Abstract
Stress responses depend on the correct regulation of gene expression. The discovery that abiotic as well as biotic stresses can regulate miRNA levels, coupled with the identification and functional analyses of stress-associated genes as miRNA targets, provided clues about the vital role that several miRNAs may play in modulating plant resistance to stresses. Nitrogen availability seriously affects crops productivity and environment and the understanding of the miRNA-guided stress regulatory networks should provide new tools for the genetic improvement of nitrogen use efficiency of crops. A recent study revealed the potential role of a number of nitrate-responsive miRNAs in the maize adaptation to nitrate fluctuations. In particular, results obtained suggested that a nitrate depletion might regulate the expression of genes involved in the starvation adaptive response, by affecting the spatio-temporal expression patterns of specific miRNAs.
Keywords: ISH, miRNAs, nitrate sensing, nutritional stress
Nitrogen (N) is the main mineral element in plant tissues, and its availability to plant roots is often an important limitation for plant growth. It represents about 2–4% of total plant dry matter, but in some environmental conditions, it can oscillate between 0.03% and 7%.1 The limited bio-availability of N and the dependence of crop growth on this mineral have spawned a massive N-based fertilizer industry worldwide, with annual N-fertilizer consumption currently close to 80 × 1012 g N (International Fertiliser Industry Association, www.fertilizer.org/ifa/statistics.asp).2 The excessive application of N fertilizers has enormous environmental and economic costs3,4 and led to the selection of genotypes highly productive if grown with high nitrogen supply, but characterized by a low efficiency of uptake and use of this nutrient.5
For these reasons the knowledge of the physiological and molecular machinery underlying the plant’s response to low nitrogen inputs has become fundamental to identify and choose crop cultivars able to uptake and metabolize nitrogen more efficiently, being consequently more adaptable to low nutritional input conditions. In order to survive in a range of different nutritional soil conditions, plants have evolved different strategies for nitrogen acquisition, but, in general, in a typical agricultural soil, nitrate represents the main nitrogen absorbed form and its uptake is mediated by an active transport process through the root cells.6,7 The plant overall response to nitrate involves a number of molecular events leading to the fine tuning of the inducible nitrate uptake systems,8,9 but affecting also several developmental aspects, as for example the lateral roots development and growth.10-12
Indeed, besides its role as a nutrient, nitrate acts importantly as a signaling molecule regulating the expression of genes involved in growth and developmental processes through transcriptional and post-translational modifications.13-15 However, the molecular regulation of nitrate sensing and signaling pathways are still only partially unravelled. The recent discovery of microRNAs (miRNA) and small interfering RNAs (siRNAs) revealed another ubiquitous mode of post-transcriptional regulation.
miRNAs and Nutritional Stress
Mature miRNAs are small RNAs ~22 nucleotides long, deriving from genome coded RNA precursors (pri-miRNA) cleaved twice by the RNase III enzyme DICER-Like 1.16 miRNAs control gene expression at the post-transcriptional level in a sequence specific manner,17 and they play essential roles in the regulation of gene expression during plant development, and by ensuring normal growth in response to environmental changes, as well as in the process of adaptation to biotic and abiotic stresses. During abiotic stresses plants regulate expression of thousands of genes at both transcriptional and post-transcriptional levels. Sunkar et al. (2007)18 discovered miRNAs that are upregulated or downregulated by abiotic stresses, being therefore probably involved in the stress-responsive gene expression regulation and stress adaptation. The miR398 family, for instance, is involved in the response to oxidative stress, by finely regulating the expression of two closely related Cu/Zn-Superoxide Dismutase (SOD) genes, the cytosolic one (CSD1) and the chloroplast-localized (CSD2).19,20
Furthermore, further studies showed that miRNAs are also involved in the Arabidopsis adaptive response to sulfate,20,21 phosphate22-25 and nitrate starvation26 respectively. Sulfur is an important component of biological compounds27 and it is taken up mainly as inorganic sulfate (SO42-). To adapt to fluctuating availabilities of sulfate in soil plants have evolved a series of physiological and molecular changes to maintain the S homeostasis in their tissues. In Arabidopsis these processes involve also the miR395 which seems to play a role in coordinating changes in sulfate translocation and assimilation, by targeting both the ATP-sulfurylase (APS) and a low-affinity sulfate transporter (SULTR2;1).20,21
Phosphorus is a major constituent of several important cell components and the soil unavailability of this nutrient induces adaptive responses including changes in expression profiles of numerous genes. Phosphate starvation strongly induces the expression of the genes encoding miRNAs of the miR399 family, which are, then, rapidly turned off immediately after the phosphate addition.22 Functional studies indicated that miR399 affects phosphate translocation, and regulates a phosphate transporter, by repressing the UBC24 (ubiquitin E2 conjugase24), which is in turn involved in protein transporters degradations.25
As far as nitrate is concerned microarray analyses showed that target transcripts of miRNAs are regulated by nitrate and/or sucrose treatments in Arabidopsis roots,28 suggesting that post-transcriptional gene expression control by miRNAs can be a general mechanism integrating nutritional signals into developmental changes. More recently, miR393 and miR167 and their respective targets, AFB3 and ARF8 (positive regulators of auxin signaling), were shown to be part of a N-responsive regulatory network that control primary root architecture and lateral root initiation in Arabidopsis.26, 29 The key role of miRNAs in Arabidopsis development and nitrate response has been then confirmed also by Pant et al. (2009).30
miRNAs Tissue Localization
In light of the importance of miRNAs on the molecular regulation of cellular processes, reliable protocols have been developed aimed at measuring the relative amount of their transcripts and their tissue localization, in response to developmental or environmental cues. The different nature of miRNA structure has implications on technical aspects of protocols for miRNAs quantification or localization. In fact, miRNAs are short, have an heterogeneous GC content, share their sequence with their targets mRNAs and their immediate precursors (pre-miRNAs) and they can differ for a single base with respect to other miRNA family members.31 These features require fine tuning strategies compared with those used for mRNA.
A recent method was developed for miRNA in situ detection based on labeled extension on ultramer templates,32 but LNA (locked nucleic acid)33 oligonucleotides are still the most widely and successfully used method for both qPCR34 and in Situ hybridization (ISH).35 LNA is a modified oligonucleotide made of pentafuranose monomers with an additional 2′-C, 4′-C-oxymethylene linker. A critical issue concerning LNA based applications for miRNA profiling is the influence exerted by the number and position of LNA residues on the specificity of recognition. To deepen this phenomenon a study aimed at improving discrimination has been performed by You and co-authors.36
Recently, a promising new type of modified oligonucleotides consisting of a short chain of oligospermine conjugates blocks attached on the 5′-end has been introduced and termed ZNA (zip nucleic acids).37 Conjugated oligospermine are positively charged, conferring to ZNA oligonucleotides the ability to attract DNA species, that are negatively charged, thus decreasing the repulsion between the probe and the target strands, both of polyanionic nature.37 Recently, the ZNA-modified oligonucleotides have been tested for their potential use in in situ hybridization applications, for miRNA localization in maize roots.38 Results obtained show that ZNA oligonucleotides may represent an alternative tool to LNA for the in situ detection of miRNA expression in plant, and probably also in human or animal tissues.
All the three methods mentioned use probes labeled with digoxigenin (DIG) that is subsequently antibody detected. The quality of signal obtained is depending on several factors including the quantity of incorporated DIG in the probe. For standard ISH, DIG-labeled nucleotides are incorporated on average every 13 nt, which are recognized by anti-DIG antibodies conjugated to alkaline phosphatase, enhancing signals. For LNA probes it is possible to label 3′-end or 5′-end with one DIG molecule. In addition it is also possible to label both 3′ and 5′-end, that is equivalent to 1 DIG every ~11 nt. For ZNA probes, at the moment only one DIG modification can be added to the 5′ end of the probe, resulting in just one DIG molecule hybridizing to each target miRNA per probe (1 DIG every ~22 nt), which may results in lowering the sensitivity of probe detection. Nevertheless, in our study a detectable hybridization signal, comparable to that measured with LNA probes, was obtained for all miRNA analyzed (Fig. 1).
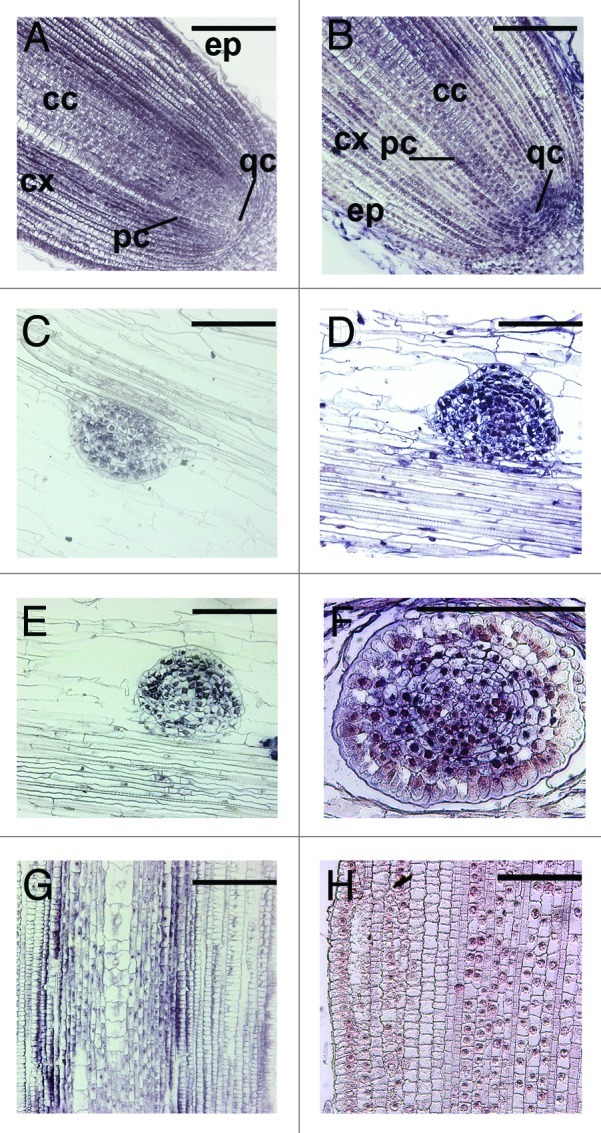
Figure 1. Comparison of in situ hybridization signal detections of zma-miR528a/b obtained with LNA (A, C, E and G) or ZNA (B, D, F and H) 5′ end DIG labeled probes. Longitudinal sections of maize root apices (A and B), longitudinal sections of maize lateral roots primordia (C–F) and longitudinal sections of maize root differentiated zones (G and H) are reported. Six slides (including at least five sections each) per probe concentration (at least two probe concentrations were tested for each hybridization combination) per experimental condition were performed in order to obtain reliable hybridization signal. Among all the concentrations tested, the one corresponding to 20 nM was showed (cc, central cylinder; qc, quiescent center; pc, pericycle; ep, epidermis; cx, cortex). Scale bars 200 μm.
By setting up an optimized protocol, we showed that ZNA probes can be used as cost effective tools for detecting abundant and medium abundant microRNAs in Zea mays paraffin embedded root sections. This protocol, being based on the use of oligonucleotides does not require probe synthesis and may thus be used at high-throughput for systematic studies aimed at miRNA profiling in plants.
Maize Nitrate-Responsive miRNAs
Nitrate availability seriously affects crops productivity and environment. The understanding of the miRNA-mediated molecular control of plant response to nitrate is essential to improve nitrogen use efficiency of crops and will provide new knowledge relevant for genetic modification of plant stress tolerance.39,40
In a our recent paper,38 a Zea mays miRNAs-microarray platform was used to identify six mature miRNAs putatively involved in the maize root response to nitrate. Concurrently, a parallel study41 reported a list of nitrate regulated miRNAs, partially overlapping with those identified in our research. qPCR and ISH results then pointed out that the response to nitrate starvation may be at least partially regulated post-transcriptionally through the downregulation of specific miRNAs. In particular, the repression of the transcription of miR528a/b, miR528a*/b*, miR169i/j/k, miR169i*/j*/k*, miR166j/k/n and miR408/b upon nitrate shortage could represent a crucial step integrating nitrate signals into developmental changes in maize roots. Indeed, most of the investigated miRNAs appear to accumulate in a highly specific manner in the root tip and LRP, which represent tissues actively dividing and differentiating and strongly decrease the amount detected in tissues where the developmental process has been completed, suggesting that these miRNAs may be crucial for tissue-fate establishment and differentiation.
Moreover ISH revealed that the presence of nitrate has a considerable influence on both the amount and the localization of the miRNAs investigated (Fig. 2). Our data suggest a predominant localization of miR169i/j/k at the vasculature level, as also evidenced by Li et al.42 in Arabidopsis and support the already hypothesized role of miR169 family members as novel candidates for long-distance signaling of the N status in the plant system.30,42 An analog function could be hypothesized for miR166j/k/n which evidenced a similar distribution among tissues, even if it displayed a different regulation of expression in response to the nitrate supply. The importance of these two families of miRNAs for vascular tissue differentiation in response to nitrogen was also supported by the results obtained from both the miRNA’s target prediction and the analysis of cis-elements in their promoter.
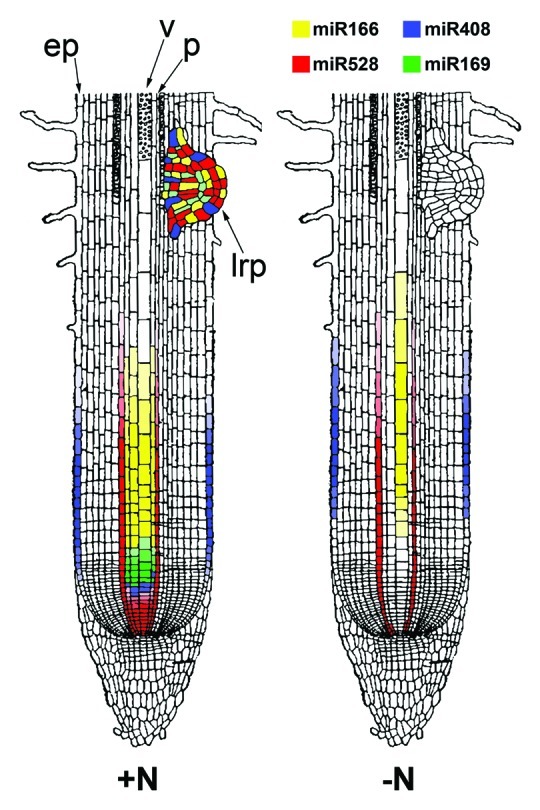
Figure 2. A graphic representation of localization of N-responsive miRNAs in a longitudinal section of both nitrate-supplied (+N) and nitrate-depleted (-N) maize roots. The apical meristem and the root cap of N-supplied seedlings are characterized by the strong expression of miR528a/b, while the vascular tissue is the site of expression of miRNA166j/k/n and miRNA169i/j/k. MiR408/b and miR528a/b were found in the epidermis cells of N-supplied apical meristem. Lateral roots are interested by the localization of all the four miRNAs showed. In general, hybridization signals became weaker in nitrate-depleted roots for all miRNAs investigated. Probe accumulations were not found in apical meristems or in lateral root primordia of N-depleted roots (ep, epidermis; v, vascular tissue; p, perycicle; lrp, lateral root primordia). Color codes of miRNAs are also indicated.
MiR408/b and miR528a/b besides localizing in the vasculature of N-supplied roots, were preferentially expressed in root tips, in epidermal cells of primary root and in LRP and were similarly regulated in response to nitrate provision. Basing also on their localization we have hypothesized that both of them could take part in the adjustment of root architecture in response to nitrate. Moreover, the results of the prediction of their putative targets led us to hypothesize that this transduction pathway could be mediated by the ROS signaling. A link between nutrient deprivation sensing and ROS accumulation has been already demonstrated by Schachtman and Shin.43 This preliminary study revealed the potential role of nitrate-responsive miRNAs in the metabolic, physiological and morphological adjustments achieved by maize seedling to adapt to the nitrate starvation.
Conclusions
The nitrogen deficiency represents a serious threat to agriculture and the scientific community should give high priority to breeding programs for nutrient stress tolerance. The molecular control of nutrient stress tolerance engages the regulation of specific genes involved in the whole sequence of biological processes underlying the stress response.44 Together with the knowledge of stress-responsive genes, a better understanding of the role of miRNAs during nitrate response will contribute to delineate the strategies aimed at improving nitrogen use efficiency in crop plants. Since single miRNAs could regulate at the same time the expression of more than one gene implicated in the physiological adaptation to the low nitrogen input, they represent excellent candidate targets for the improvement of the tolerance to nutritional deficiencies.
Acknowledgments
This research was supported by Progetto di Ateneo, Università di Padova, 2008—prot. CPDA088137 and ex-60% 2010 and by European Project “AUTOSCREEN” (LSHG-CT-2007-037897)
Glossary
Abbreviations:
- DIG
digoxigenin
- N
nitrogen
- nt
nucleotide
- ISH
In situ hybridization
- LRP
lateral root primordia
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20462
References
- 1.Marschner H, Kirby H, Engels EA. Importance of cycling and recycling of mineral nutrients within plants for growth and develpment. Bot Acta. 1997;110:265–73. [Google Scholar]
- 2.Miller AJ, Cramer MD. Root nitrogen acquisition and assimilation. Plant Soil. 2004;274:1–36. doi: 10.1007/s11104-004-0965-1. [DOI] [Google Scholar]
- 3.Frink CR, Waggoner PE, Ausubel JH. Nitrogen fertilizer: retrospect and prospect. Proc Natl Acad Sci U S A. 1999;96:1175–80. doi: 10.1073/pnas.96.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth W, Cowling EB, et al. The nitrogen cascade. Bioscience. 2003;53:341–55. doi: 10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2. [DOI] [Google Scholar]
- 5.Hirel B, Bertin P, Quilleré I, Bourdoncle W, Attagnant C, Dellay C, et al. Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 2001;125:1258–70. doi: 10.1104/pp.125.3.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford NM, Glass ADM. Molecular and physiological aspects of nitrate uptake in plants. Plant Sci. 1998;10:389–95. [Google Scholar]
- 7.Hirsch RE, Sussman MR. Improving nutrient capture from soil by the genetic manipulation of crop plants. Trends Biotechnol. 1999;17:356–61. doi: 10.1016/S0167-7799(99)01332-3. [DOI] [PubMed] [Google Scholar]
- 8.Krouk G, Tillard P, Gojon A. Regulation of the high-affinity NO3- uptake system by NRT1.1-mediated NO3- demand signaling in Arabidopsis. Plant Physiol. 2006;142:1075–86. doi: 10.1104/pp.106.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM. Nitrate transport and signalling. J Exp Bot. 2007;58:2297–306. doi: 10.1093/jxb/erm066. [DOI] [PubMed] [Google Scholar]
- 10.Forde BG. Local and long-range signaling pathways regulating plant responses to nitrate. Annu Rev Plant Biol. 2002;53:203–24. doi: 10.1146/annurev.arplant.53.100301.135256. [DOI] [PubMed] [Google Scholar]
- 11.Walch-Liu P, Ivanov II, Filleur S, Gan Y, Remans T, Forde BG. Nitrogen regulation of root branching. Ann Bot. 2006;97:875–81. doi: 10.1093/aob/mcj601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remans T, Nacry P, Pervent M, Girin T, Tillard P, Lepetit M, et al. A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol. 2006;140:909–21. doi: 10.1104/pp.105.075721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palenchar PM, Kouranov A, Lejay LV, Coruzzi GM. Genome-wide patterns of carbon and nitrogen regulation of gene expression validate the combined carbon and nitrogen (CN)-signaling hypothesis in plants. Genome Biol. 2004;5:R91. doi: 10.1186/gb-2004-5-11-r91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R, Tischner R, Gutiérrez RA, Hoffman M, Xing X, Chen M, et al. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol. 2004;136:2512–22. doi: 10.1104/pp.104.044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu KH, Tsay YF. Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J. 2003;22:1005–13. doi: 10.1093/emboj/cdg118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 17.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 18.Sunkar R, Chinnusamy V, Zhu J, Zhu JK. Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci. 2007;12:301–9. doi: 10.1016/j.tplants.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet E, Wuyts J, Rouzé P, Van de Peer Y. Detection of 91 potential conserved plant microRNAs in Arabidopsis thaliana and Oryza sativa identifies important target genes. Proc Natl Acad Sci U S A. 2004;101:11511–6. doi: 10.1073/pnas.0404025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones-Rhoades MW, Bartel DP. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell. 2004;14:787–99. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Kawashima CG, Yoshimoto N, Maruyama-Nakashita A, Tsuchiya YN, Saito K, Takahashi H, et al. Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J. 2009;57:313–21. doi: 10.1111/j.1365-313X.2008.03690.x. [DOI] [PubMed] [Google Scholar]
- 22.Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol. 2005;15:2038–43. doi: 10.1016/j.cub.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ. pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol. 2006;141:1000–11. doi: 10.1104/pp.106.078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bari R, Datt Pant B, Stitt M, Scheible WR. PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 2006;141:988–99. doi: 10.1104/pp.106.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiou TJ. The role of microRNAs in sensing nutrient stress. Plant Cell Environ. 2007;30:323–32. doi: 10.1111/j.1365-3040.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 26.Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD. Cell-specific nitrogen responses mediate developmental plasticity. Proc Natl Acad Sci U S A. 2008;105:803–8. doi: 10.1073/pnas.0709559105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leustek T. Sulfate metabolism. In: The Arabidopsis Book. Somerville CR, Meyerowitz EM (eds). The American Society of Plant Biologists, Rockville, MD, USA. 2002; doi: 10.1199/ tab.0017, www.aspb.org/publications/arabidopsis/ [DOI] [PMC free article] [PubMed]
- 28.Gutiérrez RA, Gifford ML, Poultney C, Wang R, Shasha DE, Coruzzi GM, et al. Insights into the genomic nitrate response using genetics and the Sungear Software System. J Exp Bot. 2007;58:2359–67. doi: 10.1093/jxb/erm079. [DOI] [PubMed] [Google Scholar]
- 29.Vidal EA, Araus V, Lu C, Parry G, Green PJ, Coruzzi GM, et al. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2010;107:4477–82. doi: 10.1073/pnas.0909571107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pant BD, Musialak-Lange M, Nuc P, May P, Buhtz A, Kehr J, et al. Identification of nutrient-responsive Arabidopsis and rapeseed microRNAs by comprehensive real-time polymerase chain reaction profiling and small RNA sequencing. Plant Physiol. 2009;150:1541–55. doi: 10.1104/pp.109.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benes V, Castoldi M. Expression profiling of microRNA using real-time quantitative PCR, how to use it and what is available. Methods. 2010;50:244–9. doi: 10.1016/j.ymeth.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Nuovo G, Lee EJ, Lawler S, Godlewski J, Schmittgen T. In situ detection of mature microRNAs by labeled extension on ultramer templates. Biotechniques. 2009;46:115–26. doi: 10.2144/000113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh SK, Wengel J. Universality of LNA-mediated high-affinity nucleic acid recognition. Chem Commun (Camb) 1998:1247–8. doi: 10.1039/a801571f. [DOI] [Google Scholar]
- 34.Raymond CK, Roberts BS, Garrett-Engele P, Lim LP, Johnson JM. Simple, quantitative primer-extension PCR assay for direct monitoring of microRNAs and short-interfering RNAs. RNA. 2005;11:1737–44. doi: 10.1261/rna.2148705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kloosterman WP, Wienholds E, de Bruijn E, Kauppinen S, Plasterk RHA. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat Methods. 2006;3:27–9. doi: 10.1038/nmeth843. [DOI] [PubMed] [Google Scholar]
- 36.You Y, Moreira BG, Behlke MA, Owczarzy R. Design of LNA probes that improve mismatch discrimination. Nucleic Acids Res. 2006;34:e60. doi: 10.1093/nar/gkl175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noir R, Kotera M, Pons B, Remy J-S, Behr J-P. Oligonucleotide-oligospermine conjugates (zip nucleic acids): a convenient means of finely tuning hybridization temperatures. J Am Chem Soc. 2008;130:13500–5. doi: 10.1021/ja804727a. [DOI] [PubMed] [Google Scholar]
- 38.Trevisan S, Nonis A, Begheldo M, Manoli A, Palme K, Caporale G, et al. Expression and tissue-specific localization of nitrate-responsive miRNAs in roots of maize seedlings. Plant Cell Environ. 2011;34:1127–40. doi: 10.1111/j.1365-3040.2011.02478.x. [DOI] [PubMed] [Google Scholar]
- 39.Sreenivasulu N, Sopory SK, Kavi Kishor PB. Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene. 2007;388:1–13. doi: 10.1016/j.gene.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Mittler R, Blumwald E. Genetic engineering for modern agriculture: challenges and perspectives. Annu Rev Plant Biol. 2010;61:443–62. doi: 10.1146/annurev-arplant-042809-112116. [DOI] [PubMed] [Google Scholar]
- 41.Xu Z, Zhong S, Li X, Li W, Rothstein SJ, Zhang S, et al. Genome-wide identification of microRNAs in response to low nitrate availability in maize leaves and roots. PLoS One. 2011;6:e28009. doi: 10.1371/journal.pone.0028009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Fu Y, Ji L, Wu C, Zheng C. Characterization and expression analysis of the Arabidopsis mir169 family. Plant Sci. 2010;178:271–80. doi: 10.1016/j.plantsci.2010.01.007. [DOI] [Google Scholar]
- 43.Schachtman DP, Shin R. Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol. 2007;58:47–69. doi: 10.1146/annurev.arplant.58.032806.103750. [DOI] [PubMed] [Google Scholar]
- 44.Shukla LI, Chinnusamy V, Sunkar R. The role of microRNAs and other endogenous small RNAs in plant stress responses. Biochem Biophys Acta 2008; 1779:743-48. [DOI] [PubMed]


