Abstract
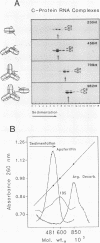
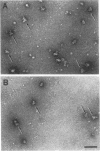
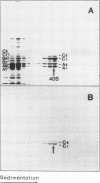
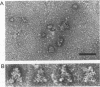
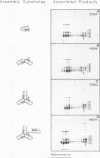
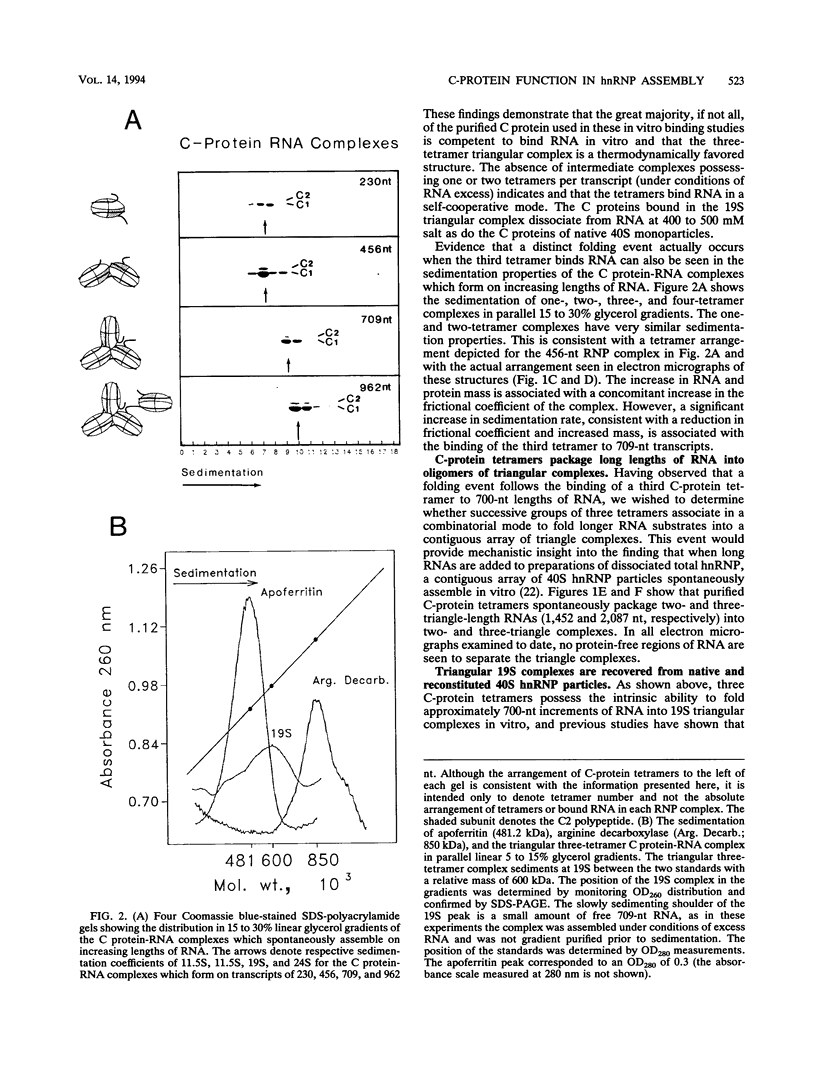
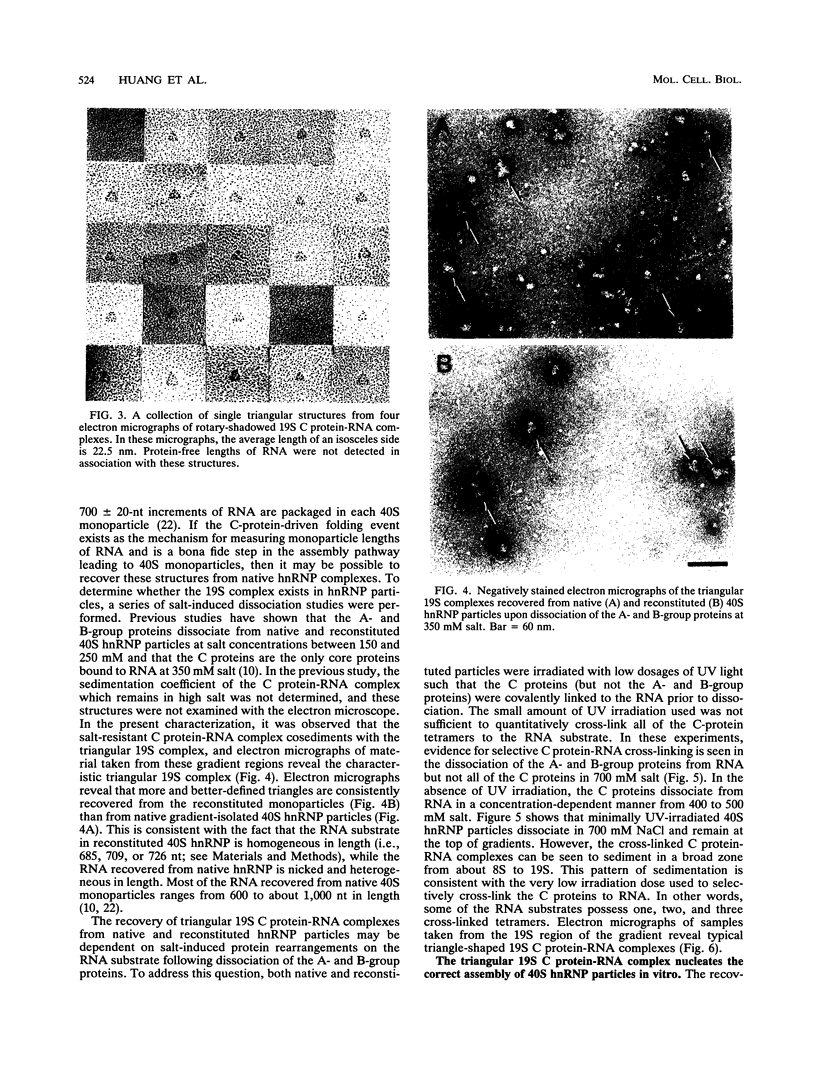
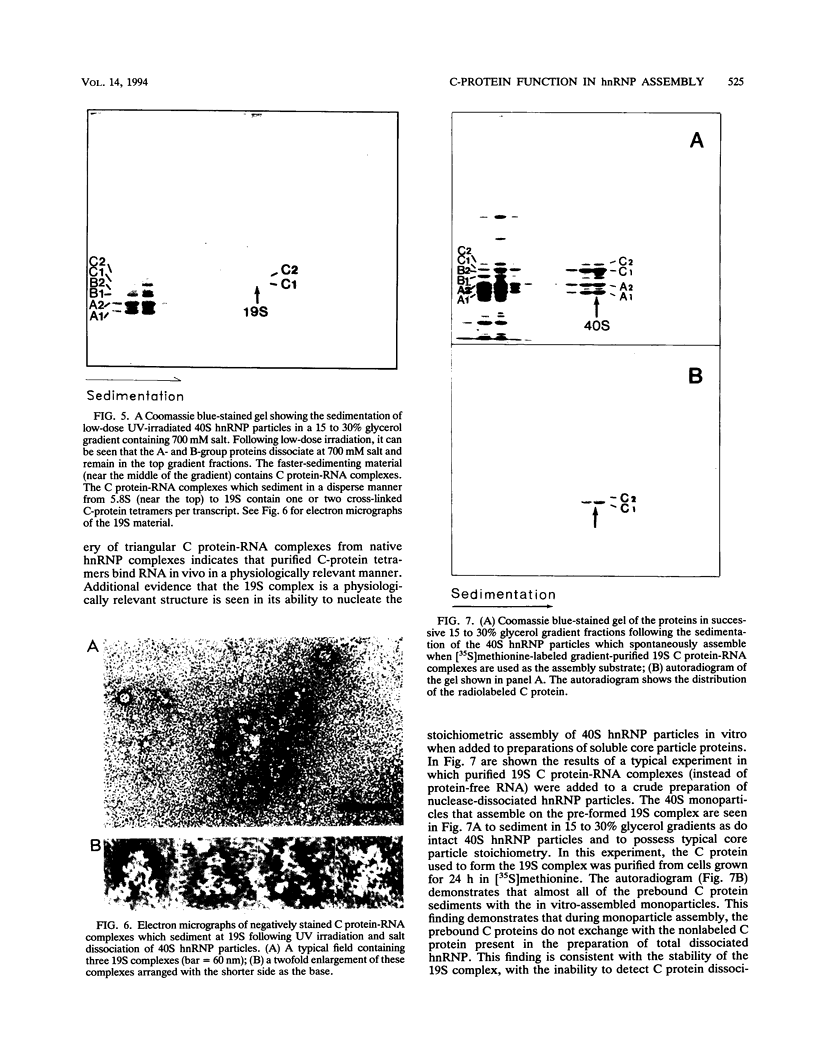
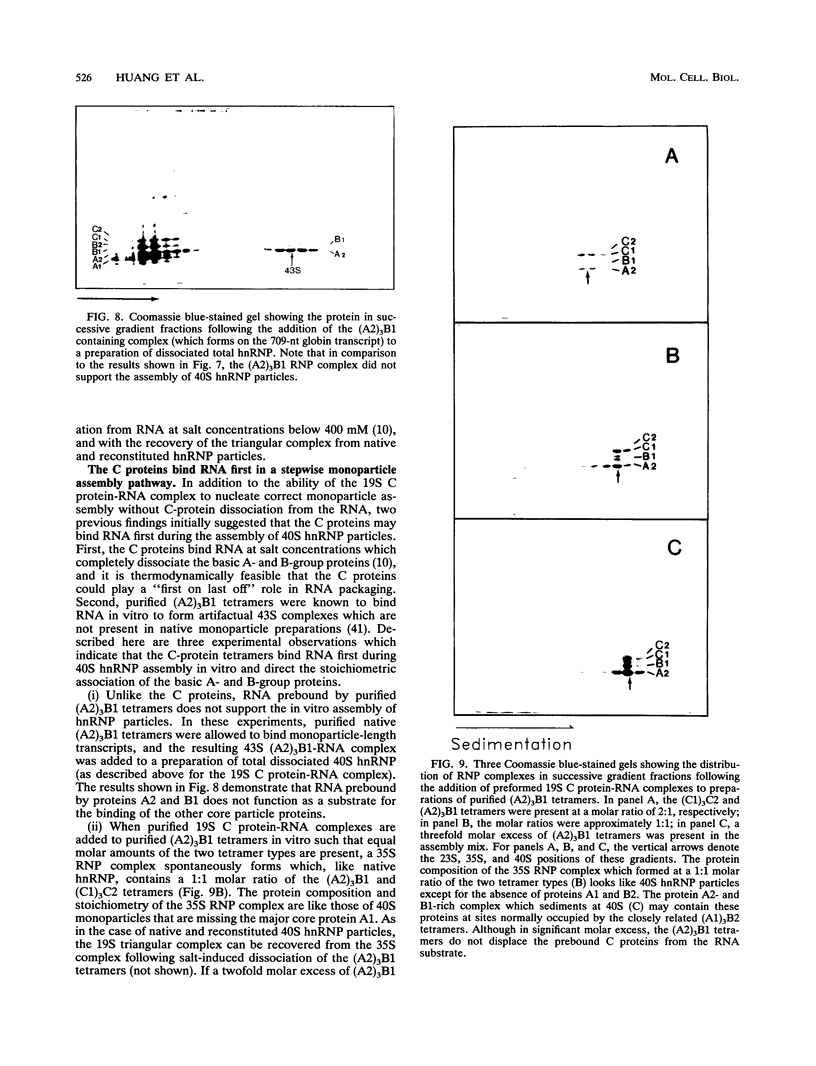
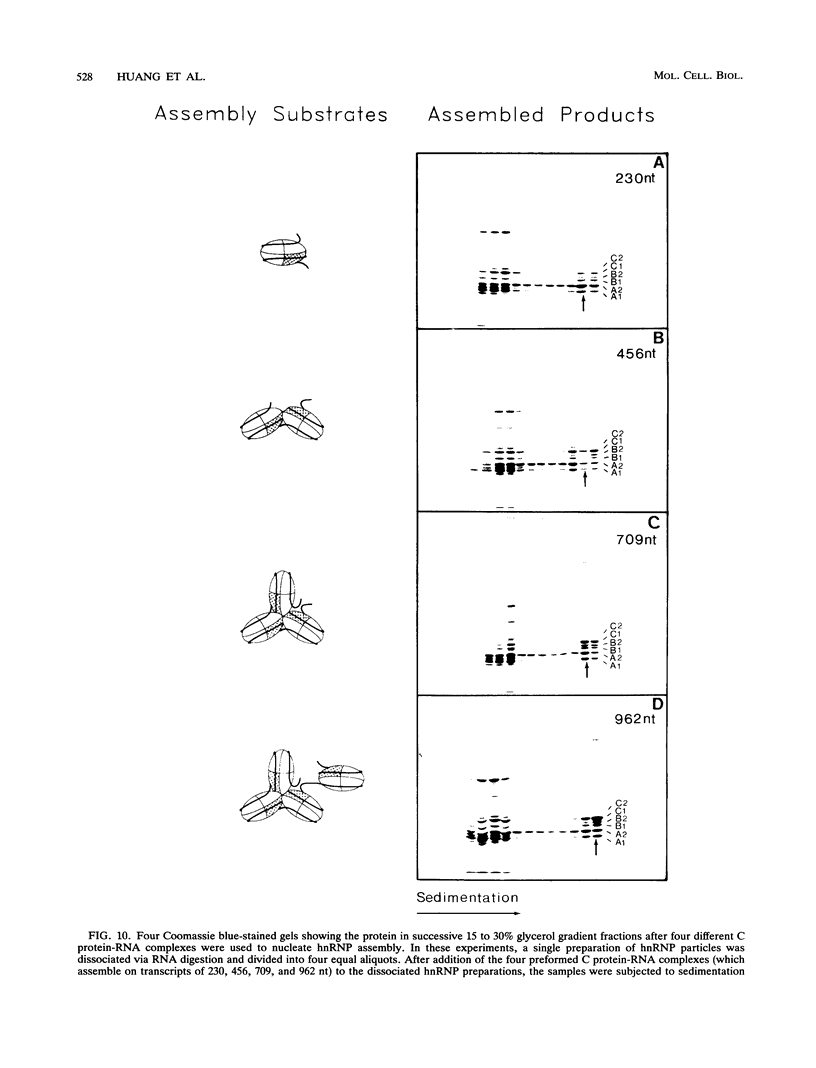
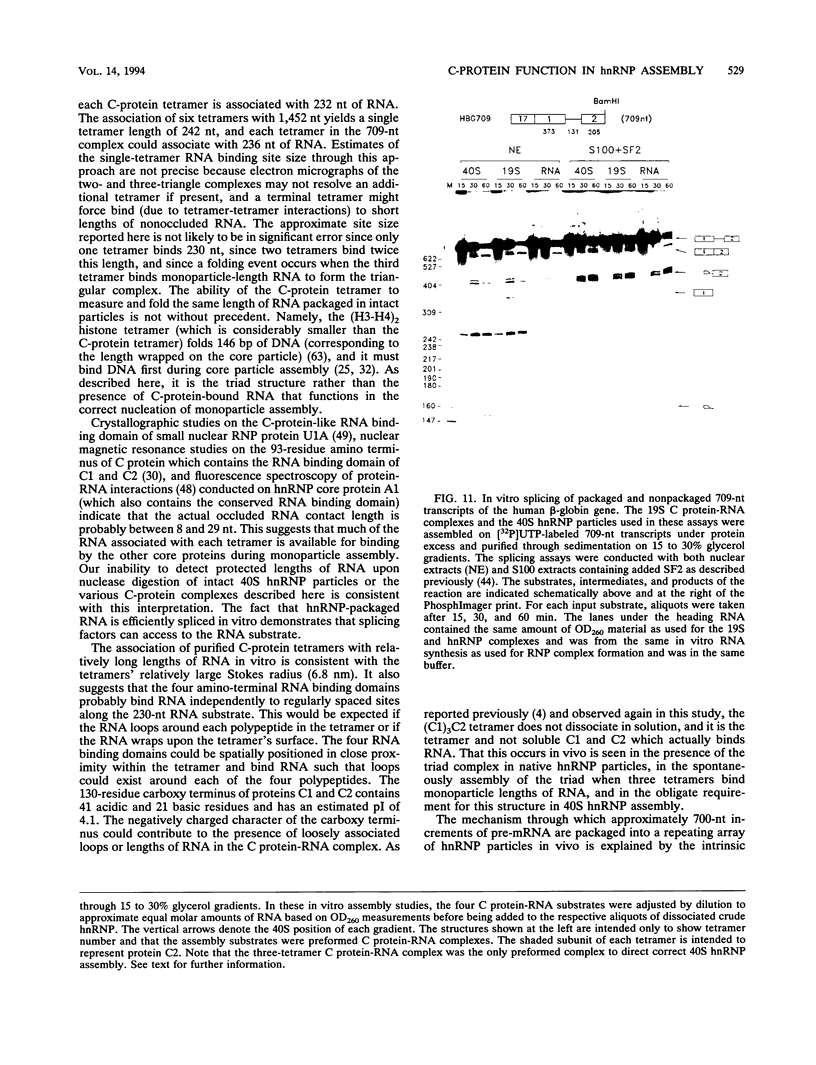
A series of in vitro protein-RNA binding studies using purified native (C1)3C2 and (A2)3B1 tetramers, total soluble heterogeneous nuclear ribonucleoprotein (hnRNP), and pre-mRNA molecules differing in length and sequence have revealed that a single C-protein tetramer has an RNA site size of 230 to 240 nucleotides (nt). Two tetramers bind twice this RNA length, and three tetramers fold monoparticle lengths of RNA (700 nt) into a unique 19S triangular complex. In the absence of this unique structure, the basic A- and B-group proteins bind RNA to form several different artifactual structures which are not present in preparations of native hnRNP and which do not function in hnRNP assembly. Three (A2)3B1 tetramers bind the 19S complex to form a 35S assembly intermediate. Following UV irradiation to immobilize the C proteins on the packaged RNA, the 19S triangular complex is recovered as a remnant structure from both native and reconstituted hnRNP particles. C protein-RNA complexes composed of three, six, or nine tetramers (one, two, or three triangular complexes) nucleate the stoichiometric assembly of monomer, dimer, and trimer hnRNP particles. The binding of C-protein tetramers to RNAs longer than 230 nt is through a self-cooperative combinatorial mode. RNA packaged in the 19S complex and in 40S hnRNP particles is efficiently spliced in vitro. These findings demonstrate that formation of the triangular C protein-RNA complex is an obligate first event in the in vitro and probably the in vivo assembly the 40S hnRNP core particle, and they provide insight into the mechanism through which the core proteins package 700-nt increments of RNA. These findings also demonstrate that unless excluded by other factors, the C proteins are likely to be located along the length of nascent transcripts.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Amero S. A., Raychaudhuri G., Cass C. L., van Venrooij W. J., Habets W. J., Krainer A. R., Beyer A. L. Independent deposition of heterogeneous nuclear ribonucleoproteins and small nuclear ribonucleoprotein particles at sites of transcription. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8409–8413. doi: 10.1073/pnas.89.18.8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ast G., Goldblatt D., Offen D., Sperling J., Sperling R. A novel splicing factor is an integral component of 200S large nuclear ribonucleoprotein (InRNP) particles. EMBO J. 1991 Feb;10(2):425–432. doi: 10.1002/j.1460-2075.1991.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett S. F., Friedman D. L., LeStourgeon W. M. The C proteins of HeLa 40S nuclear ribonucleoprotein particles exist as anisotropic tetramers of (C1)3 C2. Mol Cell Biol. 1989 Feb;9(2):492–498. doi: 10.1128/mcb.9.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett S. F., LeStourgeon W. M., Friedman D. L. Rapid purification of native C protein from nuclear ribonucleoprotein particles. J Biochem Biophys Methods. 1988 May;16(1):87–97. doi: 10.1016/0165-022x(88)90106-6. [DOI] [PubMed] [Google Scholar]
- Barnett S. F., Northington S. J., LeStourgeon W. M. Isolation and in vitro assembly of nuclear ribonucleoprotein particles and purification of core particle proteins. Methods Enzymol. 1990;181:293–307. doi: 10.1016/0076-6879(90)81130-m. [DOI] [PubMed] [Google Scholar]
- Barnett S. F., Theiry T. A., LeStourgeon W. M. The core proteins A2 and B1 exist as (A2)3B1 tetramers in 40S nuclear ribonucleoprotein particles. Mol Cell Biol. 1991 Feb;11(2):864–871. doi: 10.1128/mcb.11.2.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David Y., Bani M. R., Chabot B., De Koven A., Bernstein A. Retroviral insertions downstream of the heterogeneous nuclear ribonucleoprotein A1 gene in erythroleukemia cells: evidence that A1 is not essential for cell growth. Mol Cell Biol. 1992 Oct;12(10):4449–4455. doi: 10.1128/mcb.12.10.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M., Piñol-Roma S., Staknis D., Dreyfuss G., Reed R. Differential binding of heterogeneous nuclear ribonucleoproteins to mRNA precursors prior to spliceosome assembly in vitro. Mol Cell Biol. 1992 Jul;12(7):3165–3175. doi: 10.1128/mcb.12.7.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A. L., Christensen M. E., Walker B. W., LeStourgeon W. M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977 May;11(1):127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- Brunel C., Lelay M. N. Two-dimensional analysis of proteins associated with heterogenous nuclear RNA in various animal cell lines. Eur J Biochem. 1979 Sep;99(2):273–283. doi: 10.1111/j.1432-1033.1979.tb13254.x. [DOI] [PubMed] [Google Scholar]
- Burd C. G., Swanson M. S., Görlach M., Dreyfuss G. Primary structures of the heterogeneous nuclear ribonucleoprotein A2, B1, and C2 proteins: a diversity of RNA binding proteins is generated by small peptide inserts. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9788–9792. doi: 10.1073/pnas.86.24.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvoli M., Biamonti G., Tsoulfas P., Bassi M. T., Ghetti A., Riva S., Morandi C. cDNA cloning of human hnRNP protein A1 reveals the existence of multiple mRNA isoforms. Nucleic Acids Res. 1988 May 11;16(9):3751–3770. doi: 10.1093/nar/16.9.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvoli M., Cobianchi F., Bestagno M. G., Mangiarotti A., Bassi M. T., Biamonti G., Riva S. Alternative splicing in the human gene for the core protein A1 generates another hnRNP protein. EMBO J. 1990 Apr;9(4):1229–1235. doi: 10.1002/j.1460-2075.1990.tb08230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvoli M., Cobianchi F., Bestagno M., Bassi M. T., Biamonti G., Riva S. A second A1-type protein is encoded by the human hnRNP A1 gene. Mol Biol Rep. 1990;14(2-3):83–84. doi: 10.1007/BF00360425. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Bravo R., Arenstorf H. P., LeStourgeon W. M. Identification of proliferation-sensitive human proteins amongst components of the 40 S hnRNP particles. Identity of hnRNP core proteins in the HeLa protein catalogue. FEBS Lett. 1986 Jan 1;194(1):101–109. doi: 10.1016/0014-5793(86)80059-x. [DOI] [PubMed] [Google Scholar]
- Choi Y. D., Dreyfuss G. Isolation of the heterogeneous nuclear RNA-ribonucleoprotein complex (hnRNP): a unique supramolecular assembly. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7471–7475. doi: 10.1073/pnas.81.23.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobianchi F., SenGupta D. N., Zmudzka B. Z., Wilson S. H. Structure of rodent helix-destabilizing protein revealed by cDNA cloning. J Biol Chem. 1986 Mar 15;261(8):3536–3543. [PubMed] [Google Scholar]
- Conway G., Wooley J., Bibring T., LeStourgeon W. M. Ribonucleoproteins package 700 nucleotides of pre-mRNA into a repeating array of regular particles. Mol Cell Biol. 1988 Jul;8(7):2884–2895. doi: 10.1128/mcb.8.7.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominski Z., Kole R. Selection of splice sites in pre-mRNAs with short internal exons. Mol Cell Biol. 1991 Dec;11(12):6075–6083. doi: 10.1128/mcb.11.12.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F., van Holde K. E. Nucleosome positioning is determined by the (H3-H4)2 tetramer. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10596–10600. doi: 10.1073/pnas.88.23.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. Assembly of nuclear ribonucleoprotein particles during in vitro transcription. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1469–1473. doi: 10.1073/pnas.79.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. Structure of nuclear ribonucleoprotein: heterogeneous nuclear RNA is complexed with a major sextet of proteins in vivo. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1599–1602. doi: 10.1073/pnas.80.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiferman I., Hamilton M. G., Pogo A. O. Nucleoplasmic ribonucleoprotein particles of rat liver. I. Selective degradation by nuclear nucleases. Biochim Biophys Acta. 1970 Apr 15;204(2):550–563. doi: 10.1016/0005-2787(70)90175-9. [DOI] [PubMed] [Google Scholar]
- Görlach M., Wittekind M., Beckman R. A., Mueller L., Dreyfuss G. Interaction of the RNA-binding domain of the hnRNP C proteins with RNA. EMBO J. 1992 Sep;11(9):3289–3295. doi: 10.1002/j.1460-2075.1992.tb05407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M., Baumeister W. High resolution negative staining of ferritin molecules on vermiculite single crystal supports. Biochim Biophys Acta. 1974 Nov 5;371(1):267–274. doi: 10.1016/0005-2795(74)90176-7. [DOI] [PubMed] [Google Scholar]
- Hansen J. C., van Holde K. E., Lohr D. The mechanism of nucleosome assembly onto oligomers of the sea urchin 5 S DNA positioning sequence. J Biol Chem. 1991 Mar 5;266(7):4276–4282. [PubMed] [Google Scholar]
- Harris S. G., Hoch S. O., Smith H. C. Chemical cross-linking of Sm and RNP antigenic proteins. Biochemistry. 1988 Jun 28;27(13):4595–4600. doi: 10.1021/bi00413a002. [DOI] [PubMed] [Google Scholar]
- Harris S. G., Martin T. E., Smith H. C. Reversible chemical cross-linking and ribonuclease digestion analysis of the organization of proteins in ribonucleoprotein particles. Mol Cell Biochem. 1988 Nov;84(1):17–28. doi: 10.1007/BF00235189. [DOI] [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Isolation and characterization of ribonucleoprotein particles containing heterogeneous nuclear RNA. Methods Cell Biol. 1978;17:377–399. doi: 10.1016/s0091-679x(08)61155-3. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T., Ruskin B., Green M. R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984 Apr;36(4):993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- LeStourgeon W. M., Beyer A. L., Christensen M. E., Walker B. W., Poupore S. M., Daniels L. P. The packaging proteins of core hnRNP particles and the maintenance of proliferative cell states. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):885–898. doi: 10.1101/sqb.1978.042.01.090. [DOI] [PubMed] [Google Scholar]
- Leser G. P., Martin T. E. Changes in heterogeneous nuclear RNP core polypeptide complements during the cell cycle. J Cell Biol. 1987 Nov;105(5):2083–2094. doi: 10.1083/jcb.105.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothstein L., Arenstorf H. P., Chung S. Y., Walker B. W., Wooley J. C., LeStourgeon W. M. General organization of protein in HeLa 40S nuclear ribonucleoprotein particles. J Cell Biol. 1985 May;100(5):1570–1581. doi: 10.1083/jcb.100.5.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukanidin E. M., Zalmanzon E. S., Komaromi L., Samarina O. P., Georgiev G. P. Structure and function of informofers. Nat New Biol. 1972 Aug 16;238(85):193–197. doi: 10.1038/newbio238193a0. [DOI] [PubMed] [Google Scholar]
- Malcolm D. B., Sommerville J. The structure of nuclear ribonucleoprotein of amphibian oocytes. J Cell Sci. 1977 Apr;24:143–165. doi: 10.1242/jcs.24.1.143. [DOI] [PubMed] [Google Scholar]
- Mayeda A., Krainer A. R. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell. 1992 Jan 24;68(2):365–375. doi: 10.1016/0092-8674(92)90477-t. [DOI] [PubMed] [Google Scholar]
- Merrill B. M., Barnett S. F., LeStourgeon W. M., Williams K. R. Primary structure differences between proteins C1 and C2 of HeLa 40S nuclear ribonucleoprotein particles. Nucleic Acids Res. 1989 Nov 11;17(21):8441–8449. doi: 10.1093/nar/17.21.8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P., Martin T. E., Riehl R. M. Nucleic acid binding characteristics of group A/B hnRNP proteins. Biochem Biophys Res Commun. 1991 Apr 30;176(2):747–755. doi: 10.1016/s0006-291x(05)80248-7. [DOI] [PubMed] [Google Scholar]
- Minoo P., Sullivan W., Solomon L. R., Martin T. E., Toft D. O., Scott R. E. Loss of proliferative potential during terminal differentiation coincides with the decreased abundance of a subset of heterogeneous ribonuclear proteins. J Cell Biol. 1989 Nov;109(5):1937–1946. doi: 10.1083/jcb.109.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler S. G., Merrill B. M., Roberts W. J., Keating K. M., Lisbin M. J., Barnett S. F., Wilson S. H., Williams K. R. Interactions of the A1 heterogeneous nuclear ribonucleoprotein and its proteolytic derivative, UP1, with RNA and DNA: evidence for multiple RNA binding domains and salt-dependent binding mode transitions. Biochemistry. 1991 Mar 19;30(11):2968–2976. doi: 10.1021/bi00225a034. [DOI] [PubMed] [Google Scholar]
- Nagai K., Oubridge C., Jessen T. H., Li J., Evans P. R. Crystal structure of the RNA-binding domain of the U1 small nuclear ribonucleoprotein A. Nature. 1990 Dec 6;348(6301):515–520. doi: 10.1038/348515a0. [DOI] [PubMed] [Google Scholar]
- Nakagawa T. Y., Swanson M. S., Wold B. J., Dreyfuss G. Molecular cloning of cDNA for the nuclear ribonucleoprotein particle C proteins: a conserved gene family. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2007–2011. doi: 10.1073/pnas.83.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planck S. R., Listerud M. D., Buckley S. D. Modulation of hnRNP A1 protein gene expression by epidermal growth factor in Rat-1 cells. Nucleic Acids Res. 1988 Dec 23;16(24):11663–11673. doi: 10.1093/nar/16.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Schenkel J., Sekeris C. E., Alonso A., Bautz E. K. RNA-binding properties of hnRNP proteins. Eur J Biochem. 1988 Feb 1;171(3):565–569. doi: 10.1111/j.1432-1033.1988.tb13825.x. [DOI] [PubMed] [Google Scholar]
- Spann P., Feinerman M., Sperling J., Sperling R. Isolation and visualization of large compact ribonucleoprotein particles of specific nuclear RNAs. Proc Natl Acad Sci U S A. 1989 Jan;86(2):466–470. doi: 10.1073/pnas.86.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Kamen R. Arrangement of 30S heterogeneous nuclear ribonucleoprotein on polyoma virus late nuclear transcripts. Mol Cell Biol. 1981 Jan;1(1):21–34. doi: 10.1128/mcb.1.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988 May;8(5):2237–2241. doi: 10.1128/mcb.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. RNA binding specificity of hnRNP proteins: a subset bind to the 3' end of introns. EMBO J. 1988 Nov;7(11):3519–3529. doi: 10.1002/j.1460-2075.1988.tb03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Nakagawa T. Y., LeVan K., Dreyfuss G. Primary structure of human nuclear ribonucleoprotein particle C proteins: conservation of sequence and domain structures in heterogeneous nuclear RNA, mRNA, and pre-rRNA-binding proteins. Mol Cell Biol. 1987 May;7(5):1731–1739. doi: 10.1128/mcb.7.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanev R. G., Djondjurov L. P. Ultrastructure of free ribonucleoprotein complexes in spread mammalian nuclei. J Cell Biol. 1982 Sep;94(3):662–666. doi: 10.1083/jcb.94.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk H. E., Angeli G., Schäfer K. P. In vitro reconstitution of 35S ribonucleoprotein complexes. Biochemistry. 1983 Sep 13;22(19):4592–4600. doi: 10.1021/bi00288a038. [DOI] [PubMed] [Google Scholar]
- Wilk H. E., Werr H., Friedrich D., Kiltz H. H., Schäfer K. P. The core proteins of 35S hnRNP complexes. Characterization of nine different species. Eur J Biochem. 1985 Jan 2;146(1):71–81. doi: 10.1111/j.1432-1033.1985.tb08621.x. [DOI] [PubMed] [Google Scholar]
- de Haën C. Molecular weight standards for calibration of gel filtration and sodium dodecyl sulfate-polyacrylamide gel electrophoresis: ferritin and apoferritin. Anal Biochem. 1987 Nov 1;166(2):235–245. doi: 10.1016/0003-2697(87)90570-7. [DOI] [PubMed] [Google Scholar]
- van Eekelen C., Ohlsson R., Philipson L., Mariman E., van Beek R., van Venrooij W. Sequence dependent interaction of hnRNP proteins with late adenoviral transcripts. Nucleic Acids Res. 1982 Nov 25;10(22):7115–7131. doi: 10.1093/nar/10.22.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]