Abstract
Polyhemoglobin-superoxide dismutase-catalase-carbonic anhydrase (PolyHb-SOD-CAT-CA) is a therapeutic antioxidant that also transports both oxygen and carbon dioxide. This is formed by crosslinking Hb with SOD, CAT, and CA using glutaraldehyde. Crosslinking stroma free Hb from red blood cell (rbc) reduces CA activity to 55%. Addition of more CA resulted in a preparation with the same CA activity as RBC. PolyHb in the complex acts as a buffer to prevent large pH changes as carbon dioxide is converted to carbonic acid. We then prepare and optimize a novel PolyHb-SOD-CAT-CA, a therapeutic antioxidant that also transports both oxygen and carbon dioxide.
INTRODUCTION
Increased demand for blood transfusions in emergency and surgical settings has led to augmented need for red blood cell (RBC) substitutes. Different types of oxygen-carrying blood substitutes are thus being investigated [1–3]. One of these products is the nanobiotechnology-based polyhemoglobin (PolyHb).
Crosslinking hemoglobin (Hb) with bifunctional agents such as diacid [4] or glutaraldehyde [5] results in the formation of PolyHb [4]. The complex of 4–5 Hb molecules has been shown to successfully replace blood loss by maintaining Hb levels in a safe range during surgery and perform adequate oxygen transport [6]. Although two of these nanobiotechnological approaches are currently in Phase III clinical trials [6, 7], research efforts are at present focused on the development of new generations of blood substitutes that ressemble a more complete RBC.
In addition to the transport of oxygen from the lungs to tissues by the RBCs, the transport of carbon dioxide (CO2) from the tissues to the lungs for removal is an essential function. Elevated CO2 content in the body leads to increased plasma proton (H+) concentrations, which can result in acidosis, malfunction of the central nervous system, coma, and even death [8]. CO2 is more soluble than oxygen in the plasma, but cells in the body produce much more CO2 than the level that can be carried in the dissolved form. Only approximately 7% of CO2 is carried by venous blood. The remaining 93% of CO2 diffuse into RBCs where the gas is either bound to hemoglobin in the carbamate form, or is converted by RBC enzyme carbonic anhydrase (CA) into carbonic acid, which in turn dissociates into an H+ ion and an HCO3− ion [8, 9]. The conversion of approximately 70% of CO2 to HCO3− ions in the circulation serves two important physiological functions. Firstly, it provides a more efficient means of transport of CO2 from tissue cells to the lungs. Secondly, the HCO3− ions produced act in a buffer system for metabolic acids. In the absence of a catalyst, the conversion of CO2 to H+ and HCO3− is a very slow reaction. Thus, CO2 and carbonic acid exist in the blood in a ratio of 400:1, and the proportion of CO2 to HCO3− is 20:1 [10]. The hydration reaction of CO2 would therefore occur much too slowly to be of physiological importance were it not for the presence of the zinc-containing metalloenzyme carbonic anhydrase (CA) in the RBCs [11, 12]. CA is involved in the crucial process of CO2 transport by catalyzing the reversible hydration of CO2 to carbonic acid, H+ and HCO3−, thus playing a fundamental role in the maintenance of acid-base balance of the body.
While antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) have been crosslinked with PolyHb to produce oxygen carriers that can confer antioxidant properties [13], CA has not been incorporated into a PolyHb-enzyme system in sufficient amount for the transport of CO2. The aim of this study is thus to prepare PolyHb crosslinked with CA to produce further improved blood substitutes that can confer the other important function of RBC – transport of CO2.
In this study we propose that it is possible to employ the concept of nanobiotechnology to assemble Hb and CA into PolyHb-CA in the nanodimensions. This complex should also have the ability to transport oxygen and have adequate CA activity, and exhibit buffering properties. To achieve our aim to produce such a PolyHb-CA system, our first experiments were involved in the methods of PolyHb-enzyme preparation. Subsequently, experiments served to determine the functional properties of cross-linked Hb and CA by comparing with uncrosslinked stroma-free Hb (SFHb), which is RBC hemolysate containing Hb and RBC enzymes with the stroma removed. Crosslinked SFHb, or PolySFHb, was also used in functional characterization tests. Glutaraldehyde, a non-specific bifunctional crosslinking agent that preferentially targets lysine residues, is used for the polymerization of Hb and CA. Kinetic properties of the PolyHb-CA system were also studied in order to further characterize the properties of this novel molecule.
After completing the study on PolySFHb-CA, we proceeded with further study to construct a PolySFHb-SOD-CAT-CA that is also based on nanobiotechnology. This new nanobiotechnological polymer is an oxygen carrier (PolyHb) with antioxidant activity (SOD, CAT) and also the ability to facilitate the transport and acid-base functions (CA activity). The SOD and CAT levels in this PolySFHb-SOD-CAT-CA is much higher that those in rbc. This way, it can also act as a therapeutic agent against ischemia-reperfusion injuries. In the present study we perfect the method for preparing polySFHb-SOD-CAT-CA. Different procedures were used to prepared samples until the optimal system has been obtained with suitable enzyme activities for SOD, CAT, and CA, Moreover, samples of polySFHb-SOD-CAT-CA of different molecular weight fractions were collected and assessed. We are continuing with further detailed in vitro and in vivo studies.
MATERIALS AND METHODS
Materials
The Hb used in our experiments is SFHb extracted from bovine blood provided by the McGill University McDonald Campus Cattle Complex (Sainte-Annede-Bellevue, Canada). Glutaraldehyde (25%) was obtained from Poly-sciences (Warrington, PA). Drabkin’s reagent, L-lysine (monohydrochloride, Sigma Ultra >99%), SOD, CAT, and bovine CA (EC 4.2.1.1, 2720 units/mg solid manufacturer’s stated activity) were purchased from Sigma-Aldrich (Ontario, Canada). All other chemicals or reagents of analytical grade were purchased from Sigma-Aldrich.
Preparation of SFHb
SFHb was prepared using established methods described [2]. Fresh bovine blood with heparin as an anticoagulant was centrifuged at 6000 rpm (4000 g) for 20 minutes at 4°C. The plasma supernatant and buffy coat containing the white blood cells and platelets were aspired carefully. The sedimented RBCs were then washed four times using three times their volumes of sterile, ice-cold, isotonic saline. RBCs were lysed by adding two volumes of hypotonic phosphate buffer (15 mM, pH 7.4) to one volume of packed cells. The resulting solution was mixed by repeated inversion and swirling for approximately 3 minutes. The mixture was allowed to stand for 20 to 30 minutes, then 0.5 volume of cold, reagent-grade toluene was added. After mixing vigorously for 5 minutes, the suspension was left standing for three hours at 4°C. The upper layer, containing toluene, extracted stroma lipid, and cellular debris, was then removed by aspiration, and the remaining solution was centrifuged at 25 000 g at 4°C for one hour. The SFHb solution was dialyzed against Ringer’s lactated solution at 4°C. A dialysis membrane (Sigma) was used to form a dialysis tube with both ends carefully sealed. Ringer’s lactated solution can be replaced by an isotonic, buffered dialysate solution (pH 7.35) containing NaCl 5.85 g/L; NaHCO3 3.61 g/L; NaH2PO4 · H2O 0.44 g/L; KCl 0.37 g/L; CaCl2 · 2H2O 0.22 g/L; MgCl2 · 5H2O 0.08 g/L. The SFHb was stored at −80°C [1].
Preparation of PolySFHb, PolySFHb-CA, and PolySFHb-SOD-CAT-CA
Reaction mixtures were prepared containing SFHb (10 g/dL) with the addition of CA (1070 units/mL) in 0.1 M potassium phosphate buffer (pH 7.6). In the method of preparation of PolySFHb, the enzyme was replaced by an equivalent volume of buffer. And in the method of preparing PolySFHb-SOD-CAT-CA, SOD (1050 units/mL), catalase (21,000 units/mL), and carbonic anhydrase (1070 units/mL) were added to the 20 mL solution containing stroma-free hemoglobin (7 g/dl) in 50mM sodium phosphate buffer (pH 7.4) to construct PolySFHb-SOD-CAT-CA. Before the initiation of the crosslinking reaction, 1.3 M lysine was added at a molar ration of 7:1 lysine/Hb. Nitrogen gas was used to flush the reaction vessel to prevent formation of methemoglobin. Crosslinking was started with the addition of 5% glutaraldehyde at a molar ration of 16:1 glutaraldehyde/Hb. Glutaraldehyde was added in four equal aliquots over a period of 15 minutes, and the reaction was allowed to crosslink for 24 hours at 4°C with constant stirring under aerobic conditions. Crosslinking was stopped by adding 2.0 M lysine at a molar ratio of 200:1 lysine/Hb. The preparation was then filtered using a sterile 0.45 μM filter, and subsequently dialyzed overnight using molecular porous dialysis membrane (MWCO: 12 000–14 000) against Ringer’s lactated solution. 100 kDa microconcentrators (Amicon, Beverly, MA) were used to concentrate 500 μL aliquots of the crosslinked solution by centrifuging at 2500 g for 55 minutes at 23°C. The retentate was subsequently collected by centrifuging at 1000 g for 3 minutes.
Molecular Weight Distribution
For the analysis of molecular weight distribution of Poly-Hb-SOD-CAT-CA, we used a Sephacryl-300 HR column (Vtotal = 560 ml) at a flow rate of 36 ml/hour. This column was equilibrated with 0.1M Tris-HCl and 0.15M NaCl (pH 7.4) elution buffer. The molecular weight distribution was recorded by a 280nm UV detector at a recording velocity of 1mm/min. Fractions collected were: (1) molecular weights higher than 450kDa; (2) 100–450kDa; and (3) less than 100kDa. For animal studies, the less than 100kDa fraction will be discarded. Dialysis membrane (MWCO: 12 000–14 000) and spectra/gel absorbent were used to concentrate the sample and the hemoglobin concentration were determined by Drabkin’s method. The aliquoted samples were stored at −80 °C.
Quantitative Determination of Hb Concentration
The Hb concentration in the PolySFHb and PolySFHb-CA preparations was colorimetrically determined by reacting the samples with Drabkin’s reagent (Sigma-Aldrich), then measuring the concentration of the resulting cyanmethe-moglobin solution by spectrophotometry at 540 nm.
Determination of CA Activity
The hydration activity of carbon dioxide by CA was determined by an electrometric delta pH assay based on the methods of Henry [14], and of Wilbur and Anderson [15]. One Wilbur-Anderson (W-A) unit of CA activity is defined as the amount of enzyme that causes the pH of a 0.02 M Tris buffer to drop from pH 8.3 to 6.3 per minute. The reagent solution consisted of test samples containing CA and 0.02 M Tris. HCl buffer (pH 8.0) was kept between 0–4°C before use. Dissolved CO2 was prepared by bubbling CO2 through distilled water to obtain the substrate for the assay. The reaction was initiated by the addition of substrate, and the time (T) needed for the pH of the reaction mixture to drop from pH 8.3 to 6.3 was recorded. A Fisher Accumet Basic pH meter (Fisher Scientific, Pittsburgh, PA) with MI-407 (P) Needle pH electrode (Microelectrodes Inc., Bedford, NH) was used to measure the change in pH caused by the hydration reaction of CO2 catalyzed by CA. The control (T0) for the assay consisted of the same mixture without the test sample. The measurements in seconds were converted into W-A units according to the following formula:
The units were then plotted versus the Hb/CA concentration (mg/mL).
Determination of Kinetics Properties
Michaelis-Menten kinetics parameters were determined from data obtained with constant enzyme concentration and increasing substrate concentration until a steady rate of activity was observed. Six different substrate concentrations were selected and CA activity was assessed as previously described. Kinetic experiments were done between 0–4°C, and in triplicates for statistical analysis. Lineweaver-Burk plots (double reciprocal plots of substrate and rate of reaction) were then used as a graphical method of analysis of the Michaelis-Menten constants. On such a graph, the inverse of the y intercept is Vmax and inverse of x intercept is Km.
Determination of CAT Activity
The method for catalase measurement was according to the paper of Zhu and Chang [16]. Briefly, UV 240nm spectrophotometer was used to measure the rate of disappearance of H2O2. The reaction mixture contained 2mL of 50mM phosphate buffer, pH 7.0, and 1mL of 30mM H2O2. The decrease of the H2O2 level was monitored at 240nm for 15s. The same concentrations of blood samples or phosphate buffer mixtures without H2O2 were applied as blank. The catalase activity was expressed in units per grams of haemoglobin.
Determination of SOD Activity
The measurement for SOD activity was based on the reduction of cytochrome c by superoxide [13]. The reagent solution consisted of xantine (50 μM), cytochrome c (10μM), and CAT (500 units/mL) in 50 mL potassium phosphate buffer, 0.1 mM EDTA, pH 7.8. The reaction system consisted of test samples and reagent solution. Xanthine oxidase was added to start the reaction. 0.154 M NaCL was used as blank. The cytochrome c reduction was recorded at 550nm by a spectrophotometer [13].
RESULTS
Effect of Crosslinking with Glutaraldehyde on CA Activity
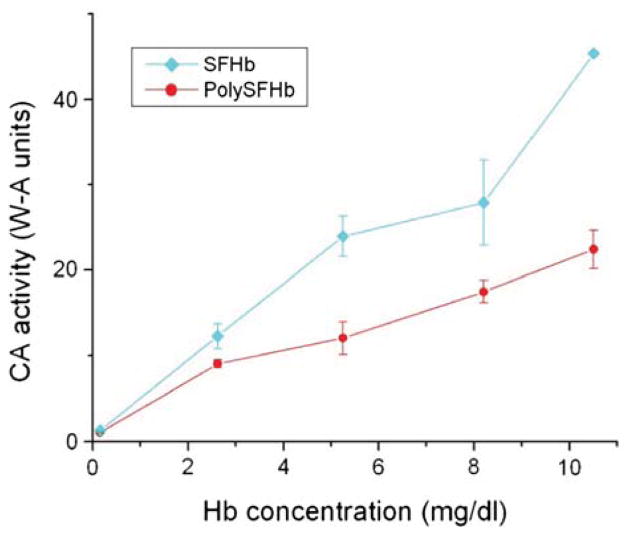
To determine the effect of the crosslinking reaction with glutaraldehyde on enzyme activity, the CO2 hydration activity of Hb solutions before and after crosslinking was compared. After removing the RBC stroma material, which is toxic to kidneys, PolySFHb was prepared from extracted SFHb from bovine blood. Stroma-free hemolysate consists of the contents of RBC including CA. The SFHb solution without the addition of glutaraldehyde was considered to contain 100% of the original CA enzyme activity. Figure 1 shows the CA activity for SFHb and PolySFHb. Six Hb solution concentrations between 0.2–10.5 mg/mL were selected for the current experiment. These concentrations were chosen after screening different amounts of Hb to determine the appropriate detectable range for the assay. These sample concentrations were also used for all additional experiments described in this report involving Hb solutions, unless stated otherwise. It was found that approximately 45% of CA activity was lost after crosslinking using a glutaraldehyde: Hb molar ratio of 16:1 (Fig. 1).
Figure 1.
The CO2 hydration activity of SFHb and PolySFHb was assayed. Six concentrations between 0.2–10.5 mg/mL of sample were selected in order to obtain results in an appropriate and measurable range. The CA activity was described in W-A units. Measurements were done in triplicates to indicate statistical reproducibility.
Effect of Enzyme Concentration on CA Activity
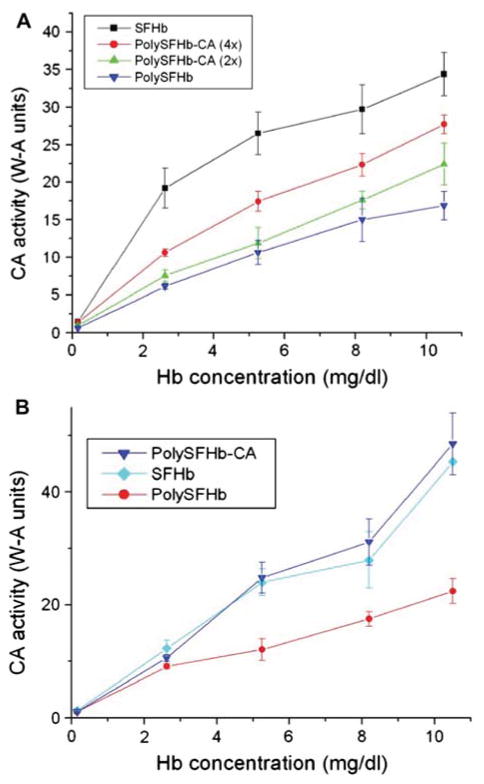
Purified CA (Sigma-Aldrich) was crosslinked to Poly SFHb in increasing enzyme concentrations. Figure 2A shows the effect of increasing the concentration of CA on enzyme activity. As shown, the rate of CO2 hydration rate increased in direct proportion with increasing concentration of CA. Figure 2B indicates that by augmenting the amount of enzyme incorporated into the PolySFHb solution, a preparation with CA activity comparable to that of SFHb (100% original RBC CA activity) can be obtained. The enzyme concentration used in the crosslink of PolyS-FHb and CA to reach the CO2 hydration activity of SFHb was 1070 units/mL.
Figure 2.
(A) PolySFHb was prepared from SFHb and crosslinked with increasing amount of CA (200 units/mL and 400 units/mL). Enzyme activity of SFHb and PolySFHb prepared from SFHb without additional CA were also assayed. (B) PolySFHb was prepared from SFHb and was assayed for CA activity. The CO2 hydration activity of PolySFHb crosslinked with CA (1070 units/mL) was also measured.
Effect of Hb on CA Activity
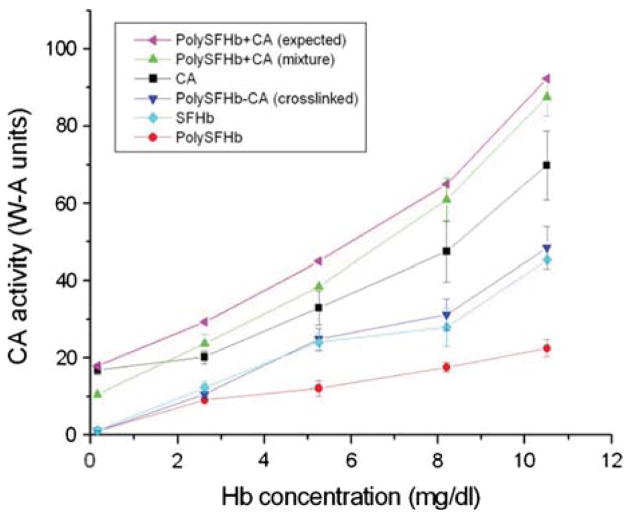
Next, the CA enzyme activities of SFHb, PolySFHb, and PolySFHb-CA (with 1070 units/mL of CA incorporated) solutions were compared with that of the CA enzyme in free form (original stock solution of 1070 units/mL). From Figure 3, SFHb, PolySFHb, and PolySFHb-CA showed similar activity as previously determined (Fig. 2B). These solutions had much lower CA activity when compared to CA in free form, which exhibited higher CO2 hydration activity even at low concentrations (Fig. 3). To determine whether the decrease in enzyme activity is solely due to crosslinking with glutaraldehyde, PolySFHb was combined in a mixture with free form CA without crosslinking, and its enzyme activity was subsequently measured. Initially, when small amounts of the mixture were tested, it was found that the mixture exhibited lower CA activity than free form CA (Fig. 3). However, with larger volumes being tested, the CA concentration of the mixture increased, and enzyme activity also increased proportionally so that the CA activity of the mixture eventually reached higher levels compared to the free form enzyme. Also seen in Figure 3 is a curve representing the expected CA activity of the PolySFHb and CA mixture, obtained by adding the individually obtained enzyme activities for PolySFHb and free form CA. A discrepancy was constantly seen between the expected and experimentally determined enzyme activity levels as the mixture of PolySFHb and CA showed less activity than the expected levels.
Figure 3.
Samples containing CA in free form and samples without free enzyme were assayed for CA activity. The expected activity for the mixture of PolySFHb and free form CA is also indicated.
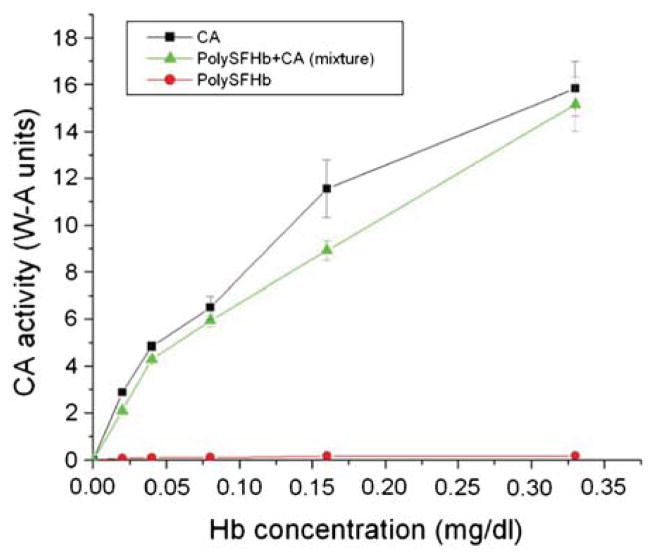
To further examine these observations, additional CA enzyme assays were carried out using sample concentrations between 0–0.35 mg/mL instead of 0.2–10.5 mg/mL. At these small concentrations, the activity level of free form CA increased rapidly with increasing sample concentration, and a similar curve was obtained for the mixture of PolySFHb and CA (Fig. 4). However, the enzyme activity of the mixture was less than that of the CA in free form. Also seen in Figure 4 is the enzyme activity of PolySFHb, which is very low as expected at these small concentrations. The observed differences between enzyme activity levels of these different samples suggest that the presence of Hb in a solution containing CA can decrease the measured CA enzyme activity.
Figure 4.
PolySFHb was mixed with CA in free form and assayed for enzyme activity. PolySFHb without additional enzyme and CA alone were also assayed. The sample concentrations were proportionally reduced and selected to be between 0–0.35 mg/mL.
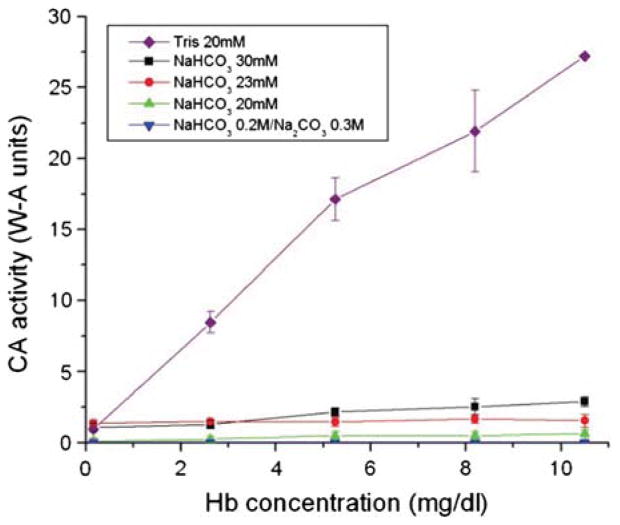
Effect of Different Buffers and Buffer Concentrations on CA Activity
An experiment simulating more physiological conditions was carried out to further investigate the effect of the buffering characteristics of Hb. In the plasma, the levels of bicarbonate and carbonate are present in the range of 23–30 mM [16]. A solution of PolySFHb was thus tested for CO2 hydration activity using buffers at physiological concentrations (Fig. 5). Bicarbonate buffer was used in the assays at 20 mM, 23 mM, and 30 mM. A solution containing 0.3 M Na2CO3 and 0.2 M NaHCO3 based on experiments described in earlier research was also used in a CA assay [17]. As a reference, 20 mM Tris buffer was used as recommended by established protocol [14]. Figure 5 shows that using the bicarbonate and carbonate buffers at all tested concentrations greatly reduced CA activity in this assay. Performing the experiment with Tris buffer gave standard results, thus the buffer is confirmed to be suitable for the assay. These results suggest that PolyS-FHb can act as a buffer to prevent dramatic pH changes caused by the CA-catalyzed formation of carbonic acid.
Figure 5.
PolySFHb was prepared from SFHb and assayed with the electrometric delta pH assay using different concentrations of bicarbonate and carbonate buffers corresponding to physiological conditions.
Kinetic Studies on CA in Free Form, Uncrosslinked SFHb, and PolySFHb-CA
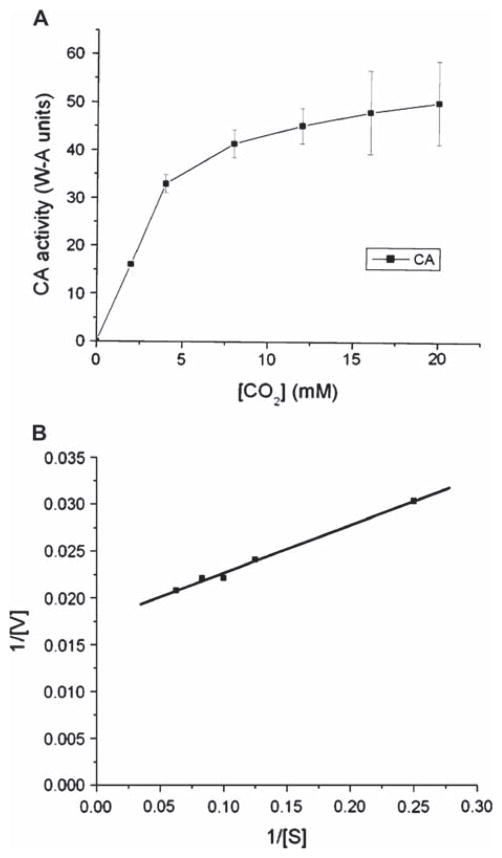
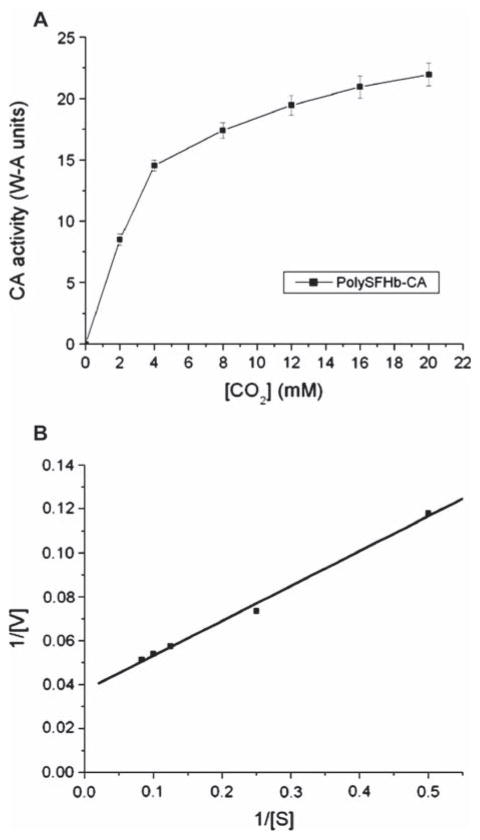
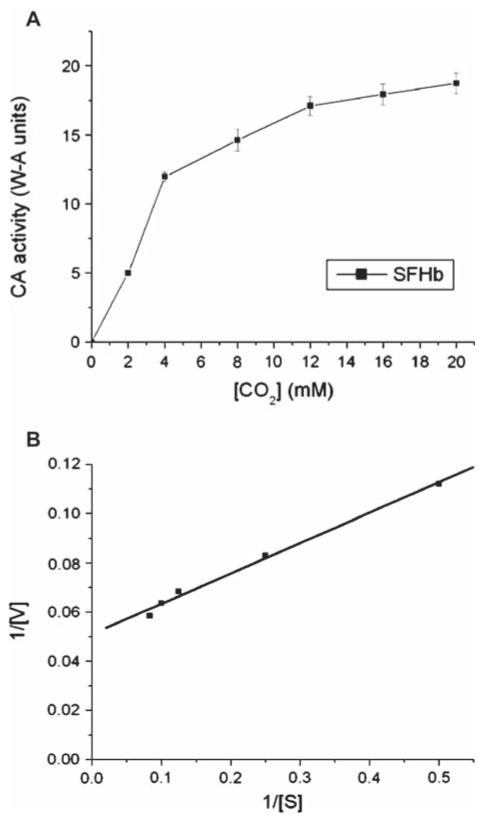
The next experiments were designed to investigate the kinetic properties of the PolySFHb-enzyme system. Apparent Michaelis-Menten parameters (Vmax and Km) of CA in free form, uncrosslinked SFHb, and PolySFHb-CA were measured. The Lineweaver-Burk plot was used as a graphical analysis tool of enzyme kinetics. Taking statistical analysis into account, apparent kinetic constants were determined from these plots (Figures 6–8).
Figure 6.
(A) Plot illustrating the Michaelis-Menten equation for CA in free form. The curve was obtained by increasing the substrate concentration until a stable rate of CO2 hydration was obtained. (B) Lineweaver-Burk plot for CA in free form. The curve was obtained by taking the reciprocal of the substrate and the rate of enzyme activity.
Figure 8.
(A) Plot illustrating the Michaelis-Menten equation for PolySFHb-CA. (B) Lineweaver-Burk plot for PolySFHb-CA.
Apparent kinetic values for the tested samples are summarized in Table 1. The Vmax of free form CA was found to be 50 W-A units, and the Km of free form CA was in the range of 11–13 mM. The Vmax of SFHb was determined to be 19 W-A units and its Km was between 10–12 mM. PolySFHb-CA exhibited a Vmax of 27 W-A units and the Km of the complex was measured to be 23–26 mM.
Table 1.
Apparent kinetic constants for CA in free form, SFHb, and PolySFHb-CA.
| Vmax (W-A units) | Km (mM) | |
|---|---|---|
| CA | 50 | 11–13 |
| SFHb | 19 | 10–12 |
| PolySFHb-CA | 27 | 23–26 |
Enzyme Activity of SFHb, polySFHb, and polySFHb-SOD-CAT-CA
PolySFHb-SOD-CAT-CA was formed by nanobiotechnology using glutaraldehyde as crosslink reagent. The enzymes activities of (1) SFHb, (2) PolySFHb, (3) PolySFHb+SOD+CAT+CA (SOD, CAT and CA added in solution to PolySFHb), and (4) PolySFHb-SOD-CAT-CA (PolySFHb crosslinked with SOD, CAT, and CA) were assessed. To eliminate errors, three batches of polySFHb and polySFHb-SOD-CAT-CA were prepared and tested separately. Crosslink reactions significantly decreased the SOD, CAT, and CA enzyme activities. Thus, PolySFHb alone did not have the same enzyme activities as RBC. The amount of SOD and CAT used in the crosslinking were much higher than those in the RBC. This is needed in order to have a therapeutic level of SOD and CAT for ischemia-reperfusion injuries. CA (1070 units/mL) also had to be added in the crosslink of PolySFHb and CA to emulate the CO2 hydration activity of SFHb in RBC. Enrichment of enzymes highly improved the activities of SOD, CAT, and CA in PolySFHb-SOD-CAT-CA. An optimal PolyHb-SOD-CAT with the following addition of enzymes requires a Hb: SOD: CAT: CA ratio of 1g: 18,000:310,000:130,000U.
Molecular Weight Distribution of PolySFHb-SOD-CAT-CA and Enzyme Activities
Sepacryl S-300 gel column chromatography was used to analyze the molecular weight distribution of PolySFHb-SOD-CAT-CA. The molecular weight distributions for PolyHb-SOD-CAT show three molecular components: (1) low (<100kDa); (2) intermediate (100–450kDa); and (3) high molecular weight (>450kDa). The sample contains about 86% components with molecular weight higher than 100kDa. The low molecular weight fraction (<100kDa) will be discarded in animal studies.
Most of the Hb, SOD, CAT, and CA activity (70, 82 ± 3,90 ± 1,84 ± 2, respectively) are in the PolyHb-SOD-CAT-CA fraction with a molecular weight of >450 kDa. The fraction with molecular weight between 100 kDa and 450 kDa contains Hb, SOD, CAT, and CA activities of 16, 11 ± 1, 9 ± 1,13 ± 2, respectively. The activities of SOD, CAT, and CA activities were even lower (14, 5 ± 1,0.5 ± 0.05,3 ± 1) in the PolyHb-SOD-CAT-CA fraction with molecular weight of less than 100 kDa. The fraction with molecular weight of less that 100 kDa will be discarded in animal studies.
DISCUSSION
Hemoglobin-based blood substitutes such as PolyHb are more advantageous than donor blood in many aspects. For instance, they do not have blood group antigens, can be sterilized, and can be stored at room temperature for more than a year [1]. Therefore, such products can be transfused into patients without delay. However, PolyHb remains only an oxygen carrier and lacks the other enzymatic functions of the biological RBC. PolyHb-SOD-CAT was thus developed as a new generation of blood substitutes able to confer additional antioxidant properties [13], but sufficient amounts of other RBC enzymes, such as the addition of carbonic anhydrase to those normally present in SFHb, have not been incorporated within a PolyHb-enzyme complex. Therefore, in this report we first studied a novel nanobiotechnology-based PolyHb-CA system. We then followed with a more complete but more complicated PolyHb-SOD-CAT-CA system.
The first part of the study was to prepare PolySFHb-CA from SFHb using glutaraldehyde. To study the effect of crosslinking on enzyme activity, we compared the CA activity before and after crosslinking with Hb, and found that crosslinking resulted in a reduction of 45% in CO2 hydration activity. This suggests that the polymerization may have decreased the number of CO2 binding sites available on the enzyme molecules. However, we were able to produce PolySFHb-CA with comparable CO2 hydration activity as SFHb by incorporating additional CA into the PolySFHb-enzyme complex.
Next, we investigated the effect of Hb on CA activity, and we observed that in the free form CA alone exhibited higher activity than the solutions containing Hb. This difference in enzyme activity cannot be solely attributed to the effect of crosslinking as the experimentally determined activity of the PolySFHb and CA mixture (no crosslinking) did not reach expected values. These observations can be explained by the buffering ability of Hb. This function of Hb is of physiological importance because maintenance of the pH of bodily fluids at an appropriate level constitutes a major homeostatic challenge. A significant change in pH can result in malfunctioning of proteins and lead to various pathological conditions. Thus, different buffer mechanisms in the body exist to remove excess H+ produced from metabolism, acting quickly to temporarily bind excess H+ ions, and removing these highly reactive molecules from solution [16, 18]. Hb is involved in the protein buffer system, the most abundant buffer in intracellular fluids and blood plasma. Since Hb have binding sites for both H+ and CO2, some of this substrate will be sequestered by Hb as soon as CO2 is added to the assay vessel, resulting in less substrate available for CA to act upon. In addition, as CA in the reaction carry out hydration of CO2 and consequently render the buffer environment more acidic, PolyHb can remove free H+ from the solution. As a result, less enzyme activity is detected since the electrometric delta pH assay measures change in pH. We also noted that the buffering effect by Hb is stronger when the CA concentration is lower in the sample. The decrease in enzyme activity can be overcome by incorporating additional CA in the solution. When CA concentration increases, the buffering effect of Hb is less detectable and, as a result, the enzyme activity increases. At higher sample concentrations, the enzyme activity of the PolySFHb and CA mixture eventually surpasses that of the free form enzyme alone because there is a larger total amount of CA molecules in the mixture compared to free form CA alone. Thus, when all the Hb molecules in the solution have reached their buffering limit and when their H+ binding sites are saturated, more enzymatic activity is detected. CA assays using bicarbonate and carbonate buffers at physiological concentrations found in the plasma provided additional support that PolySFHb preparations can prevent significant changes in pH, thus acting as buffers.
We next studied the kinetic properties of PolySF-Hb-CA. The Km values of free form CA and SFHb were found to be very similar, being 11–13 mM and 10–12 mM, respectively. This result was expected as the same unmodified enzyme should exhibit the same affinity to the same substrate under the same conditions. On the other hand, the Km for PolySFHb-CA was higher. One proposed explanation is that since PolySFHb-CA is a complex structure obtained from crosslinking individual proteins, as a result many molecules are randomly packaged together. Therefore, a higher concentration of substrate may be required to be able to diffuse into the complex to reach the enzyme molecules among the Hb molecules in order to be acted upon. More substrate is thus needed for the enzymatic reaction to occur, resulting in a higher Km. We then observed that free form CA has the highest activity rate, and SFHb the lowest. Since Hb buffers H+ ions produced by hydration of CO2, the apparent maximal rate of Hb-containing solutions is expected to be lower as the assay detects less change in pH. As SFHb contains the least amount of CA, the buffering effect is strongest, resulting in the lowest Vmax values. Similarly, PolySFHb-CA has a higher Vmax than SFHb because the PolySFHb-enzyme system contains additional CA, thus it is less influenced by the buffering effect. However, the Vmax of PolySFHb-CA is proposed to be lower than that of free form CA because the non-specific crosslinking reaction with glutaraldehyde may have reduced the number of active sites on the enzyme.
The result from the PolySFHb-CA study above can be combined with our earlier study on PolySFHb-SOD-CAT to result in a much more complete RBC substitute, PolySFHb-SOD-CAT-CA. This not only contains all the major enzymatic functions of RBC, but it also contain a much higher concentration of the enzymes to allow this to be used much more effectively as a therapeutic agent for those conditions with ischemia-reperfusion injuries. After all, RBC with the normal concentrations of enzymes is not effective in conditions of severe ischemia-reperfusion, as in stroke, severe sustained hemorrhagic shock, myocardial infarction, and other conditions [20,21].
Figure 7.
(A) Plot illustrating the Michaelis-Menten equation for SFHb. (B) Lineweaver-Burk plot for SFHb.
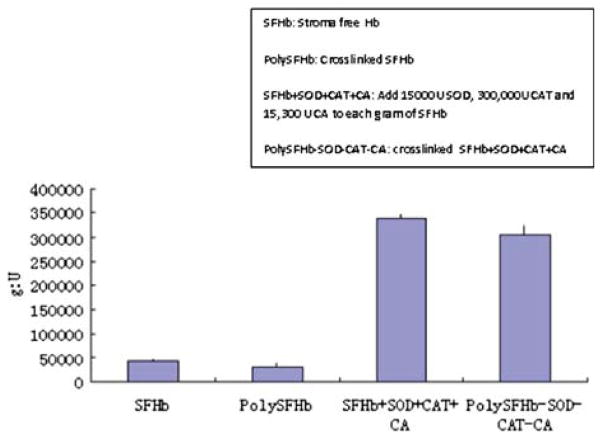
Figure 9.
Superoxide dismutase activity of SFHb, PolySFHb, SFHb+SOD+CAT+CA, and polySFHb-SOD-CAT-CA. SOD (1050 units/mL), catalase (21,000 units/mL), and carbonic anhydrase (1070 units/mL) were added to stroma-free hemoglobin (7 g/dl), then polymerized into PolySFHb-SOD-CAT-CA, resulting in a Hb: SOD ratio of 1g: 18,000 after crosslinking.
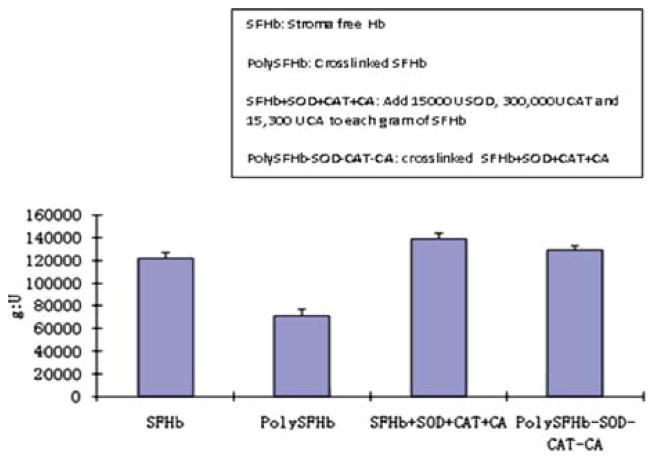
Figure 10.
Catalase activity of SFHb, PolySFHb, SFHb+SOD+CAT+CA, and polySFHb-SOD-CAT-CA. SOD (1050 units/mL), catalase (30,000 units/mL), and carbonic anhydrase (1070 units/mL) were added to stroma-free hemoglobin (7 g/dl), then polymerized into PolySFHb-SOD-CAT-CA, resulting in a Hb: SOD ratio of 1g: 310,000 after crosslinking.
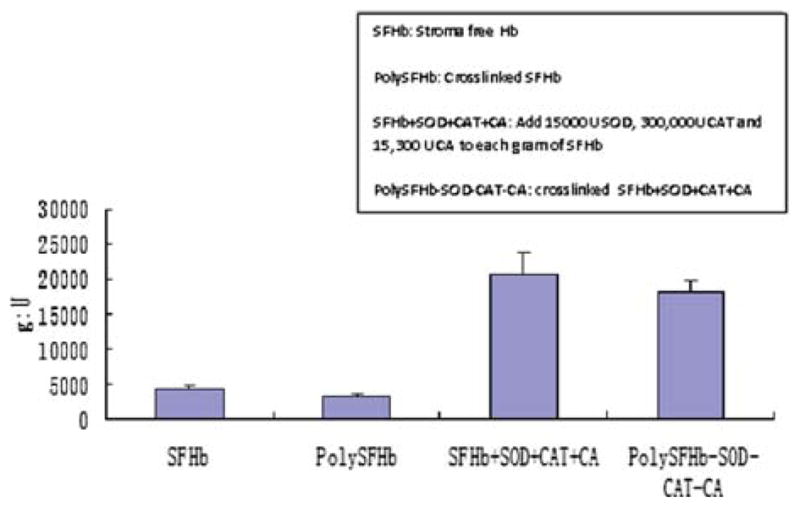
Figure 11.
Carbonic anhydrase activity of SFHb, PolySFHb, SFHb+SOD+CAT+CA, and polySFHb-SOD-CAT-CA. SOD (1050 units/mL), catalase (21,000 units/mL), and carbonic anhydrase (1070 units/mL) were added to stroma-free hemoglobin (7 g/dl), then polymerized into PolySFHb-SOD-CAT-CA, resulting in a Hb:CA ratio of 1g: 130,000U after crosslinking
Table 2.
Molecular distribution of PolySFHb-SOD-CAT-CA and enzyme activities.
| Molecular Activity Weight (%) | MW Component (%) | SOD Activity (%) | CAT Activity (%) | CA |
|---|---|---|---|---|
| 450 kDa | 70 | 82 ± 3 | 90 ± 1 | 84 ± 2 |
| 100–450 kDa | 16 | 11 ± 1 | 9 ± 1 | 13 ± 2 |
| <100kDa | 14 | 5 ± 1 | 0.5 ± 0.05 | 3 ± 1 |
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Chang TMS. Blood Substitutes: Principles, Methods, Products and Clinical Trials. Vol. 1. Basel: Karger Landes Systems; 1997. [Google Scholar]
- 2.Chang TMS. Artificial Cells: Biotechnology, Nanotechnology, Blood Substitutes, Regenerative Medicine, Bioencapsulation, Cell/StemCell Therapy. Singapore: World Science Publishers/Imperial College Press; 2007. [Google Scholar]
- 3.Winslow RM. Blood Substitutes. London: Elsevier Inc; 2005. [Google Scholar]
- 4.Chang TMS. Semipermeable microcapsules. Science. 1964;146:524–525. doi: 10.1126/science.146.3643.524. [DOI] [PubMed] [Google Scholar]
- 5.Chang TMS. Stabilization of enzyme by microencapsulation with a concentrated protein solution or by crosslinking with glutaraldehyde. Biochem Biophys Res Commun. 1971;44:1531–1533. doi: 10.1016/s0006-291x(71)80260-7. [DOI] [PubMed] [Google Scholar]
- 6.Gould SA, et al. The life-sustaining capacity of human polymerized hemoglobin when red cells might be unavailable. J Am Coll Surg. 2002;195:445–452. doi: 10.1016/s1072-7515(02)01335-2. [DOI] [PubMed] [Google Scholar]
- 7.Levy JH, et al. Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell transfusion after cardiac surgery: Results of a randomized, double-blind trial. J Thorac Cardiovasc Surg. 2002;124 (1):35–42. doi: 10.1067/mtc.2002.121505. [DOI] [PubMed] [Google Scholar]
- 8.Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Reviews. 2000;80 (2):681–715. doi: 10.1152/physrev.2000.80.2.681. [DOI] [PubMed] [Google Scholar]
- 9.Arthurs GJ, Sudhakar M. Carbon dioxide transport. Cont Ed Anaesth. 2005;5 (6):207–210. [Google Scholar]
- 10.Bauer C, Gros G, Bartels H, editors. Biophysics and Physiology of Carbon Dioxide; Symposium Held at the University of Regensburg (FRG); April 17–20, 1979; New York: Springer-Verlag; 1980. [Google Scholar]
- 11.Monanty AK, Kannan KK, Mahajan SK. Human carbonic anhydrase I: Effects of specific site mutations on its function. J Biosci. 1998;3:235–246. [Google Scholar]
- 12.Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacol Ther. 1997;74 (1):1–20. doi: 10.1016/s0163-7258(96)00198-2. [DOI] [PubMed] [Google Scholar]
- 13.D’Agnillo F, Chang TMS. Polyhemoglobin-superoxide dismutase, catalase as a blood substitute with antioxidant properties. Nature Biotechnol. 1998;16:667–671. doi: 10.1038/nbt0798-667. [DOI] [PubMed] [Google Scholar]
- 14.Henry RP. Techniques for measuring carbonic anhydrase activity in vitro. In: Dodgson SJ, Tashian RE, Gros G, Carter ND, editors. The Carbonic Anhydrases. New York: Plenum Press; 1991. pp. 119–126. [Google Scholar]
- 15.Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem. 1948;176:147–154. [PubMed] [Google Scholar]
- 16.Zhu HL, Du QQ, Chen C, Chang TMS. The immunological properties of stroma-free polyhemolysate containing catalase and superoxide dismutase activities prepared by polymerized bovine stroma-free hemolysate. Artificial Cells, Blood Substitutes, and Biotechnology. 2010;38:57–63. doi: 10.3109/10731191003634232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyton AC, Hall JE. Textbook of Medical Physiology. 10. Philadelphia: W. B. Saunders Company; 2000. pp. 1–1064. [Google Scholar]
- 18.Philpot FJ, St Philpot JL. A modified colorimetric estimation of carbonic anhydrase. Biochem J. 1937;30 (12):2191–2193. doi: 10.1042/bj0302191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hlastala MP, Berger AJ. Physiology of Respiration. 2. New York: Oxford University Press; 2001. pp. 1–275. [Google Scholar]
- 20.Chang TMS. Blood replacement with engineered hemoglobin and hemoglobin nanocapsules. Wiley Interdisciplinary Reviews on Nanomedicine and Nanobiotechnology. 2010;2:418–430. doi: 10.1002/wnan.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang TMS. Nanobiotechnology for hemoglobin based blood substitutes. Critical Care Clinics. 2009;25:373–382. doi: 10.1016/j.ccc.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]