Abstract
The plant SNF1-related kinase (SnRK1) is the α-subunit of the SnRK1 heterotrimeric compleses. Although SnRK1 is widely known as a key regulator of plant response to various physiological processes including nutrient- and energy-sensing, regulation of global metabolism, and control of cell cycle, development, as well as abiotics stress, less is known about the function of SnRK1 during pathogen infection. Our previous work has demonstrated that a tomato SNF1-related kinase (SlSnRK1) can interact with and phosphorylate βC1, a pathogenesis protein encoded by tomato yellow leaf curl China betasatellite. Our results also showed that the plant SnRK1 can affect genimivirus infection in plant and reduce viral DNA accumulation. Phosphorylation of βC1 protein negatively impacts its function as a pathogenicity determinant. Here we provide more information on interaction between βC1 and SlSnRK1 and propose a mechanistic model for the SlSnRK1-mediated defense responses against geminiviruses and the potential role of SnRK1 in plant resistance to geminivirus.
Keywords: SlSnRK1, geminivirus, phosphorylation, plant defense, protein interaction, βC1
SUCROSE NON-FERMENTING1 (SNF1) was initially identified in a mutant yeast (Saccharomyces cerevisiae) defective in derepressing the Glc-regulated genes and thus unable to grow on media with sugars other than Glc.1 The plant SNF1-related kinase (SnRK1) belongs to a conserved kinases family and consists of an α catalytic subunit, and a β and γ regulatory subunit. The plant SnRK1 also shares great homology with the mammalian AMP-activated protein kinase (AMPK).2,3 Yeast SNF1, mammalian AMPK and plant SnRK1 are all known to function in regulating carbon metabolism and energy balance in eukaryotes,3-8 and are metabolic sensors of Glc availability as well as the AMP to ATP ratios.4 Activity of the plant SnRK1 can be stimulated by metabolic stresses including sugar starvation and dark treatment.9 In addition, the SnRK1 can phosphorylate and negatively regulate key metabolic enzymes such as sucrose-phosphate synthase (SPS), 3-hydroxy-3-methylglutaryl-coenzyme A reductase, nitrate reductase and trehalose-6-phosphate synthase important for modulating plant metabolism.10-13 Baena-Gonzalez et al. indicated previously that the SnRK1 can act as a master regulator of global gene expression in plant grown under the starvation and energy deprivation conditions. They also indicated that many genes regulated by SnRK1 are involved in plant primary and secondary metabolisms.9 The plant SnRK1 is also known to play roles in development,14-19 and in regulation of essential signaling pathways through interacting with proteosome and ubiquitin ligase components.20 Although the SnRK1-mediated metabolic changes were considered to be important in plant defense against viruses,21 the molecular role of SnRK1 in plant innate defense systems is largely unknown.
Geminiviridae is a large family of viruses with circular, single-stranded DNA genomes. This virus family contains four genera, and Begomovirus is the largest genus, consisting more than 200 different species, which cause devastating diseases in many economically important crops world-wide.22,23 Begomoviruses have either monopartite or bipartite single-stranded DNA genomes.22,24 In recent years many monopartite begomoviruses have been identified to have a betasatellite molecule (e.g., a circular, single-stranded DNA molecule of approximately 1,350 nucleotides). The betasatellite molecule is essential component in induction of typical disease symptoms in plants.25-29 All known betasatellite molecules encode a single protein known as βC1. Many studies have identified the βC1 as the determinant of pathogenicity and suppressor of post-transcriptional gene silencing (PTGS).30-32 A previous study by Yang et al. showed that the βC1 of tomato yellow leaf curl China betasatellite (TYLCCNB) can interact with Arabidopsis ASYMMETRIC LEAVES1 to cause morphological changes in leaf, and suppress specific jasmonic acid responses.33 The βC1 of cotton leaf curl Multan betasatellite was shown to interact with a tomato ubiquitin-conjugating enzyme, SlUBC3 and this interaction is required for the βC1 pathogenesis.34 In addition, the βC1 protein can inhibit methylation-mediated transcriptional gene silencing (TGS) by interacting with and inactivating S-adenosyl homocysteine hydrolase (SAHH), a methyl cycle enzyme required for TGS.35
Through yeast two-hybrid screen using a tomato cDNA library, we recently identified a tomato SNF1-related kinase (SlSnRK1) as a novel protein that interacts with βC1. We also determined that the βC1-interaction site is located in a region having an internal Ubiquitin-Associated domain (UBA) and/or a self-regulating AIS domain of SlSnRK1. Interestingly the conserved Serine/Threonine protein kinases catalytic domain (S_TKc) is not involved in the binding with βC1 as previously described.36
The catalytic domain is the core region of protein kinases and it consists of two lobes: a smaller N-terminal lobe (N-lobe) and a larger C-terminal lobe (C-lobe). These two lobes form a cleft that serves as a docking site for ATP. An activation segment was reported to present in the C-lobe and it regulates the catalytic activity of many protein kinases through its phosphorylation, except those protein kinases that can form catalytically active conformations in the absence of the activation segment phosphorylation.37,38 More recently the SNF1 kinase found in the budding yeast was shown to be activated by three related but functionally redundant kinases (e.g., SAK1, TOS3 and ELM1).7,40,41 In animals the SNF1 homolog, AMP-activated protein kinase (AMPK), was reported to be activated by two upstream protein kinases known as LKB1 and CaMKKβ.7,42-47 In plants, two Arabidopsis kinases (e.g., GRIK1 and GRIK2) can activate the SnRK1.48-50 The activation of SNF1 kinase through phosphorylation may occur after a conformational change which leads to a change of the activation loop position and allows the access to the kinase active site. The active site of protein kinase is known to be highly conserved and can be exemplified by the well-characterized cyclin-dependent kinase and mitogen-activated protein kinase (MAPK) cascades. Certain protein kinases can be activated or inhibited by specific polypeptide cofactors. For example, cyclin can partially activate the cyclin-dependent kinase Cdk2 by binding to the C helix and orients the αC helix to form an active conformation.51 In contrast Src-homology SH2 and SH3 domains can inactivate Src tyrosine kinase by binding to the αC helix and an inhibitory p-tyrosine in the C-terminal tail, resulting in an inactive conformation.52 Many reports have indicated that some proteins produced by plant pathogens can alter functions of certain protein kinases. For example, the AL2 from tomato golden mosaic virus (TGMV; genus Begomovirus) and L2 from beet curly top virus (BCTV; genus Curtovirus) were shown to inhibit the activity of an SNF1-related kinase (SnRK) through protein-protein interactions.21 The NSP from cabbage leaf curl virus (CaLCuV) was also shown to inhibit protein kinase NIKs by binding to the putative active site within the NIK1 domain, and to the activation loop for Ser/Thr kinase. Binding to these two sites plays critical roles in both controlling the activity of protein kinases and substrate recognition.53 Phytophthora infestans INF1 was also shown to alter NbLRK1 kinase activity by binding to the VIb subdomain and then suppressing its autophosphorylation.54 These published information together with our previous results, showing that the βC1-interacting domain in the SlSnRK1 is not a kinase domain, suggest that interaction between βC1 and SlSnRK1 may not alter SlSnRK1’s kinase activity. To test this hypothesis, we performed yeast complementation assays and our results show that indeed βC1 does not inhibit the kinase activity of SlSnRK1 in yeast cells.36
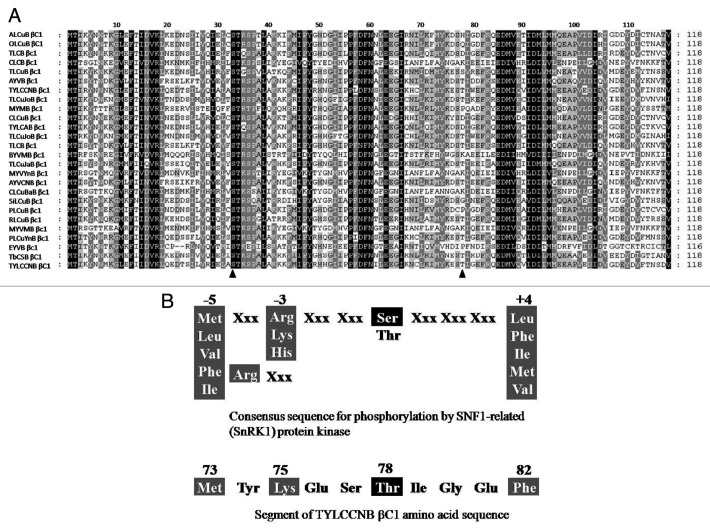
Furthermore, we used the NetPhos 2.0 server (www.cbs.dtu.dk/services/NetPhos/) to predict for the serine, threonine and tyrosine phosphorylation sites in the βC1 protein and our results show that Ser-33 and Thr-78 are likely the phosphorylation sites. Our amino acid sequence analysis revealed that the potential TYLCCNB βC1 phosphorylation site, Ser-33, is conserved among βC1 proteins encoded by different geminiviruses and the Thr-78 site is less conserved (Fig. 1A). The amino acid context of the βC1 Thr-78 (Fig. 1B) exhibits a substrate recognition motif similar to that reported for the SnRK1.55 Our results show that the interaction between the βC1 and SlSnRK1 leads to phosphorylation of the βC1 protein but does not inhibit the kinase activity of SlSnRK1. Results of our previous in vitro kinase assay also indicated that the SlSnRK1 protein could phosphorylate βC1 mainly on the threonine at position 78 and serine at position 33.36
Figure 1. Analyses of TYLCCNB βC1 amino acid sequence. (A) Ser-33 is conserved among the geminivirus βC1 proteins. Predicted βC1 amino acid sequences of Ageratum yellow leaf curl betasatellite (ALCuB) (AJ316027), okra leaf curl betasatellite (OLCuB) (AJ316031), tomato leaf curl betasatellite (TLCB) (AJ316036), cotton leaf curl betasatellite (CLCB) (AJ316038), tomato leaf curl betasatellite (TLCuB) (AJ542492), Ageratum yellow vein betasatellite (AYVB) (AJ542497), tomato yellow leaf curl China betasatellite (TYLCCNB) (AJ781301), tomato leaf curl Joydebpur betasatellite (TLCuJoB) (AJ966244), Malvastrum yellow mosaic betasatellite (MYMB) (AM236769), chilli leaf curl betasatellite (CLCuB) (AM258978), tomato yellow leaf curl associated betasatellite (TYLCAB) (DQ644567), tomato leaf curl Joydebpur betasatellite (TLCuJoB) (EF190216), tomato leaf curl betasatellite (TLCB) (EU286799), Bhendi yellow vein mosaic betasatellite (BYVMB) (NC_003405), tomato leaf curl Java betasatellite (TLCuJaB) (NC_005497), Malvastrum yellow vein Yunnan betasatellite (MYVYnB) (NC_006632), Ageratum yellow vein China betasatellite (AYVCNB) (NC_007067), cotton leaf curl Bangalore betasatellite (CLCuBaB) (NC_007219), Sida leaf curl betasatellite (SiLCuB) (NC_007639), pepper leaf curl betasatellite (PLCuB) (NC_010235), radish leaf curl betasatellite (RLCuB) (NC_010239), Malachra yellow vein mosaic betasatellite (MYVMB) (NC_010328), pepper leaf curl Yunnan betasatellite (PLCuYnB) (NC_010619), Emilia yellow vein betasatellite (EYVB) (NC_012666), tobacco curly shoot betasatellite (TbCSB) (AJ421484) and tomato yellow leaf curl China betasatellite (TYLCCNB) (AJ781299) were aligned using the Clustal method with PAM250 residue weight (DNASTAR Inc.). Shading indicates extent of conservation/similarity. The black triangle represents Ser-33 and Thr-78. (B) A recognition motif for SnRK1 and the amino acid context of βC1 potential phosphorylation sites.
We demonstrated previously that SnRK1 could impact geminivirus infection and viral DNA accumulation in plant and phosphorylation of the βC1 protein could delay geminivirus infection in plant, attenuate disease symptoms and reduce viral DNA accumulation.36 With our new findings and previously published results4,29,31,33,35,56 we propose a mechanistic model for the SlSnRK1-mediated defense response against geminiviruses (Fig. 2). This model shows that SlSnRK1 attenuates geminivirus infection by interacting with and phosphorylating the pathogenicity determinant βC1 protein. Future investigations are needed to determine the effect of phosphorylation on βC1 protein’s ability to suppress RNA silencing and/or methylation-mediated TGS. An investigation on βC1 protein degradation by the 26S proteasome may provide critical information to this model.
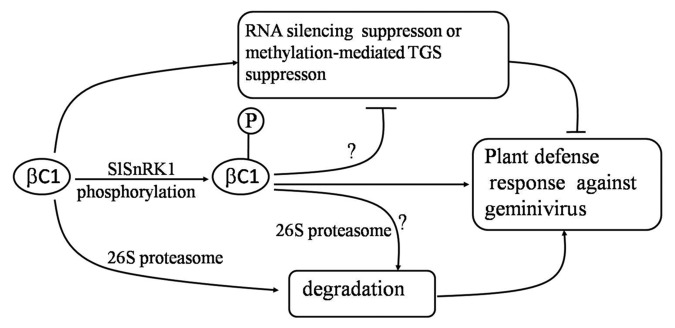
Figure 2. A proposed model for functions of SlSnRK1 in plant defense response against geminivirus. The SlSnRK1 interacts with and phosphorylates the βC1 protein, and phosphorylation of βC1 may negatively affect the βC1 function on RNA silencing suppression or methylation-mediated TGS suppression, which further leads to attenuation of genimivirus infection. Alternatively, SlSnRK1 may phosphorylate βC1 for degradation by the 26S proteasome which also leads to attenuation of disease symptoms and reduction of virus infection. Arrows represent activation and T lines represent repression.
Acknowledgments
This work was supported by the National Key Basic Research and Development Program of China (2012CB114004) and the National High Technology Research and Development Program of China (2012CB114004).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/20646
References
- 1.Celenza JL, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233:1175–80. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- 2.Le Guen L, Thomas M, Bianchi M, Halford NG, Kreis M. Structure and expression of a gene from Arabidopsis thaliana encoding a protein related to SNF1 protein kinase. Gene. 1992;120:249–54. doi: 10.1016/0378-1119(92)90100-4. [DOI] [PubMed] [Google Scholar]
- 3.Polge C, Thomas M. SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends Plant Sci. 2007;12:20–8. doi: 10.1016/j.tplants.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–55. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 5.Halford NG, Hey S, Jhurreea D, Laurie S, McKibbin RS, Paul M, et al. Metabolic signalling and carbon partitioning: role of Snf1-related (SnRK1) protein kinase. J Exp Bot. 2003;54:467–75. doi: 10.1093/jxb/erg038. [DOI] [PubMed] [Google Scholar]
- 6.Halford NG, Hey S, Jhurreea D, Laurie S, McKibbin RS, Zhang Y, et al. Highly conserved protein kinases involved in the regulation of carbon and amino acid metabolism. J Exp Bot. 2004;55:35–42. doi: 10.1093/jxb/erh019. [DOI] [PubMed] [Google Scholar]
- 7.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–85. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 8.Baena-González E, Sheen J. Convergent energy and stress signaling. Trends Plant Sci. 2008;13:474–82. doi: 10.1016/j.tplants.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baena-González E, Rolland F, Thevelein JM, Sheen J. A central integrator of transcription networks in plant stress and energy signalling. Nature. 2007;448:938–42. doi: 10.1038/nature06069. [DOI] [PubMed] [Google Scholar]
- 10.McMichael RW, Jr., Bachmann M, Huber SC. Spinach Leaf Sucrose-Phosphate Synthase and Nitrate Reductase Are Phosphorylated/Inactivated by Multiple Protein Kinases in Vitro. Plant Physiol. 1995;108:1077–82. doi: 10.1104/pp.108.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker JH, Slocombe SP, Ball KL, Hardie DG, Shewry PR, Halford NG. Evidence that barley 3-hydroxy-3-methylglutaryl-coenzyme a reductase kinase is a member of the sucrose nonfermenting-1-related protein kinase family. Plant Physiol. 1996;112:1141–9. doi: 10.1104/pp.112.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugden C, Donaghy PG, Halford NG, Hardie DG. Two SNF1-related protein kinases from spinach leaf phosphorylate and inactivate 3-hydroxy-3-methylglutaryl-coenzyme A reductase, nitrate reductase, and sucrose phosphate synthase in vitro. Plant Physiol. 1999;120:257–74. doi: 10.1104/pp.120.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harthill JE, Meek SE, Morrice N, Peggie MW, Borch J, Wong BH, et al. Phosphorylation and 14-3-3 binding of Arabidopsis trehalose-phosphate synthase 5 in response to 2-deoxyglucose. Plant J. 2006;47:211–23. doi: 10.1111/j.1365-313X.2006.02780.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Shewry PR, Jones H, Barcelo P, Lazzeri PA, Halford NG. Expression of antisense SnRK1 protein kinase sequence causes abnormal pollen development and male sterility in transgenic barley. Plant J. 2001;28:431–41. doi: 10.1046/j.1365-313X.2001.01167.x. [DOI] [PubMed] [Google Scholar]
- 15.Lovas A, Bimbó A, Szabó L, Bánfalvi Z. Antisense repression of StubGAL83 affects root and tuber development in potato. Plant J. 2003;33:139–47. doi: 10.1046/j.1365-313X.2003.016015.x. [DOI] [PubMed] [Google Scholar]
- 16.McKibbin RS, Muttucumaru N, Paul MJ, Powers SJ, Burrell MM, Coates S, et al. Production of high-starch, low-glucose potatoes through over-expression of the metabolic regulator SnRK1. Plant Biotechnol J. 2006;4:409–18. doi: 10.1111/j.1467-7652.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 17.Radchuk R, Radchuk V, Weschke W, Borisjuk L, Weber H. Repressing the expression of the SUCROSE NONFERMENTING-1-RELATED PROTEIN KINASE gene in pea embryo causes pleiotropic defects of maturation similar to an abscisic acid-insensitive phenotype. Plant Physiol. 2006;140:263–78. doi: 10.1104/pp.105.071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu CA, Lin CC, Lee KW, Chen JL, Huang LF, Ho SL, et al. The SnRK1A protein kinase plays a key role in sugar signaling during germination and seedling growth of rice. Plant Cell. 2007;19:2484–99. doi: 10.1105/tpc.105.037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosnoblet C, Aubry C, Leprince O, Vu BL, Rogniaux H, Buitink J. The regulatory gamma subunit SNF4b of the sucrose non-fermenting-related kinase complex is involved in longevity and stachyose accumulation during maturation of Medicago truncatula seeds. Plant J. 2007;51:47–59. doi: 10.1111/j.1365-313X.2007.03116.x. [DOI] [PubMed] [Google Scholar]
- 20.Farrás R, Ferrando A, Jásik J, Kleinow T, Okrész L, Tiburcio A, et al. SKP1-SnRK protein kinase interactions mediate proteasomal binding of a plant SCF ubiquitin ligase. EMBO J. 2001;20:2742–56. doi: 10.1093/emboj/20.11.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao L, Wang H, Sunter G, Bisaro DM. Geminivirus AL2 and L2 proteins interact with and inactivate SNF1 kinase. Plant Cell. 2003;15:1034–48. doi: 10.1105/tpc.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fauquet CM, Bisaro DM, Briddon RW, Brown JK, Harrison BD, Rybicki EP, et al. Revision of taxonomic criteria for species demarcation in the family Geminiviridae, and an updated list of begomovirus species. Arch Virol. 2003;148:405–21. doi: 10.1007/s00705-002-0957-5. [DOI] [PubMed] [Google Scholar]
- 23.Fauquet CM, Briddon RW, Brown JK, Moriones E, Stanley J, Zerbini M, et al. Geminivirus strain demarcation and nomenclature. Arch Virol. 2008;153:783–821. doi: 10.1007/s00705-008-0037-6. [DOI] [PubMed] [Google Scholar]
- 24.Hanley-Bowdoin L, Settlage SB, Orozco BM, Nagar S, Robertson D. Geminiviruses: models for plant DNA replication, transcription, and cell cycle regulation. Crit Rev Biochem Mol Biol. 2000;35:105–40. [PubMed] [Google Scholar]
- 25.Saunders K, Bedford ID, Briddon RW, Markham PG, Wong SM, Stanley J. A unique virus complex causes Ageratum yellow vein disease. Proc Natl Acad Sci U S A. 2000;97:6890–5. doi: 10.1073/pnas.97.12.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briddon RW, Mansoor S, Bedford ID, Pinner MS, Saunders K, Stanley J, et al. Identification of dna components required for induction of cotton leaf curl disease. Virology. 2001;285:234–43. doi: 10.1006/viro.2001.0949. [DOI] [PubMed] [Google Scholar]
- 27.Jose J, Usha R. Bhendi yellow vein mosaic disease in India is caused by association of a DNA Beta satellite with a begomovirus. Virology. 2003;305:310–7. doi: 10.1006/viro.2002.1768. [DOI] [PubMed] [Google Scholar]
- 28.Saunders K, Bedford ID, Yahara T, Stanley J. Aetiology: The earliest recorded plant virus disease. Nature. 2003;422:831. doi: 10.1038/422831a. [DOI] [PubMed] [Google Scholar]
- 29.Cui X, Tao X, Xie Y, Fauquet CM, Zhou X. A DNAbeta associated with Tomato yellow leaf curl China virus is required for symptom induction. J Virol. 2004;78:13966–74. doi: 10.1128/JVI.78.24.13966-13974.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saunders K, Norman A, Gucciardo S, Stanley J. The DNA beta satellite component associated with ageratum yellow vein disease encodes an essential pathogenicity protein (betaC1) Virology. 2004;324:37–47. doi: 10.1016/j.virol.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Cui X, Li G, Wang D, Hu D, Zhou X. A Begomovirus DNAbeta-encoded protein binds DNA, functions as a suppressor of RNA silencing, and targets the cell nucleus. J Virol. 2005;79:10764–75. doi: 10.1128/JVI.79.16.10764-10775.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeed M, Behjatnia SA, Mansoor S, Zafar Y, Hasnain S, Rezaian MA. A single complementary-sense transcript of a geminiviral DNA beta satellite is determinant of pathogenicity. Mol Plant Microbe Interact. 2005;18:7–14. doi: 10.1094/MPMI-18-0007. [DOI] [PubMed] [Google Scholar]
- 33.Yang JY, Iwasaki M, Machida C, Machida Y, Zhou X, Chua NH. betaC1, the pathogenicity factor of TYLCCNV, interacts with AS1 to alter leaf development and suppress selective jasmonic acid responses. Genes Dev. 2008;22:2564–77. doi: 10.1101/gad.1682208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eini O, Dogra S, Selth LA, Dry IB, Randles JW, Rezaian MA. Interaction with a host ubiquitin-conjugating enzyme is required for the pathogenicity of a geminiviral DNA beta satellite. Mol Plant Microbe Interact. 2009;22:737–46. doi: 10.1094/MPMI-22-6-0737. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Xie Y, Raja P, Li S, Wolf JN, Shen Q, et al. Suppression of methylation-mediated transcriptional gene silencing by βC1-SAHH protein interaction during geminivirus-betasatellite infection. PLoS Pathog. 2011;7:e1002329. doi: 10.1371/journal.ppat.1002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Q, Liu Z, Song F, Xie Q, Hanley-Bowdoin L, Zhou X. Tomato SlSnRK1 protein interacts with and phosphorylates βC1, a pathogenesis protein encoded by a geminivirus β-satellite. Plant Physiol. 2011;157:1394–406. doi: 10.1104/pp.111.184648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, et al. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–32. doi: 10.1016/S0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 38.Kornev AP, Haste NM, Taylor SS, Eyck LF. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc Natl Acad Sci U S A. 2006;103:17783–8. doi: 10.1073/pnas.0607656103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci U S A. 2003;100:8839–43. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nath N, McCartney RR, Schmidt MC. Yeast Pak1 kinase associates with and activates Snf1. Mol Cell Biol. 2003;23:3909–17. doi: 10.1128/MCB.23.11.3909-3917.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland CM, Hawley SA, McCartney RR, Leech A, Stark MJ, Schmidt MC, et al. Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr Biol. 2003;13:1299–305. doi: 10.1016/S0960-9822(03)00459-7. [DOI] [PubMed] [Google Scholar]
- 42.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 44.Woods A, Vertommen D, Neumann D, Turk R, Bayliss J, Schlattner U, et al. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem. 2003;278:28434–42. doi: 10.1074/jbc.M303946200. [DOI] [PubMed] [Google Scholar]
- 45.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–6. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 47.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Shen W, Hanley-Bowdoin L. Geminivirus infection up-regulates the expression of two Arabidopsis protein kinases related to yeast SNF1- and mammalian AMPK-activating kinases. Plant Physiol. 2006;142:1642–55. doi: 10.1104/pp.106.088476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hey S, Mayerhofer H, Halford NG, Dickinson JR. DNA sequences from Arabidopsis, which encode protein kinases and function as upstream regulators of Snf1 in yeast. J Biol Chem. 2007;282:10472–9. doi: 10.1074/jbc.M611244200. [DOI] [PubMed] [Google Scholar]
- 50.Shen W, Reyes MI, Hanley-Bowdoin L. Arabidopsis protein kinases GRIK1 and GRIK2 specifically activate SnRK1 by phosphorylating its activation loop. Plant Physiol. 2009;150:996–1005. doi: 10.1104/pp.108.132787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massagué J, et al. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–20. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 52.Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–82. doi: 10.1016/S0092-8674(02)00741-9. [DOI] [PubMed] [Google Scholar]
- 53.Fontes EP, Santos AA, Luz DF, Waclawovsky AJ, Chory J. The geminivirus nuclear shuttle protein is a virulence factor that suppresses transmembrane receptor kinase activity. Genes Dev. 2004;18:2545–56. doi: 10.1101/gad.1245904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanzaki H, Saitoh H, Takahashi Y, Berberich T, Ito A, Kamoun S, et al. NbLRK1, a lectin-like receptor kinase protein of Nicotiana benthamiana, interacts with Phytophthora infestans INF1 elicitin and mediates INF1-induced cell death. Planta. 2008;228:977–87. doi: 10.1007/s00425-008-0797-y. [DOI] [PubMed] [Google Scholar]
- 55.Halford NG, Hardie DG. SNF1-related protein kinases: global regulators of carbon metabolism in plants? Plant Mol Biol. 1998;37:735–48. doi: 10.1023/A:1006024231305. [DOI] [PubMed] [Google Scholar]
- 56.Sugden C, Crawford RM, Halford NG, Hardie DG. Regulation of spinach SNF1-related (SnRK1) kinases by protein kinases and phosphatases is associated with phosphorylation of the T loop and is regulated by 5′-AMP. Plant J. 1999;19:433–9. doi: 10.1046/j.1365-313X.1999.00532.x. [DOI] [PubMed] [Google Scholar]