. 10- and 100-fold higher, respectively, than previously reported activities.21 Finally, we also found that SuSy activities in the leaves and stems of the sus1/sus2/sus3/sus4 mutants were ca. 85% of those of WT plants, thus concluding that SuSy activity in sus1/sus2/sus3/sus4 mutants is sufficient to support normal cellulose and starch biosynthesis in Arabidopsis.22 Contrary to the claims of Barratt et al.,21 Angeles-Núñez and Tiessen23 showed that developing seeds of single sus2 and sus3 mutants accumulate considerably reduced levels of transitory starch. This and the fact that AtSUS2 is strongly associated with plastids24 would support the idea that SUS is involved in a specific route for ADPG synthesis in Arabidopsis seeds.
There are several possible reasons that, individually or collectively, can explain why the values of SuSy activity shown in earlier reports20,21 were a gross underestimate and differed greatly from those presented by Baroja-Ferńndez et al.22 First, Bieniawska et al.20 and Barratt et al.21 measured SuSy activity in the UDP synthetic direction, whereas Baroja-Ferńndez et al.22 measured SuSy activity in the more physiological sucrose breakdown (UDPG and ADPG synthesis) direction. Second, the SuSy reaction assay mixture employed by Bieniawska et al.20 and Barratt et al.21 contained MgCl2 and its pH was 9.0–9.5. Under these conditions, UDPG (the substrate for SuSy reaction in the sucrose synthetic direction) is highly unstable, being spontaneously converted into UMP and glucose-1,2-monophosphate.22 Third, the SuSy reaction assay mixture employed by Bieniawska et al.20 and Barratt et al.21 contained 6 mM fructose, a concentration which is comparable, or even lower, than the reported Km values for fructose in SuSy from many species.25,26 Such low fructose concentration in the SuSy reaction assay mixture would prevent substrate inhibition by both fructose and UDPG, a phenomenon reported by several independent groups when measuring UDP-producing SuSy activity from UDPG and fructose.20,26-29
As a first step to investigate the optimum fructose and UDPG concentrations in the reaction mixture for assaying maximum SuSy activity in crude extracts of Arabidopsis, in this work we performed kinetic analyses of recombinant AtSUS1 in the sucrose synthetic direction in two steps: (1) SuSy reaction, and (2) measurement of sucrose produced during the reaction in step 1. The SuSy reaction assay mixture contained 50 mM HEPES, 3 mM MgCl2, the indicated amounts of fructose and UDPG, and recombinant AtSUS1 obtained as previously described.22 After 3 min at 25°C (still under initial velocity conditions), reactions were stopped by boiling the assay mixture for 1 min. Sucrose was then measured by HPLC with pulsed amperometric detection on a DX-500 system (Dionex) fitted to a CarboPac PA10 column. Preliminary analyses of optimum pH in the absence of MgCl2 revealed that sucrose producing AtSUS1 activity has a broad pH optimum between 7.5 and 9.5, which is consistent with previous reports.27,30,31 Because UDPG is highly unstable in the presence of MgCl2 at pH values higher than 7.5,22 the step one reaction in our kinetic analyses was conducted at pH 7.5.
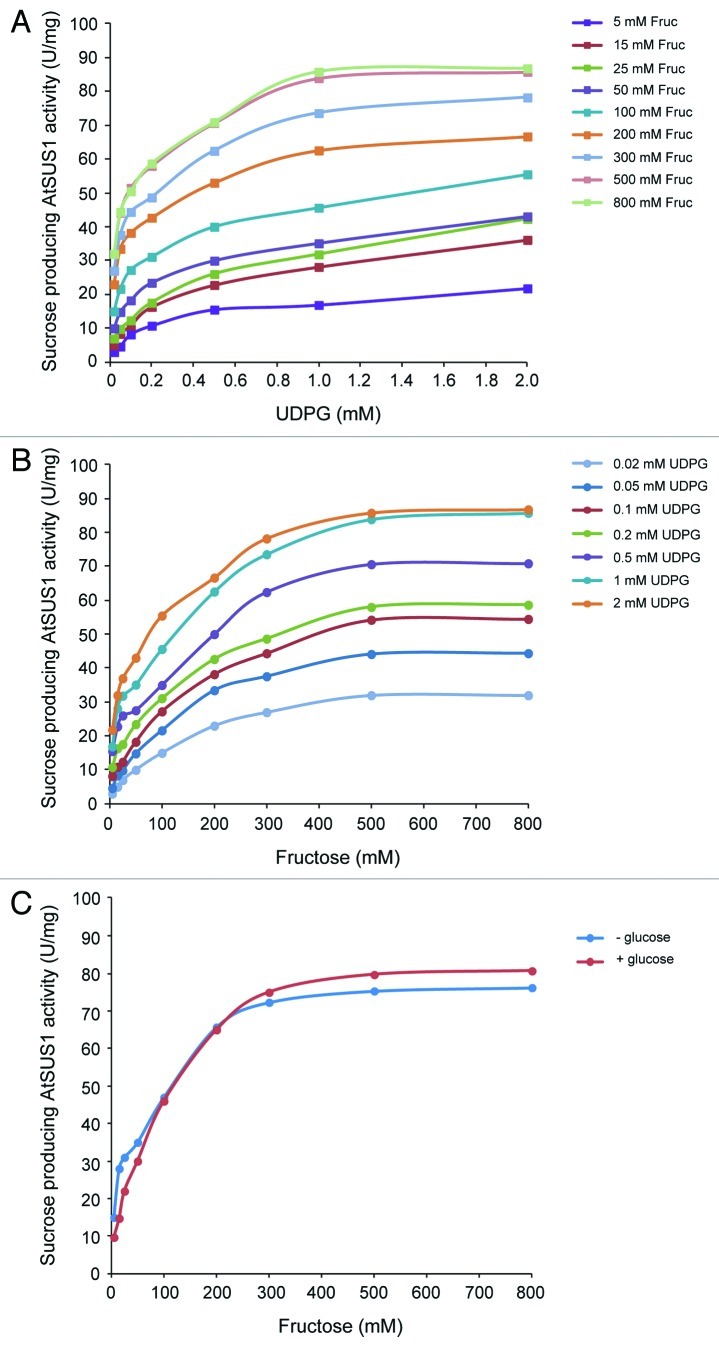
Kinetic studies of sucrose producing AtSUS1 activity revealed a typical Michaelis-Menten behavior with respect to UDPG (Fig. 1A) and fructose (Fig. 1B), with Km values of 50 μM and 25 mM, respectively, as calculated using Lineweaver-Burk plots. These analyses revealed no substrate inhibition of SuSy by high concentrations of UDPG or fructose. Also, we found that glucose did not exert any inhibitory effect on AtSUS1 (Fig. 1C). Essentially the same results were obtained using SuSy purified from developing maize endosperm (not shown). Although consistent with previous reports showing the absence of substrate inhibition of SuSy by fructose and UDPG,31-34 our results conflict with those of an earlier report20 obtained using a one-step continuous method for the enzymatic assay of UDP-producing SuSy in the presence of 3 mM MgCl2 at pH 9.5. Results included in this report20 showed inhibitory effect of UDPG, fructose and glucose on UDP-producing AtSUS1 activity, and pointed to the occurrence of an ordered kinetic mechanism where (1) UDPG binds to AtSUS1 first and UDP dissociates last, (2) fructose binds to the AtSUS1-UDP complex forming a dead-end ternary complex, and (3) glucose binds to the AtSUS1-UDP complex, probably at the site vacated by sucrose.
Figure 1. Kinetic studies of sucrose-producing activity of recombinant AtSUS1. (A) UDPG-dependent AtSUS1 activity was measured in the presence of different concentrations of fructose (5–800 mM). (B) Fructose-dependent AtSUS1 activity was measured in the presence of different concentrations of UDPG (0.02–2 mM). (C) AtSUS1 activity was measured in the presence or absence of 200 mM glucose, the indicated fructose concentrations and 1 mM UDPG. We define 1 unit (U) of enzyme activity as the amount of enzyme that catalyzes the production of 1 μmol of product per min.
Based on the kinetic properties obtained above, we measured maximum sucrose-producing SuSy activity in crude extracts from leaves and stems of WT and sus1/sus2/sus3/sus4 mutants using the above described two-step assay method for SuSy activity. These analyses revealed that total sucrose-producing SuSy activities in leaves and stems of WT plants were 119 ± 13 mU g FW−1 and 68 ± 4 mU g FW−1, respectively (Fig. 2), which are exceedingly higher than those shown in previous reports.20,21 Furthermore, consistent with our earlier studies of SuSy activity in the cleavage direction,22 we found that total sucrose-producing SuSy activities in the leaves and stems of sus1/sus2/sus3/sus4 mutants were 80–90% of those of WT plants (Fig. 2).
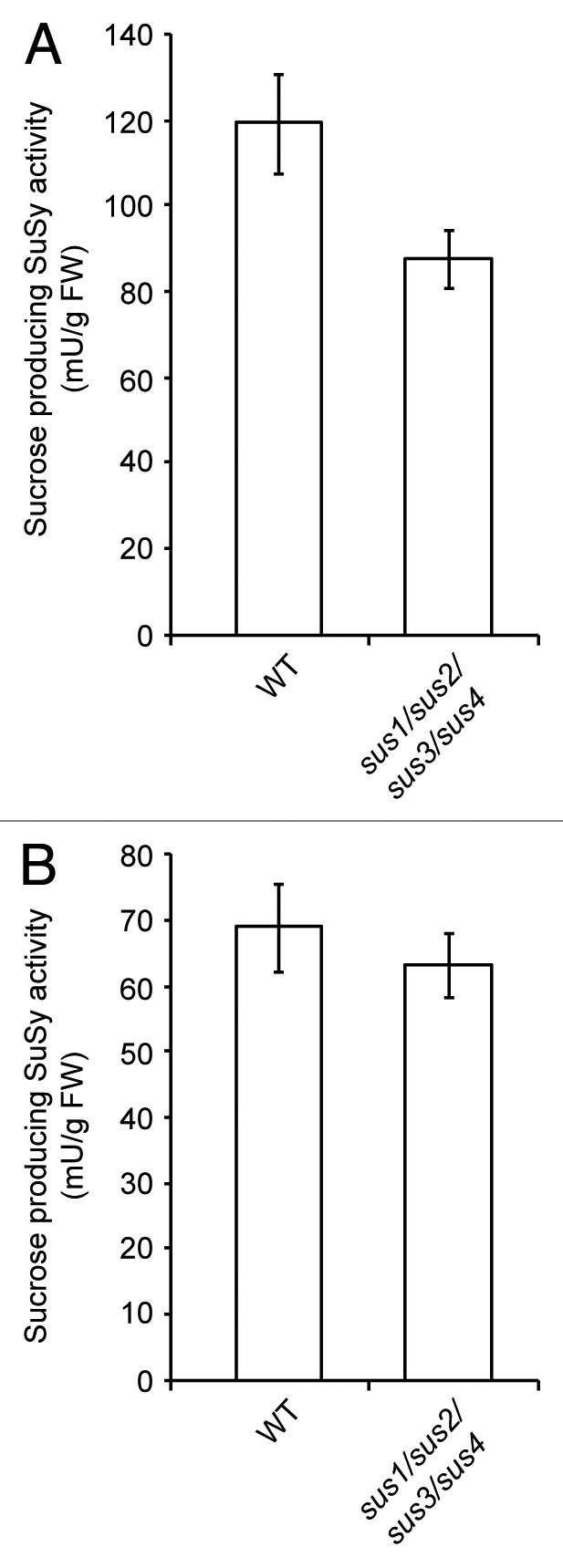
Figure 2. Sucrose-producing SuSy activity in crude extracts of (A) leaves and (B) stems of WT and sus1/sus2/sus3/sus4 Arabidopsis plants. SuSy activity was measured following the two-step assay method described in the main text. The SuSy reaction assay mixture of step 1 included 50 mM HEPES (pH 7.5), 3 mM MgCl2, 1 mM UDPG and 200 mM fructose. After 3 min at 25°C (still under initial velocity conditions), reactions were stopped by boiling the assay mixture for 1 min. Sucrose was then measured by HPLC with pulsed amperometric detection on a DX-500 system (Dionex) fitted to a CarboPac PA10 column. The results are the mean ± SD of three independent experiments.
Acknowledgments
This research was partially supported by the grant BIO2010-18239 from the Comisión Interministerial de Ciencia y Tecnología and Fondo Europeo de Desarrollo Regional (Spain) and by Iden Biotechnology S.L. G.A. acknowledges a fellowship from the Public University of Navarra.
Glossary
Abbreviations:
- ADPG
ADP-glucose
- SuSy
sucrose synthase
- U
unit of enzyme activity
- UDPG
UDP-glucose, WT, wild type
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20601
References
- 1.Fu H, Park WD. Sink- and vascular-associated sucrose synthase functions are encoded by different gene classes in potato. Plant Cell. 1995;7:1369–85. doi: 10.1105/tpc.7.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purcell PC, Smith AM, Halford NG. Antisense expression of a sucrose non-fermenting-1-related protein kinase sequence in potato results in decreased expression of sucrose synthase in tubers and loss of sucrose-inducibility of sucrose synthase transcripts in leaves. Plant J. 1998;14:195–202. doi: 10.1046/j.1365-313X.1998.00108.x. [DOI] [Google Scholar]
- 3.Déjardin A, Sokolov LN, Kleczkowski LA. Sugar/osmoticum levels modulate differential abscisic acid-independent expression of two stress-responsive sucrose synthase genes in Arabidopsis. Biochem J. 1999;344:503–9. doi: 10.1042/0264-6021:3440503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciereszko I, Klezkowski LA. Glucose and mannose regulate the expression of a major sucrose synthase gene in Arabidopsis via hexokinase-dependent mechanisms. Plant Physiol Biochem. 2002;40:907–11. doi: 10.1016/S0981-9428(02)01452-3. [DOI] [Google Scholar]
- 5.Hardin SC, Winter H, Huber SC. Phosphorylation of the amino terminus of maize sucrose synthase in relation to membrane association and enzyme activity. Plant Physiol. 2004;134:1427–38. doi: 10.1104/pp.103.036780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chourey PS, Nelson OE. The enzymatic deficiency conditioned by the shrunken-1 mutations in maize. Biochem Genet. 1976;14:1041–55. doi: 10.1007/BF00485135. [DOI] [PubMed] [Google Scholar]
- 7.Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci U S A. 1995;92:9353–7. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zrenner R, Salanoubat M, Willmitzer L, Sonnewald U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.) Plant J. 1995;7:97–107. doi: 10.1046/j.1365-313X.1995.07010097.x. [DOI] [PubMed] [Google Scholar]
- 9.Chourey PS, Taliercio EW, Carlson SJ, Ruan YL. Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol Gen Genet. 1998;259:88–96. doi: 10.1007/s004380050792. [DOI] [PubMed] [Google Scholar]
- 10.Delmer DP, Haigler CH. The regulation of metabolic flux to cellulose, a major sink for carbon in plants. Metab Eng. 2002;4:22–8. doi: 10.1006/mben.2001.0206. [DOI] [PubMed] [Google Scholar]
- 11.Ruan Y-L, Llewellyn DJ, Furbank RT. Suppression of sucrose synthase gene expression represses cotton fiber cell initiation, elongation, and seed development. Plant Cell. 2003;15:952–64. doi: 10.1105/tpc.010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baroja-Ferńndez E, Muñoz FJ, Montero M, Etxeberria E, Sesma MT, Ovecka M, et al. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADPglucose and UDPglucose and total yield. Plant Cell Physiol. 2009;50:1651–62. doi: 10.1093/pcp/pcp108. [DOI] [PubMed] [Google Scholar]
- 13.Thévenot C, Simond-Côte E, Reyss A, Manicacci D, Trouverie J, Le Guilloux M, et al. QTLs for enzyme activities and soluble carbohydrates involved in starch accumulation during grain filling in maize. J Exp Bot. 2005;56:945–58. doi: 10.1093/jxb/eri087. [DOI] [PubMed] [Google Scholar]
- 14.Rong J, Pierce GJ, Waghmare VN, Rogers CJ, Desai A, Chee PW, et al. Genetic mapping and comparative analysis of seven mutants related to seed fiber development in cotton. Theor Appl Genet. 2005;111:1137–46. doi: 10.1007/s00122-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Guo W, Zhu H, Ruan Y-L, Zhang T. Overexpression of GhSusA1 increases plant biomass and improves cotton fiber yield and quality. Plant Biotechnol J. 2012;10:301–12. doi: 10.1111/j.1467-7652.2011.00662.x. [DOI] [PubMed] [Google Scholar]
- 16.Tang G-Q, Sturm A. Antisense repression of sucrose synthase in carrot (Daucus carota L.) affects growth rather than sucrose partitioning. Plant Mol Biol. 1999;41:465–79. doi: 10.1023/A:1006327606696. [DOI] [PubMed] [Google Scholar]
- 17.Baroja-Ferńndez E, Muñoz FJ, Zandueta-Criado A, Morán-Zorzano MT, Viale AM, Alonso-Casajús N, et al. Most of ADP x glucose linked to starch biosynthesis occurs outside the chloroplast in source leaves. Proc Natl Acad Sci U S A. 2004;101:13080–5. doi: 10.1073/pnas.0402883101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz FJ, Baroja-Ferńndez E, Morán-Zorzano MT, Viale AM, Etxeberria E, Alonso-Casajús N, et al. Sucrose synthase controls both intracellular ADP glucose levels and transitory starch biosynthesis in source leaves. Plant Cell Physiol. 2005;46:1366–76. doi: 10.1093/pcp/pci148. [DOI] [PubMed] [Google Scholar]
- 19.Baud S, Vaultier M-N, Rochat C. Structure and expression profile of the sucrose synthase multigene family in Arabidopsis. J Exp Bot. 2004;55:397–409. doi: 10.1093/jxb/erh047. [DOI] [PubMed] [Google Scholar]
- 20.Bieniawska Z, Paul Barratt DH, Garlick AP, Thole V, Kruger NJ, Martin C, et al. Analysis of the sucrose synthase gene family in Arabidopsis. Plant J. 2007;49:810–28. doi: 10.1111/j.1365-313X.2006.03011.x. [DOI] [PubMed] [Google Scholar]
- 21.Barratt DHP, Derbyshire P, Findlay K, Pike M, Wellner N, Lunn J, et al. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc Natl Acad Sci U S A. 2009;106:13124–9. doi: 10.1073/pnas.0900689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baroja-Ferńndez E, Muñoz FJ, Li J, Bahaji A, Almagro G, Montero M, et al. Sucrose synthase activity in the sus1/sus2/sus3/sus4 Arabidopsis mutant is sufficient to support normal cellulose and starch production. Proc Natl Acad Sci U S A. 2012;109:321–6. doi: 10.1073/pnas.1117099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angeles-Núñez JG, Tiessen A. Arabidopsis sucrose synthase 2 and 3 modulate metabolic homeostasis and direct carbon towards starch synthesis in developing seeds. Planta. 2010;232:701–18. doi: 10.1007/s00425-010-1207-9. [DOI] [PubMed] [Google Scholar]
- 24.Núñez JG, Kronenberger J, Wuillème S, Lepiniec L, Rochat C. Study of AtSUS2 localization in seeds reveals a strong association with plastids. Plant Cell Physiol. 2008;49:1621–6. doi: 10.1093/pcp/pcn117. [DOI] [PubMed] [Google Scholar]
- 25.Tanase K, Yamaki S. Purification and characterization of two sucrose synthase isoforms from Japanese pear fruit. Plant Cell Physiol. 2000;41:408–14. doi: 10.1093/pcp/41.4.408. [DOI] [PubMed] [Google Scholar]
- 26.Barratt DHP, Barber L, Kruger NJ, Smith AM, Wang TL, Martin C. Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol. 2001;127:655–64. doi: 10.1104/pp.010297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morell M, Copeland L. Sucrose synthase of soybean nodules. Plant Physiol. 1985;78:149–54. doi: 10.1104/pp.78.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matic S, Akerlund H-E, Everitt E, Widell S. Sucrose synthase isoforms in cultured tobacco cells. Plant Physiol Biochem. 2004;42:299–306. doi: 10.1016/j.plaphy.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Doehlert DC. Substrate inhibition of maize endosperm sucrose synthase by fructose and its interaction with glucose inhibition. Plant Sci. 1987;52:153–7. doi: 10.1016/0168-9452(87)90048-3. [DOI] [Google Scholar]
- 30.Grimes WJ, Jones BL, Albersheim P. Sucrose synthetase from Phaseolus aureus seedlings. J Biol Chem. 1970;245:188–97. [PubMed] [Google Scholar]
- 31.Slabnik E, Frydman RB, Cardini CE. Some Properties of Potato Tuber UDPGd-fructose-2-glucosyltransferase (E.C. 2.4.1.14) and UDPGd-fructose-6-phosphate-2-glucosyltransferase (E.C. 2.4.1.13) Plant Physiol. 1968;43:1063–8. doi: 10.1104/pp.43.7.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su J-C, Wu J-L, Yang C-L. Purification and Characterization of Sucrose Synthetase from the Shoot of Bamboo Leleba oldhami. Plant Physiol. 1977;60:17–21. doi: 10.1104/pp.60.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avigad G. Sucrose-uridine diphosphate gucosyltransferase from Jerusalem artichoke tubers. J Biol Chem. 1964;239:3613–8. [PubMed] [Google Scholar]
- 34.Nakamura M. The sucrose-synthesizing enzyme in the beans. Bull Agr Chem Soc Japan. 1959;23:398–405. doi: 10.1271/bbb1924.23.398. [DOI] [Google Scholar]