Abstract
Calcium-dependent protein kinases (CDPKs) constitute a large multigene family in various plant species. CDPKs have been shown to have important roles in various physiological processes, including plant growth and development and abiotic and biotic stress responses in plants. Functional analysis using gain-of-function and loss-of-function mutants has revealed the biological function of CDPKs in planta. Several CDPKs have been shown to be essential factors in abiotic stress tolerance, positively or negatively regulating stress tolerance by modulating ABA signaling and reducing the accumulation of reactive oxygen species (ROS). This review summarizes recent results describing the biological function of CDPKs that are involved in abiotic stress tolerance.
Keywords: abiotic stress, abscisic acid, calcium-dependent protein kinase, reactive oxygen species, tolerance
Plants have developed a network of signal transduction pathways to control their metabolism and to adapt to their environment. Among these pathways, calcium plays an important role as a messenger in various signal transduction pathways.1-3 In plants, several Ca2+ sensors or Ca2+-binding proteins recognize transient Ca2+ elevation in response to various stimuli, including hormones, pathogens, light and abiotic stresses, and induce downstream effects, such as altered protein phosphorylation and gene expression patterns.1,4,5 Three major classes of Ca2+-binding proteins have been characterized in higher plants: calcium-dependent protein kinases (CDPKs), calmodulins (CaMs) and CaM-like proteins,6 and calcineurin B-like proteins.7-9
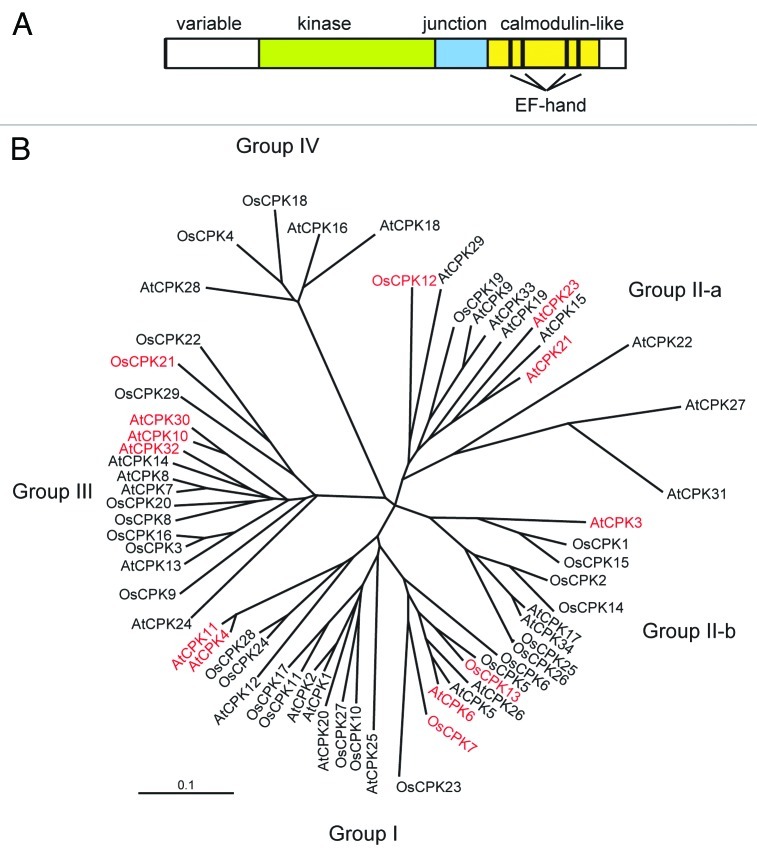
Each calcium-dependent protein kinase (CDPK) consists of a variable N-terminal domain, a protein kinase domain, an autoinhibitory region and a calmodulin-like domain with EF-hand Ca2+-binding sites.10-13 CDPKs are directly activated by the binding of Ca2+ to the calmodulin-like domain, and the activated CDPKs regulate downstream targets.14,15 CDPKs have been identified throughout the plant kingdom15,16 and in some protozoans,17 but not in animals.11,12,15 CDPKs constitute a large multigene family in various plant species;11,12,18,19 34 CDPK genes have been identified in Arabidopsis thaliana,11,12 and 29 CDPK genes have been found in Oryza sativa (rice)18 (Fig. 1). The expression and activities of CDPKs are upregulated by a variety of stimuli, such as hormones, abiotic stresses and biotic stresses.16 Furthermore, CDPKs show widespread subcellular distribution, including the plasma membrane, cytosol, nucleus, endoplasmic reticulum, peroxisomes, mitochondrial outer membrane, and oil bodies.14,20 These results suggest that CDPKs are involved in many physiological processes in various cellular compartments. In this review, we focus on recent finding describing the molecular mechanisms by which CDPKs modulate tolerance to abiotic stress in plants.
Figure 1. (A) Structure of CDPK. The junction contains an autoinhibitory sequence that blocks the active site of the CDPK under basal state. The four bars within the calmodulin-like domain indicate the EF-hand Ca2+ binding motif. (B) Phylogenetic relationships among CDPKs from rice (OsCPK1-OsCPK29) and Arabidopsis (AtCPK1-AtCPK34). Red letters indicate CDPKs involved in abiotic stress signaling.
The Molecular Functions of CDPKs in ABA and Abiotic Stress Responses
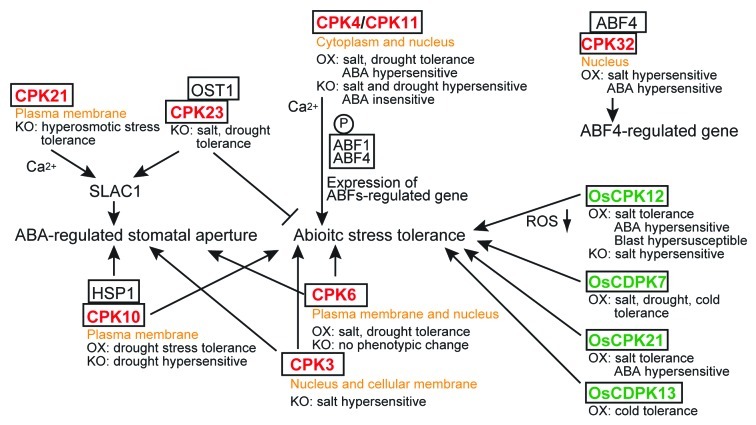
The phytohormone abscisic acid (ABA) plays important roles in tolerance to abiotic stress in plants.21-23 Some Arabidopsis CDPKs have been reported to be involved in abiotic and ABA signaling pathways. CPK3 and CPK6 were shown to be involved in the regulation of guard cell ion channels and in ABA-regulated stomatal signaling in experiments using Arabidopsis loss-of-function mutants. These mutants maintained normal ABA response during seed germination or early seedling growth.24 Later, CPK3 and CPK6 were reported to be positive regulators of the abiotic stress response. cpk3 mutants have a salt-sensitive phenotype. CPK3 is found to be associated with the plasma membrane and the vacuole. CPK3 is likely to regulate abiotic stress signaling independently of the MAPK-mediated signaling pathway.25 Meanwhile, CPK6-overexpressing plants have enhanced tolerance to salt and drought stresses, whereas cpk6 mutants have no obvious phenotypes. CPK6 is likely to act as a positive regulator of the tolerance to salt and drought stresses, but CPK6 is functionally redundant in abiotic stress signaling in Arabidopsis.26 Arabidopsis CPK10 is involved in tolerance to drought stress. Furthermore, cpk10 mutants exhibit impaired induction of stomatal closure and inhibition of stomatal opening in response to ABA and Ca2+.27 Therefore, CPK10 may play important roles in the ABA- and Ca2+-mediated regulation of stomatal movements.
Arabidopsis CDPKs are reported to be involved in the ABA signaling pathway by phosphorylating ABA-responsive element binding factors (ABFs), a subfamily of basic leucine zipper class transcription factor proteins. The closely related Arabidopsis CPK4 and CPK11 are involved in ABA-regulated physiological processes, including seed germination, seedling growth, stomatal movement, and tolerance to salt and drought stresses. CPK4 and CPK11 phosphorylate two ABA-responsive transcription factors, ABF1 and ABF4,28 indicating that they are positive regulators in the CDPK-mediated ABA signaling pathway. Arabidopsis CPK32 interacts with ABF4 and phosphorylates ABF4 in vitro. The overexpression of CPK32 in plants affects the expression of ABF4 regulated genes, the ABA response, and salt sensitivity. Thus, CPK32 participates in ABA and stress responses by regulating the ABA-responsive gene expression via ABF4.29 The Arabidopsis cpk23 mutant shows markedly enhanced tolerance to drought and salt stresses and reduced stomatal apertures, while CPK23 overexpressing lines are more sensitive to drought and salt stresses and have increased stomatal apertures.30 Arabidopsis seedlings with a loss-of-function of CPK21, which is the closest homolog of CPK23, show increased tolerance to hyperosmotic stress. In contrast, the salt tolerance of the cpk21 mutants is similar to that of wild-type plants.31 The closely related CPK21 and CPK23 proteins have partially overlapping functions in ABA and abiotic stress signaling and appear to act as negative regulators of the plant abiotic stress response. Independently, using Xenopus laevis oocytes and Arabidopsis protoplast systems, CPK21 and CPK23 were reported to control the activation state of the slow guard cell anion channel SLAC1 in response to different Ca2+ concentrations as well as in a Ca2+-independent manner. Open stomata 1 protein kinase (OST1)/CPK23 and ABA-INSENSITIVE1 (ABI1) seem to be associated with the calcium-independent steps of ABA signaling, whereas CPK21/ABI1 regulates SLAC1 in response to cytosolic calcium signaling.32 Thus, these contradictory results may be attributed to differences in the experimental systems used. This observation suggests that a complex CDPK-mediated signaling pathway functions in the ABA and abiotic stress responses in plants, and it is clear that several additional factors function in concert with CPK21/CPK23 in this signaling pathway.
In contrast to Arabidopsis CDPKs, the activities of rice CDPKs in abiotic stress and ABA signaling are largely unknown. The overexpression of OsCDPK7 in rice has been shown to enhance tolerance to cold, salt and drought stresses33; meanwhile, the overexpression of OsCDPK13 in rice has been shown to increase cold stress tolerance.34 The overexpression of OsCPK21 in rice has been shown to increase tolerance to salt stress and sensitivity to ABA. Therefore, OsCPK21 is likely to be involved in the positive regulation of ABA and salt signaling pathways.35
Abiotic Stress Tolerance is Mediated through the Decreased Accumulation of Reactive Oxygen Species
Abiotic stress induces the accumulation of reactive oxygen species (ROS).36-38 ROS are toxic molecules that cause oxidative damage, although some ROS function as signal transduction molecules in many biological processes.36,37,39,40 Plants have evolved defense systems to minimize and/or prevent oxidative damage to cells by ROS and to maintain cellular redox homeostasis under abiotic stress conditions. Various studies have shown that ROS-scavenging enzymes such as ascorbate peroxidase (APx), superoxide dismutase, catalase, and glutathione peroxidase are involved in tolerance to abiotic stress in plants.38,41-45
NADPH oxidase is reported to be involved in ABA-induced ROS production and ABA-induced stomatal closure.46 In addition, ROS produced by NADPH oxidase plays a central role in the oxidative burst and is responsible for triggering defense responses in plants. Solanum tuberosum (potato) CDPK4 and CDPK5 were reported to regulate ROS production by phosphorylating NADPH oxidase.47 Arabidopsis CPK5/CPK6 and CPK4/CPK11 also appear to regulate ROS production.48 Thus, CDPKs seem to function as positive regulators of ROS production in biotic stress signaling. The rice CDPK OsCPK12 is reported to be an essential positive regulator of tolerance to salt stress.49 In contrast to the abovementioned potato and Arabidopsis CDPKs, OsCPK12 is thought to promote tolerance to salt stress by reducing the accumulation of ROS. The overexpression of OsCPK12 confers increased tolerance to salt stress and decreased the accumulation of hydrogen peroxide (H2O2) in planta under high salinity conditions. Genes encoding ROS-scavenging enzymes (OsAPx2 and OsAPx8) were more highly expressed in OsCPK12-overexpressing plants than in wild-type plants, whereas the expression of the NADPH oxidase gene OsrbohI was decreased in OsCPK12-overexpressing plants under high salinity conditions. These results suggest that OsCPK12 positively regulates ROS detoxification by controlling the expression of OsAPx2, OsAPx8 and OsrbohI. In addition, OsCPK12 overexpression confers increased sensitivity to exogenous ABA and enhanced susceptibility to blast fungus, most likely due to the repression of ROS production and/or the involvement of OsCPK12 in the ABA signaling pathway. Collectively, these data indicate that OsCPK12 functions in multiple signaling pathways and inversely modulates salt stress tolerance and blast disease resistance.
Conclusions and Perspectives
Several CDPKs have been shown to be involved in abiotic stress tolerance in various plants, and these proteins have been especially well characterized in Arabidopsis. CDPKs appear to have important roles in mediating tolerance to abiotic stress through their involvement in ABA signaling and ROS detoxification. In addition, the CDPKs are localized to various compartments in plant cells. These data suggest that CDPKs may have multiple functions that enhance tolerance to abiotic stress. Although several target proteins, that interact with CDPKs and/or are phosphorylated by CDPKs, have been identified, the CDPK-mediated signaling pathways underlying the response to ABA and abiotic stress remain largely unknown. Furthermore, opposing functions for CPK21 and CPK23 in ABA signaling and abiotic stress signaling have been reported. This suggests that the system regulating CDPK-mediated ABA and abiotic stress responses is likely complex. Further physiological and biological analyses are necessary to elucidate the functions of CDPKs in abiotic stress responses in more detail. Understanding the mechanism by which increased cytosolic Ca2+ triggers the abiotic stress response and identifying additional components that act downstream of CDPKs or cooperate with CDPKs in abiotic stress signaling will facilitate the resolution of the complex regulatory networks involved in CDPK-mediated ABA and abiotic stress signaling.
Figure 2. Summary of the function of CDPKs in ABA and abiotic stress responses, as reported by several authors.20,24-35,49,50 The subcellular localization of each CDPK and the phenotypes of overexpression (OX) or knockout or knockdown (KO) lines are described. Red letters, Arabidopsis CDPKs; green letters, rice CDPKs; P, phosphorylation; ABF, ABA-responsive element binding factor; HSP1, a heat shock protein; OST1, open stomata 1 protein kinase.
Acknowledgments
This work was supported by grants from the Japan Society for the Promotion of Science (Grant-in-Aid No. 17780013 for Young Scientists to T.A.) and the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN to R.O.).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20351
References
- 1.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14(Suppl):S401–17. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudla J, Batistic O, Hashimoto K. Calcium signals: the lead currency of plant information processing. Plant Cell. 2010;22:541–63. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 4.Evans NH, McAinsh MR, Hetherington AM. Calcium oscillations in higher plants. Curr Opin Plant Biol. 2001;4:415–20. doi: 10.1016/S1369-5266(00)00194-1. [DOI] [PubMed] [Google Scholar]
- 5.Knight H, Knight MR. Abiotic stress signalling pathways: specificity and cross-talk. Trends Plant Sci. 2001;6:262–7. doi: 10.1016/S1360-1385(01)01946-X. [DOI] [PubMed] [Google Scholar]
- 6.McCormack E, Tsai YC, Braam J. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 2005;10:383–9. doi: 10.1016/j.tplants.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Kolukisaoglu U, Weinl S, Blazevic D, Batistic O, Kudla J. Calcium sensors and their interacting protein kinases: genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol. 2004;134:43–58. doi: 10.1104/pp.103.033068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luan S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009;14:37–42. doi: 10.1016/j.tplants.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Weinl S, Kudla J. The CBL-CIPK Ca(2+)-decoding signaling network: function and perspectives. New Phytol. 2009;184:517–28. doi: 10.1111/j.1469-8137.2009.02938.x. [DOI] [PubMed] [Google Scholar]
- 10.Harper JF, Sussman MR, Schaller GE, Putnam-Evans C, Charbonneau H, Harmon AC. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science. 1991;252:951–4. doi: 10.1126/science.1852075. [DOI] [PubMed] [Google Scholar]
- 11.Cheng SH, Willmann MR, Chen HC, Sheen J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002;129:469–85. doi: 10.1104/pp.005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hrabak EM, Chan CWM, Gribskov M, Harper JF, Choi JH, Halford N, et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003;132:666–80. doi: 10.1104/pp.102.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harmon AC, Gribskov M, Harper JF. CDPKs—a kinase for every Ca2+ signal? Trends Plant Sci. 2000;5:154–9. doi: 10.1016/S1360-1385(00)01577-6. [DOI] [PubMed] [Google Scholar]
- 14.Harper JF, Breton G, Harmon A. Decoding Ca(2+) signals through plant protein kinases. Annu Rev Plant Biol. 2004;55:263–88. doi: 10.1146/annurev.arplant.55.031903.141627. [DOI] [PubMed] [Google Scholar]
- 15.Harper JF, Harmon A. Plants, symbiosis and parasites: a calcium signalling connection. Nat Rev Mol Cell Biol. 2005;6:555–66. doi: 10.1038/nrm1679. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig AA, Romeis T, Jones JDG. CDPK-mediated signalling pathways: specificity and cross-talk. J Exp Bot. 2004;55:181–8. doi: 10.1093/jxb/erh008. [DOI] [PubMed] [Google Scholar]
- 17.Ward P, Equinet L, Packer J, Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics. 2004;5:79. doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asano T, Tanaka N, Yang G, Hayashi N, Komatsu S. Genome-wide identification of the rice calcium-dependent protein kinase and its closely related kinase gene families: comprehensive analysis of the CDPKs gene family in rice. Plant Cell Physiol. 2005;46:356–66. doi: 10.1093/pcp/pci035. [DOI] [PubMed] [Google Scholar]
- 19.Li AL, Zhu YF, Tan XM, Wang X, Wei B, Guo HZ, et al. Evolutionary and functional study of the CDPK gene family in wheat (Triticum aestivum L.) Plant Mol Biol. 2008;66:429–43. doi: 10.1007/s11103-007-9281-5. [DOI] [PubMed] [Google Scholar]
- 20.Dammann C, Ichida A, Hong B, Romanowsky SM, Hrabak EM, Harmon AC, et al. Subcellular targeting of nine calcium-dependent protein kinase isoforms from Arabidopsis. Plant Physiol. 2003;132:1840–8. doi: 10.1104/pp.103.020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol. 2010;61:651–79. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 22.Finkelstein RR, Gampala SSL, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14(Suppl):S15–45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–73. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, Andreoli S, et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 2006;4:1749–62. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehlmer N, Wurzinger B, Stael S, Hofmann-Rodrigues D, Csaszar E, Pfister B, et al. The Ca2+-dependent protein kinase CPK3 is required for MAPK-independent salt-stress acclimation in Arabidopsis. Plant J. 2010;63:484–98. doi: 10.1111/j.1365-313X.2010.04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Tian YS, Peng RH, Xiong AS, Zhu B, Jin XF, et al. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta. 2010;231:1251–60. doi: 10.1007/s00425-010-1122-0. [DOI] [PubMed] [Google Scholar]
- 27.Zou JJ, Wei FJ, Wang C, Wu JJ, Ratnasekera D, Liu WX, et al. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 2010;154:1232–43. doi: 10.1104/pp.110.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu SY, Yu XC, Wang XJ, Zhao R, Li Y, Fan RC, et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell. 2007;19:3019–36. doi: 10.1105/tpc.107.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi HI, Park HJ, Park JH, Kim S, Im MY, Seo HH, et al. Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant Physiol. 2005;139:1750–61. doi: 10.1104/pp.105.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma SY, Wu WH. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol Biol. 2007;65:511–8. doi: 10.1007/s11103-007-9187-2. [DOI] [PubMed] [Google Scholar]
- 31.Franz S, Ehlert B, Liese A, Kurth J, CazalA(c) AC, Romeis T. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol Plant. 2011;4:83–96. doi: 10.1093/mp/ssq064. [DOI] [PubMed] [Google Scholar]
- 32.Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci U S A. 2010;107:8023–8. doi: 10.1073/pnas.0912030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000;23:319–27. doi: 10.1046/j.1365-313x.2000.00787.x. [DOI] [PubMed] [Google Scholar]
- 34.Abbasi F, Onodera H, Toki S, Tanaka H, Komatsu S. OsCDPK13, a calcium-dependent protein kinase gene from rice, is induced by cold and gibberellin in rice leaf sheath. Plant Mol Biol. 2004;55:541–52. doi: 10.1007/s11103-004-1178-y. [DOI] [PubMed] [Google Scholar]
- 35.Asano T, Hakata M, Nakamura H, Aoki N, Komatsu S, Ichikawa H, et al. Functional characterisation of OsCPK21, a calcium-dependent protein kinase that confers salt tolerance in rice. Plant Mol Biol. 2011;75:179–91. doi: 10.1007/s11103-010-9717-1. [DOI] [PubMed] [Google Scholar]
- 36.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 37.Miller G, Shulaev V, Mittler R. Reactive oxygen signaling and abiotic stress. Physiol Plant. 2008;133:481–9. doi: 10.1111/j.1399-3054.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- 38.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–67. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 39.Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–75. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Badawi GH, Kawano N, Yamauchi Y, Shimada E, Sasaki R, Kubo A, et al. Over-expression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol Plant. 2004;121:231–8. doi: 10.1111/j.0031-9317.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 42.Kotchoni SO, Kuhns C, Ditzer A, Kirch HH, Bartels D. Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant Cell Environ. 2006;29:1033–48. doi: 10.1111/j.1365-3040.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 43.Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 2007;144:1777–85. doi: 10.1104/pp.107.101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng MJ, Liu CW, Yiu JC. Enhanced tolerance to sulfur dioxide and salt stress of transgenic Chinese cabbage plants expressing both superoxide dismutase and catalase in chloroplasts. Plant Physiol Biochem. 2007;45:822–33. doi: 10.1016/j.plaphy.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H. A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell. 1999;11:1195–206. doi: 10.1105/tpc.11.7.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–33. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, et al. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell. 2007;19:1065–80. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, et al. Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature. 2010;464:418–22. doi: 10.1038/nature08794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asano T, Hayashi N, Kobayashi M, Aoki N, Miyao A, Mitsuhara I, et al. A rice calcium-dependent protein kinase OsCPK12 oppositely modulates salt-stress tolerance and blast disease resistance. Plant J. 2012;69:26–36. doi: 10.1111/j.1365-313X.2011.04766.x. [DOI] [PubMed] [Google Scholar]
- 50.Benetka W, Mehlmer N, Maurer-Stroh S, Sammer M, Koranda M, NeumA1/4ller R, et al. Experimental testing of predicted myristoylation targets involved in asymmetric cell division and calcium-dependent signalling. Cell Cycle. 2008;7:3709–19. doi: 10.4161/cc.7.23.7176. [DOI] [PubMed] [Google Scholar]