Abstract
Acetyl coenzyme A carboxylase B gene (ACACB) single nucleotide polymorphism (SNP) rs2268388 is reproducibly associated with type 2 diabetes (T2DM)-associated nephropathy (DN). ACACB knock-out mice are also protected from obesity. This study assessed relationships between rs2268388, body mass index (BMI) and gene expression in multiple populations, with and without T2DM. Among subjects without T2DM, rs2268388 DN risk allele (T) associated with higher BMI in Pima Indian children (n = 2021; p-additive = 0.029) and African Americans (AAs) (n = 177; p-additive = 0.05), with a trend in European Americans (EAs) (n = 512; p-additive = 0.09), but not Germans (n = 858; p-additive = 0.765). Association with BMI was seen in a meta-analysis including all non-T2DM subjects (n = 3568; p-additive = 0.02). Among subjects with T2DM, rs2268388 was not associated with BMI in Japanese (n = 2912) or EAs (n = 1149); however, the T allele associated with higher BMI in the subset with BMI≥30 kg/m2 (n = 568 EAs; p-additive = 0.049, n = 196 Japanese; p-additive = 0.049). Association with BMI was strengthened in a T2DM meta-analysis that included an additional 756 AAs (p-additive = 0.080) and 48 Hong Kong Chinese (p-additive = 0.81) with BMI≥30 kg/m2 (n = 1575; p-additive = 0.0033). The effect of rs2268388 on gene expression revealed that the T risk allele associated with higher ACACB messenger levels in adipose tissue (41 EAs and 20 AAs with BMI>30 kg/m2; p-additive = 0.018) and ACACB protein levels in the liver tissue (mixed model p-additive = 0.03, in 25 EA bariatric surgery patients with BMI>30 kg/m2 for 75 exams). The T allele also associated with higher hepatic triglyceride levels. These data support a role for ACACB in obesity and potential roles for altered lipid metabolism in susceptibility to DN.
Introduction
There is a world-wide epidemic of type 2 diabetes mellitus (T2DM) and chronic diabetes complications are major public health concerns [1]. Dyslipidemia associates with risk for T2DM and its complications, particularly diabetic nephropathy (DN) [2]. ACACB is the rate-limiting enzyme for fatty acid oxidation, and single nucleotide polymorphism (SNP) rs2268388 in the acetyl coenzyme A carboxylase B gene (ACACB) is reproducibly associated with T2DN and diabetic end-stage renal disease (ESRD) [3], [4]. Relative to the C allele, a 29-bp DNA fragment containing the T risk allele of rs2268388 demonstrated greater enhancer activity in cultured human renal proximal tubular epithelial cells, indicating higher ACACB expression in risk allele carriers [3]. Genome-wide association studies (GWAS) and the HapMap database have not revealed other SNPs in high genotypic concordance with rs2268388. In addition, the T allele of rs2268388 is overrepresented in obese women with T2DM and had higher transcription binding affinity than the C allele [5]. Therefore, rs2268388 could contribute to regulation of metabolism, alterations in lipids, and adiposity. We prioritized this SNP in order to explore potential links between predisposition to DN and lipid dysregulation in subjects with T2DM.
We previously examined association of rs2268388 and ten other common coding ACACB variants with metabolic traits and gene expression [6]. Although significant associations were detected with gene expression and several metabolic traits for coding variants, we failed to associate rs2268388 with expression quantitative trait loci (QTL) or metabolic traits. This may have been due to low allele frequencies with reduced power or undetected gene*environment interactions [6].
To assess the role of the DN-associated ACACB SNP rs2268388 on body mass index (BMI), we tested for association between this SNP and BMI in four population groups, including Europeans and European Americans (EAs), African Americans (AAs), Pima Indians and Asians. Subjects with and without T2DM were assessed, with a focus on those with high BMI. Effects on gene expression were investigated in multiple tissues and cell types.
Materials and Methods
Study samples
Table 1 contains demographic data in study participants. All participants provided written informed consent.
Table 1. Demographic data of the study cohorts.
| Cohort | Status | N(M/F) | BMI(kg/m2) | Age(yrs) |
| Leipzig German | Non-diabetic | 858(272/586) | 28.70±5.70 | 49.0±13.5 |
| Arkansas EAs | Non-diabetic | 404(129/275) | 30.30±5.92 | 38.96±10.36 |
| Utah EAs | Non-diabetic | 108(42/66) | 27.51±5.63 | 40.13±11.32 |
| Arkansas AAs | Non-diabetic | 177(71/106) | 30.68±6.07 | 39.38±9.44 |
| Pima Indians | Non-diabetic children | 2021(891/1130) | 27.01±6.31* | 13.85±3.98** |
| Arkansas adipose biopsy sample | Non-diabetic (All EAs) | 105(43/62) | 27.78±5.59 | 40.13±10.91 |
| Non-diabetic (All AAs) | 44(27/17) | 30.20±6.38 | 43.32±9.14 | |
| Non-diabetic (EAs BMI>30 kg/m2) | 41(14/27) | 34.50±3.79 | 42.30±9.60 | |
| Non-diabetic (AAs BMI>30 kg/m2) | 20(9/11) | 35.82±4.37 | 43.70±9.39 | |
| Wake Forest liver biopsy sample EAs | 26(20/6) | 43.14±11.61 | 50.08±9.91 | |
| Wake Forest liver biopsy sample AAs | 3(1/2) | 43.36±8.82 | 45.00±10.54 | |
| Wake Forest EAs | Diabetic (All) | 1149(555/594) | 31.02±7.13 | 64.21±9.82 |
| Diabetic (BMI>30 kg/m2) | 568(236/332) | 36.57±5.60 | 61.65±8.76 | |
| Wake Forest AAs | Diabetic (All) | 1446(593/853) | 32.43±14.29 | 59.41±10.41 |
| Diabetic (BMI>30 kg/m2) | 756(259/497) | 38.56±17.10 | 57.27±9.85 | |
| Japanese | Diabetic (All) | 2912(1697/1215) | 24.0±3.8 | 63.2±10.7 |
| Diabetic (BMI>30 kg/m2) | 196(82/114) | 32.8±3.3 | 55.3±13.1 | |
| HK Chinese | Diabetic (All) | 596(317/279) | 24.56±4.20 | 66.82±10.90 |
| Diabetic (BMI>30 kg/m2) | 48(29/19) | 33.83±4.53 | 60.32±12.20 | |
| Pima Indians | Non-diabetic Pima children eventually developed DM | 642(259/383) | max adult BMI 40.64±8.79***BMI at first DM visit 38.88±8.41 | age at max adult BMI 33.67±9.97age at first DM visit 32.78±9.45 |
| Pima Indians All | 3197(1353/1844) | max BMI 37.02±8.75 | age at max BMI 35.06±14.21 |
EA: European American; AA: African American.
The analysis used age & sex standardized z scores (mean: 0.288±1.04). Mean shown is mean of max z-scores converted to BMI units using mean & SD of 12 year-old females.
Age shown is age at max BMI z-score; not necessarily same as age at max BMI.
max BMI from age >15 yrs. N = 637 since 5 kids had no exams after age 15.
Wake Forest School of Medicine (WFSM) Diabetes Heart Study (DHS) and African American-Diabetes Heart Study (AA-DHS)
Subjects with T2DM in the DHS (n = 1149 EAs), AA-DHS and AA T2D-ESRD studies (n = 1446 AAs) were born in North Carolina, South Carolina, Georgia, Tennessee, or Virginia. Those with a history of ketoacidosis or developing DM before the age of 25 years and receiving continuous insulin treatment since diagnosis were excluded. For patients with ESRD, maximal pre-dialysis BMI values were utilized. Study procedures were approved by the WFSM Institutional Review Board (IRB) [7], [8].
University of Utah (UT) and University of Arkansas (AR) samples
EAs (n = 512) and AAs (n = 177) without T2DM ascertained in UT and AR were evaluated under separate IRB-approved protocols at the University of Utah Health Sciences Center and University of Arkansas for Medical Sciences (UMAS) [9]. Participants had normal 75 g oral glucose tolerance tests (OGTTs). A subset of the AR sample underwent adipose biopsy (below).
Pima Indian population-based samples
A population-based sample of full-heritage Pima Indians (n = 3,197) derived from the longitudinal study of the etiology of T2DM in the Gila River Indian Community in Central Arizona was evaluated [10]. This study was approved by the National Institute of Diabetes and Digestive and Kidney Diseases IRB and included related individuals with biennial exams measuring height, weight, and a 75-g OGTT (WHO 1999 criteria). In the sample of full-heritage Pima Indians, BMI was included for exams after the age of 5 years (19,385 BMI measures in 3,197 subjects). In a subset of the full sample, Pima children aged 5–19 years had their maximum BMI recorded at a time when they were not yet diagnosed with T2DM (n = 2,021).
German samples
Non-diabetic Europeans (n = 858) recruited at University Hospital in Leipzig, Germany were included in a study approved by the ethics committee. Diabetes was defined using WHO 1999 criteria.
Japanese samples
Samples from outpatient diabetes clinics at Shiga University of Medical Science, Tokyo Women's Medical University, Kawai Clinic, and Tokai University Hospital were evaluated under IRB approved protocols [3]. Diabetes was defined using WHO 1999 criteria, and type 2 diabetes patients with normoalbuminuria or with microalbuminuria were included in the present analysis.
Hong Kong samples
Chinese subjects born in Hong Kong or southern China were evaluated [4]. T2DM was diagnosed in those treated with oral hypoglycemic agents and/or insulin, in the absence of insulin-only treatment after one year of T2DM. The study was approved by the IRBs at University of Hong Kong/Hospital Authority Hong Kong West, Kowloon Central and East Clusters of Hospitals and met criteria in the Declaration of Helsinki.
Genotyping
SNP rs2268388 was genotyped in WFSM and Hong Kong samples using the MassARRAY genotyping system (Sequenom Inc., San Diego, CA). PCR primers were designed using the MassARRAY Assay Design 3.4 Software (Sequenom Inc., San Diego, CA). The minimum SNP call rate for an individual was 98.4%. Forty-six blind duplicates were genotyped with a concordance rate of 99.6%. The SNP was genotyped in UT, AR, Pima Indian and German samples using the TaqMan genotyping reaction. DNA was amplified on a GeneAmp PCR system 9700 (95°C for 10 min, followed by 40 cycles of 95°C for 30 s and 60°C for 1 min 30 s), and fluorescence was detected on an ABI Prism 7700 or 7500 (Applied BioSystems). Sequence information for oligonucleotide primers and probes is available upon request. The overall genotyping call rate exceeded 98%. Duplicate quality control (QC) samples were randomly distributed in the UT/AR (n = 69) and Pima (n = 150) plates assuring ∼99% reproducibility. To assess genotyping reproducibility in the German cohort, a random ∼5% selection of the sample was re-genotyped and the genotypic concordance reached 100%. In the Japanese cohort, the SNP was genotyped using a multiplex-PCR-invader assay. The call rate for the SNP was 99.1% and a concordance rate in duplicate samples (n = 1,289) was 99.6% [3].
Adipose Biopsy
149 AR non-diabetic subjects underwent a fasting adipose biopsy under local lidocaine anesthesia [11]. Tissue was obtained using a Bergstrom needle from abdominal sub-cutaneous fat. Samples were rinsed in sterile saline, quick frozen in liquid nitrogen and stored at −80°C.
Liver Biopsy
Twenty-five EAs undergoing laparoscopic gastric banding or Roux-en-Y gastric bypass surgery at WFSM agreed to an intra-operative wedge liver biopsy in an IRB-approved study. Patients with liver disease other than non-alcoholic fatty liver disease (macrosteatosis in the setting of hepatic ballooning, inflammation or fibrosis), malignancy, coagulopathy or need for anticoagulation, chronic inflammatory diseases, or ethanol intake ≥105 g/week (or ≥45 g/day) were excluded. Hepatic lipid analysis methods in these wedge biopsies immediately snap frozen and stored at −80°C have been reported [12]. Briefly, lipids from 50–100 mg of minced tissue were extracted in 2∶1 chloroform∶methanol overnight and triglycerides (TGs) quantified using enzymatic assays after addition of Triton-X100 (TG and cholesterol - Roche Diagnostics; unesterified cholesterol - Wako Chemicals USA) [13], [14]. TG content was calculated as mg (TG)/g liver total cell lysate protein.
RNA isolation and gene transcription arrays
Total RNA was isolated from adipose using the RNAeasy Lipid Tissue Mini kit (Qiagen, Valencia, CA). The quantity and quality of the isolated RNA were determined by ultraviolet spectrophotometry and electrophoresis using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Genome-wide transcriptome analysis was performed on the AR adipose biopsy sample using Human HT-12 v4 Expression BeadChip (Illumina, San Diego,CA) whole-genome gene expression array according to the vendor-recommended protocol.
Western Blot
Liver samples were homogenized in 5× volume of phosphate-buffered saline relative to tissue weight and protease inhibitors added. Sodium dodecyl sulfate buffer (1% sodium dodecyl sulfate, 10 mmol/L Tris, pH 7.6) was added in the amount of the homogenate and sonicated. Equal amounts of protein for each sample were separated by 4% to 20% sodium dodecyl sulfate-polyacrylamide (SDS) gel electrophoresis, transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA) and blocked for 1 hour at room temperature with Tris-buffered saline containing 1% skim milk powder, 0.1% Tween 20. Blots were incubated overnight at 4°C with a polyclonal anti-ACACB antibody (1∶1,000, Sigma, HPA006554). Membranes were washed 3 times in Tris-buffered saline containing 0.1% Tween 20 and incubated for 1 hour in the blocking buffer with anti-rabbit IgG conjugated to horseradish peroxidase (1∶20,000; Jackson Immuno-Research, West Grove, PA, USA). The bound antibodies were visualized using enhanced chemiluminescence (Super Signal West Pico; Thermo Pierce, Rockford, IL, USA) and recorded on X-ray film. The bands were scanned and densities quantitated using Image J (http://rsbweb.nih.gov/ij/). Liver lysate samples were randomized before being loaded on the SDS gels for three batches of replicated experiments.
Statistical Analyses
Statistical analyses were performed using SAS 9.1 software (SAS Institute; Cary, NC). A generalized linear model (GENMOD) was used to assess associations between genotype and BMI, ACACB gene expression in adipose and ACACB protein level in liver. For additive model analyses, homozygotes for allele (1/1), heterozygotes (1/2), and homozygotes for allele (2/2) were coded to a continuous variable (0, 1, and 2). Generalized estimating equations (GEE) were used to account for sibships in the UT EAs, Wake Forest, and Pima subjects, in addition to adjustment for age, sex, and BMI. The SNP was analyzed using the same genetic model in all analyses, regardless of the study sample. A meta-analysis was performed using the Stouffer method [15]. Mixed model analyses were applied for multiple exams of continuous variables (BMI for Pima Indians and ACACB protein levels by Western blot) to account for repeat values. P-values<0.05 were considered to represent nominal levels of statistical significance.
Results
Association with BMI in subjects without diabetes
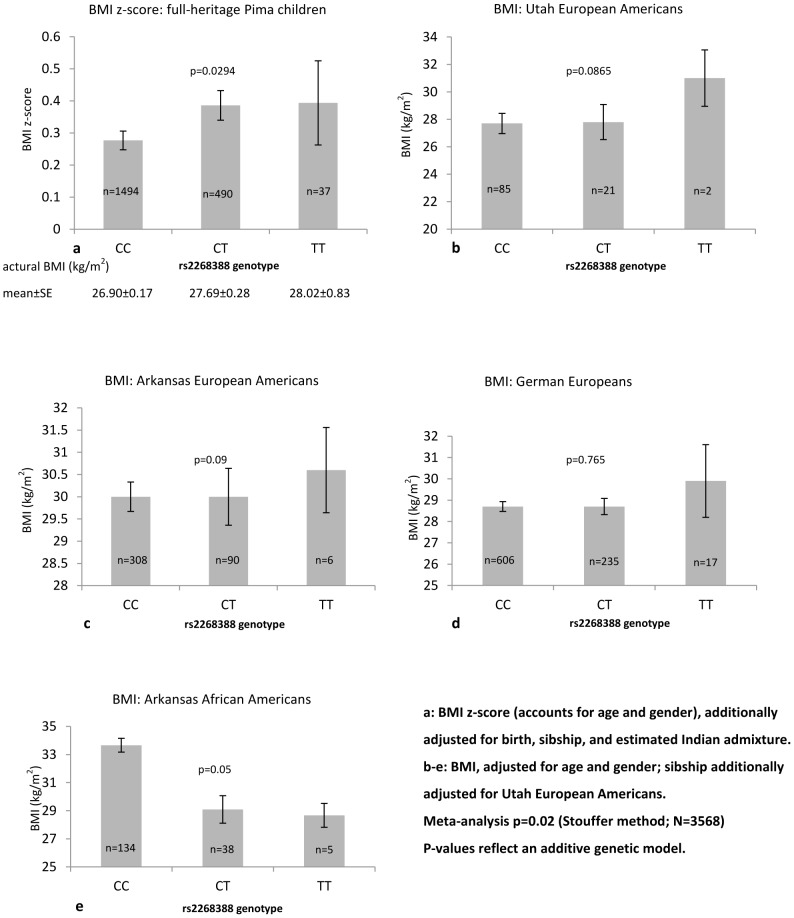
Figure 1 summarizes association results for BMI with rs2268388 in non-diabetic Germans (European), UT EAs, AR EAs, AR AAs and Pima Indians. Significant association was seen in Pima Indian children (p = 0.0294); while the three European-derived populations revealed non-statistically significant trends consistent in direction. Association in non-diabetic AR AAs was nominally significant but opposite in direction. A meta-analysis revealed significant association (p = 0.02 Stouffer method) combining all populations, mainly driven by Pima Indian children ( Figure 1 ).
Figure 1. Association of rs2268388 with BMI in non-diabetic subjects.
Association with BMI in subjects with diabetes and BMI>30 kg/m2
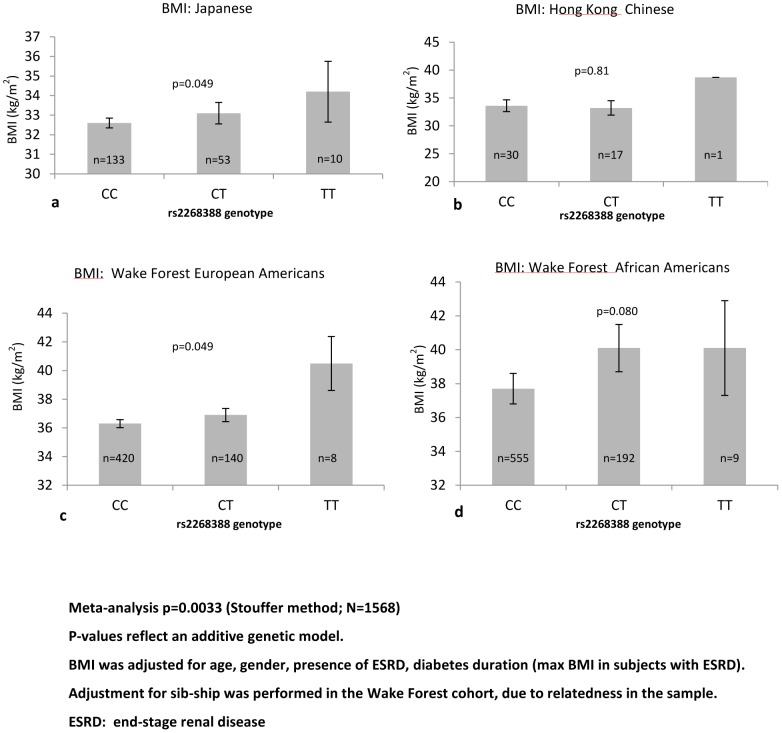
No significant association of BMI with rs2268388 was detected in diabetic subjects within each cohort or the combined sample (data not shown). However, stratified analyses revealed rs2268388 associated with BMI in subjects with T2DM and BMI≥30 kg/m2 in Japanese (p = 0.049) and WFSM EAs (p = 0.049), with a non-significant trend in WFSM diabetic AAs (p = 0.08). No association was seen in Hong Kong Chinese (p = 0.81), but the direction was consistent. A meta-analysis in combined WFSM AAs, WF EAs, Japanese and Hong Kong Chinese with T2DM and BMI≥30 kg/m2 revealed significant evidence of association (p = 0.0033; Figure 2 ).
Figure 2. Association of rs2268388 with BMI in subjects with type 2 diabetes and BMI≥30 kg/m2.
Association with BMI in Pima Indians
BMI z-scores were evaluated in all full-heritage Pima subjects <18 years of age who had at least one non-diabetic exam and who then subsequently developed T2DM (Model 1, n of subjects = 642, N of exams = 5321). In addition, all BMI exams in all recorded full-heritage Pima Indians genotyped for rs2268388 were evaluated in Model 2 (n of subjects = 3197, N of exams = 19385). BMI was not associated with rs2268388 in Model 1 (mixed model analysis p = 0.265, Table 2 ). However, when all BMI exam z-scores were examined in Model 2, a trend toward association was seen (mixed model analysis p = 0.0755; Table 2 ). Subjects in model 1 had diabetes, with BMIs recorded longitudinally after their initial non-diabetic exams. Pima Indians have a high prevalence of T2D; a majority will ultimately develop T2DM. Therefore, we included both models with the full WF AA, WF EA, Japanese and Hong Kong Chinese T2DM study samples. A meta-analysis for BMI and rs2268388 in all subjects with T2DM revealed significant association (Stouffer method p = 0.0022 with Model 1 and p = 0.01 with Model 2, respectively; Table 2 ).
Table 2. Association of rs2268388(C/T) with BMI in full-heritage Pima Indian longitudinal cohort.
| Analysis | CC | CT | TT | Beta | P-value |
| BMI z-score±SE | BMI z-score ±SE | BMI z-score ±SE | |||
| (n_CC/N_exams) | (n_CT/N_exams) | (n_TT/N_exams) | |||
| model 1 | 0.253±0.038 | 0.373±0.058 | 0.047±0.206 | 0.065 | 0.26531 |
| (470/3987) | (162/1257) | (10/77) | |||
| Meta-analysis together with 4 cohorts (Japanese, HK Chinese, Wake Forest EAs and AAs) in Figure 2 (Stouffer method) | 0.0022 | ||||
| model 2 | −0.002±0.020 | 0.063±0.031 | 0.039±0.097 | 0.052 | 0.07551 |
| (2367/14422) | (765/4565) | (65/398) | |||
| Meta-analysis together with 4 cohorts (Japanese, HK Chinese, Wake Forest EAs and AAS) in Figure 2 (Stouffer method) | 0.01 | ||||
model 1: > = 1 nondiabetic exam age <18 and subsequently developed DM; total n = 642; total exam N = 5321;
P values were adjusted for age, gender, sibship, and repeated exams (matrix: autoregressive; additive genetic model).
model 2: All individuals- all examinations; total n = 3197; total exam N = 19385;
P values were adjusted for age, gender, sibship, diabetic status, duration of diabetes, and repeated exams (matrix: autoregressive; additive genetic model).
T allele is defined as risk allele for higher BMI z-score.
n_CC, n_CT, n_TT: number of individuals in each genotypic group. N_exams: number of exams.
Association with ACACB messenger level in non-diabetic subjects
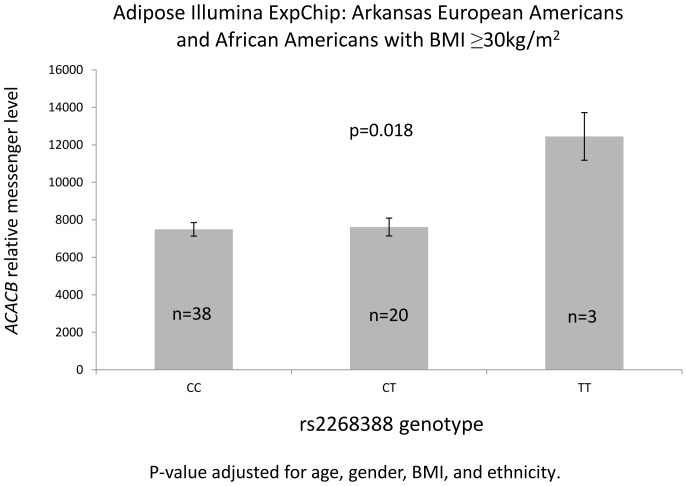
The messenger level of ACACB was measured by Human HT-12 v4 BeadChip (Illumina, San Diego,CA) whole-genome gene expression array in AR non-diabetic EAs and AAs with subcutaneous adipose tissue (all had an OGTT). Significant association was not observed in the full biopsy sample (n = 149, data not shown). A nominally significant association between ACACB messenger level and rs2268388 was seen in subjects with BMI≥30 kg/m2 (p = 0.018) adjusting for age, gender, BMI, and ethnicity ( Figure 3 ), where the (T) risk allele for higher ACACB messenger level was consistent with associations with higher BMI.
Figure 3. Adipose ACACB expression by rs2268388.
Association with liver ACACB protein level
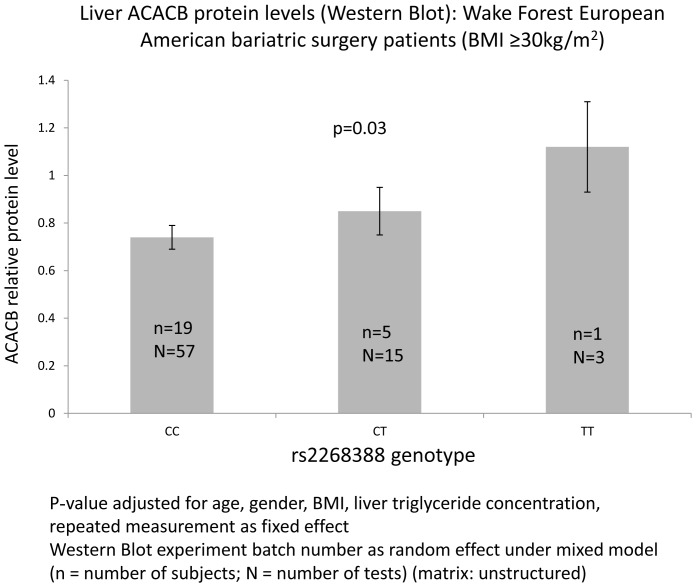
Figure 4 reveals significant association between liver ACACB protein level and rs2268388 in EAs who underwent bariatric surgery (BMI>30 kg/m2, n = 25 with 75 total Western blot exams; p = 0.03). The (T) risk allele for higher ACACB protein level was consistent with associations with higher BMI.
Figure 4. Liver ACACB expression by rs2268388.
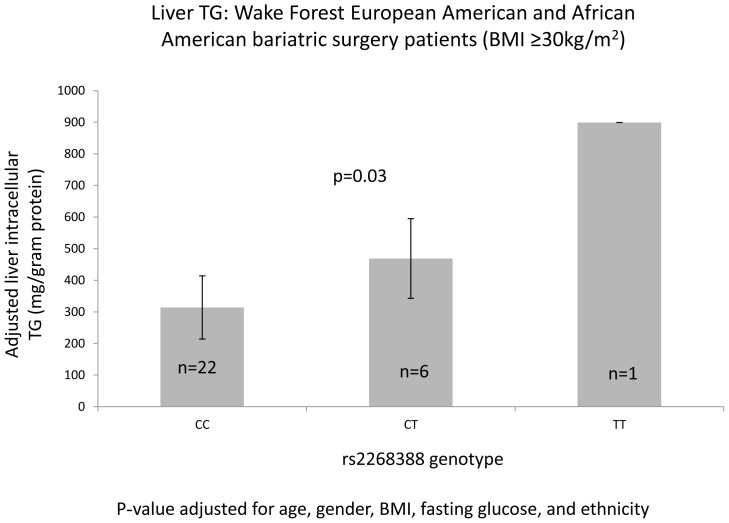
Association with hepatic triglycerides (TGs)
Figure 5 reveals that rs2268388 was associated with higher hepatic intracellular TGs in 26 EA and 3 AA subjects who underwent bariatric surgery (BMI>30 kg/m2; p = 0.03). The (T) risk allele for higher TG was consistent with associations with higher BMI.
Figure 5. Liver cell triglyceride (TG) by rs2268388.
Discussion
ACACB is a rate-limiting enzyme involved in mitochondrial fatty acid oxidation and plays a key role in fatty acid metabolism [16]–[19]. The current results reveal that ACACB SNP rs2268388 (reproducibly implicated in risk of nephropathy) associates with BMI in general populations and with obesity in subjects with T2D, as well as impacts gene expression in adipose and hepatic tissue. ACACB cis SNPs reportedly regulate ACACB gene expression, providing potential roles for effects on adiposity [6]. Adipose ACACB expression was negatively associated with adiposity (p = 0.0002, r = −0.35) in EAs [6] and Pima Indians (p = 4.3×10−12, r = −0.45, personal communication [20]). Feed-back regulation has been observed by significant down-regulation of ACACB mRNA with free fatty-acid (palmitate or oleate) treatment [21], as well as reductions in ACACB protein expression (data not shown). This implicates a complex cross-regulation between ACACB gene expression and adiposity.
Although ACACB is a physiological candidate gene for obesity and diabetes, significant association has not been observed in GWAS. This may reflect the effect of gene*gene or gene*environment interactions. A recent gene*environment interaction study of ACACB variants in metabolism suggests that undiscovered causative variants may have been overlooked [22]. Associations between ACACB and obesity in general populations can be masked by environmental factors such as lifestyle and eating behaviors linked with development of adiposity. Acacb knock-out mice are less likely to gain weight when fed high-fat diets compared to their wild type littermates; however, significant differences in body weight were not observed when fed normal chow [16]. This phenomenon has been observed in other rodent models where overweight phenotypes are conditional on diet [23], [24].
ACACB variant rs2268388 is reproducibly associated with DN [3], [4]. There is also evidence implicating altered lipid metabolism in the pathogenesis of DN [25], [26]. PPARA agonists (fibrates), up-regulating fatty acid oxidation, may improve DN in humans [27], [28] and animal models [29]. Accelerated DN was observed in mice lacking the peroxisome proliferator-activated receptor alpha [30]. The mechanism of ACACB association with DN remains unknown.
ACACB variant rs2268388 was also associated with severe obesity in Spanish women, no data were provided in men [5]. We chose to perform the association analyses for this SNP with BMI in multiple samples, including general populations of non-diabetic subjects, subjects with T2DM, and subjects with T2DM and BMI≥30 kg/m2. We selected non-diabetic Pima childhood max BMI z-score to represent non-diabetic BMI measures for Pimas, since adult BMIs are usually high and largely impacted by environmental factors, and are very close to the first visit diabetic BMIs. However, it is informative to know that significant association was not observed between rs2268388 and maximum BMI in adult Pima Indians when non-diabetic (detailed data not shown). Our finding of association with BMI in obese African American subjects with T2D was consistent with the report in Spanish women [5]. Women, not men, contributed to the significant association in AAs (Figure S1-1); whereas gender interaction was not observed in EAs (Figure S1-2). In full-heritage Pima subjects <18 years of age who had at least one non-diabetic exam and who then subsequently developed T2DM (Model 1), there was a weak trend that men, not women, with T allele were heavier, and genotype*gender interaction was borderline significant; however no significant gender interaction was observed in all full-heritage Pimas for all recorded BMI exams (Model 2) (Table S1). Due to limited sample power for obese diabetic subjects of the Japanese and Chinese cohorts, additional gene*gender interaction analysis was not attempted for those two ethnic groups. Severe obesity in the Spanish population was defined as BMI≥35 kg/m2, whereas we chose BMI≥30 kg/m2 to capture obese Japanese and Hong Kong Chinese subjects. Although association with high BMI in any single population was relatively weak, overall effects in combined samples appeared stronger. BMI data in Pima Indians from an on-going half-century long longitudinal study were included. Full-heritage Pima Indians have minimum admixture from other populations and many had BMI records starting in childhood. This allowed us to perform mixed model analyses testing association of rs2268388 with BMI z-scores from multiple exams in every individual, rather than a single exam in each subject. This was important since BMI changes during life. The majority of Pima subjects' BMI exceed 34 kg/m2 in adulthood for the younger generations (Figure S2) and 80% of those aged above 55 years have T2DM [31].
ACACB gene expression by genotype revealed the same pattern with BMI in our combined Arkansas EA and AA samples, the rs2268388 DN “T” risk allele represented higher expression levels with BMI≥30 kg/m2. Higher ACACB expression is linked to obesity and insulin resistance in a mouse model fed a high-fat diet [17]. ACACB RNA expression levels were also elevated in transformed lymphoblast cell lines from subjects with the T allele in a combined analysis of CEU (Utah European ancestry population), Japanese and Yoruba samples (HapMap-Sanger gene expression database, Figure S3) (http://www.hapmap.org & ftp://ftp.sanger.ac.uk/pub/genevar/). Two groups reported that Acacb knock-out mice had similar body weights as wild type mice [32], [33]. Molecular explanations for the phenotypic differences observed between Olson's model [32] of Acacb deletion and that of Abu-Elheiga et al [16] resistant to obesity, diabetes and insulin resistance are unclear. The Acacb biotin-binding site was deleted in both models, but using different approaches. The targeting strategy employed in the original study replaced only the exon containing the biotin binding motif [16]. RNA splicing across the targeting cassette could have left the mRNA in frame, resulting in a mutated but otherwise intact protein lacking a catalytic domain. Such a protein could have “dominant negative” activity toward ACACA [33] where both ACACB and ACACA activities are inhibited. This is consistent with the effects of soraphen, an inhibitor of both ACACA and ACACB which improved peripheral insulin sensitivity in mice fed a high-fat diet [34].
Our report benefits from a liver tissue repository allowing performance of a Western blot analysis assessing ACACB protein levels in hepatic cell lysates from obese subjects undergoing bariatric surgery (Figure S4). Subjects with the T risk allele tended to have higher ACACB protein levels in liver cells, consistent with association of this SNP with ACACB RNA levels in adipose tissue from non-diabetic EA and AA subjects with BMI≥30 kg/m2. In addition, bariatric surgery subjects with the T allele tended to have higher liver TG levels. Abu-Elheiga et al. reported that Acacb knock-out mice were also protected against fatty liver during high-fat, high-carbohydrate diet and de novo lipogenic conditions [35]. These data support that metabolic changes with mutant ACACB may be conditional on nutritional status. The ACACB pathway may be a useful target for ameliorating metabolic syndrome.
Limitations of this study were small numbers of Asian samples and all except the Pima lacked longitudinal BMI data. The sample numbers in our gene expression studies had limited statistical power. However, we estimate the liver cohort had approximately 0.80 genetic power for detecting 20% of the variation in protein expression levels (assuming type 1 error rate = 0.005) using mixed models for triplicate Western blot analyses under unstructured matrix and adjustment to account for across experiment variance.
We conclude that the T allele for ACACB SNP rs2268388 associates with higher BMI in subjects with diabetes and BMI≥30 kg/m2 and in general non-diabetic populations. The T allele also associates with higher ACACB expression in subjects with elevated BMI at the RNA (adipose) and protein levels (liver). Intracellular hepatic TGs are also elevated with this allele. GWAS for BMI, obesity and T2DM may not identify metabolism-related genes involved in these processes when nutritional status and lifestyles are assumed to be homogenous. ACACB variants may regulate gene expression and impact BMI; however, adiposity can also impact ACACB gene expression and balance the effects of genetic variation [5]. The ACACB rs2268388 association with DN has been replicated in multiple populations and the T risk allele for DN appears consistent with that of higher BMI, elevated liver TGs and metabolic cell gene expression. Alterations in lipid metabolism may jointly impact development of DN and obesity.
Supporting Information
BMI vs. rs2268388 in Wake Forest diabetic men and women. S1-1. BMI vs. rs2268388 in Wake Forest diabetic African Americans (AAs) (a: men BMI>30 kg/m2; b: women BMI>30 kg/m2; c: men; d: women). S1-2. BMI vs. rs2268388 in Wake Forest diabetic European Americans (EAs) (BMI>30 kg/m2) (a: men; b: women).
(JPG)
BMI distribution by age for different birth era groups in non-diabetic full-heritage Pima Indians.
(JPG)
ACACB expression in transformed lymphoblast cell lines in HapMap/Sanger gene expression data set (in CEU, JPT, &YRI).
(JPG)
Liver ACACB protein level (an example gel image for Western Blot) in Caucasians (underwent bariatric surgery: BMI>30 kg/m2).
(JPG)
Association of rs2268388(C/T) with BMI in full-heritage Pima Indian men and women.
(PDF)
Acknowledgments
The authors are grateful to Sanger Institute and HapMap Project investigators for generously making the gene expression and genotyping data publicly available. We would like to thank Dr. Tomoya Umezono, Dr. Masao Toyoda at Division of Nephrology and Metabolism, Department of Internal Medicine, Tokai University School of Medicine, Dr. Shin-ichi Araki at Department of Medicine, Shiga University of Medical Science, Dr. Koichi Kawai at Kawai clinic and staffs of BioBank Japan at Institute of Medical Science, University of Tokyo, for recruiting Japanese samples, and Dr. William C. Knowler and clinical staff at NIDDK Phoenix Epidemiology and Clinical Research Branch for recruiting Pima Indian subjects for the longitudinal studies, as well as members of the Gila River Indian community of Central Arizona for their long term collaboration and contribution in the longitudinal study over half a century. We thank the technical staff of WFSM Genome Center, RIKEN Center for Genomic Medicine, and NIDDK Phoenix Branch for their technical assistance. This work is dedicated to the memory of the late Dr. Steven C. Elbein.
Funding Statement
This study was supported by a Wake Forest School of Medicine Translational Science Institute Synergy Grant K99 DK081350, National Institutes of Health extramural grants R01 DK071891, R01 HL67348, R01 DK53591, R01 DK039311, and partially supported by R0171349, a Merit Grant from the Veterans Administration, and NIDDK intramural research program. The Japanese study was partly supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan (S.M.). P.K. (Leipzig) was funded by Boehringer Ingelheim Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, et al. (2004) The evolving diabetes burden in the United States. Ann Intern Med 140: 945–950. [DOI] [PubMed] [Google Scholar]
- 2. Rosario RF, Prabhakar S (2006) Lipids and diabetic nephropathy. Curr Diab Rep 6: 455–462. [DOI] [PubMed] [Google Scholar]
- 3. Maeda S, Kobayashi MA, Araki S, Babazono T, Freedman BI, et al. (2010) A single nucleotide polymorphism within the acetyl-coenzyme A carboxylase beta gene is associated with proteinuria in patients with type 2 diabetes. PLoS Genet 6: e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang SC, Leung VT, Chan LY, Wong SS, Chu DW, et al. (2010) The acetyl-coenzyme A carboxylase beta (ACACB) gene is associated with nephropathy in Chinese patients with type 2 diabetes. Nephrol Dial Transplant 25: 3931–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riancho JA, Vázquez L, García-Pérez MA, Sainz J, Olmos JM, et al. (2011) Association of ACACB polymorphisms with obesity and diabetes. Mol Genet Metab 104: 670–676. [DOI] [PubMed] [Google Scholar]
- 6. Ma L, Mondal AK, Murea M, Sharma NK, Tönjes A, et al. (2011) The effect of ACACB cis-variants on gene expression and metabolic traits. PLoS One 6: e23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agarwal S, Morgan T, Herrington DM, Xu J, Cox AJ, et al. (2011) Coronary calcium score and prediction of all-cause mortality in diabetes: the diabetes heart study. Diabetes Care 34: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Divers J, Wagenknecht LE, Bowden DW, Carr JJ, Hightower RC, et al. (2010) Ethnic differences in the relationship between pericardial adipose tissue and coronary artery calcified plaque: African-American-diabetes heart study. J Clin Endocrinol Metab 95: 5382–5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elbein SC, Chu WS, Das SK, Yao-Borengasser A, Hasstedt SJ, et al. (2007) Transcription factor 7-like 2 polymorphisms and type 2 diabetes, glucose homeostasis traits and gene expression in US participants of European and African descent. Diabetologia 50: 1621–1630. [DOI] [PubMed] [Google Scholar]
- 10. Hanson RL, Bogardus C, Duggan D, Kobes S, Knowlton M, et al. (2007) A search for variants associated with young-onset type 2 diabetes in American Indians in a 100K genotyping array. Diabetes 56: 3045–3052. [DOI] [PubMed] [Google Scholar]
- 11. Das SK, Sharma NK, Hasstedt SJ, Mondal AK, Ma L, et al. (2011) An integrative genomics approach identifies activation of thioredoxin/thioredoxin reductase-1-mediated oxidative stress defense pathway and inhibition of angiogenesis in obese nondiabetic human subjects. J Clin Endocrinol Metab 96: E1308–E1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shores NJ, Link K, Fernandez A, Geisinger KR, Davis M, et al. (2011) Non-contrasted computed tomography for the accurate measurement of liver steatosis in obese patients. Dig Dis Sci 56: 2145–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509. [PubMed] [Google Scholar]
- 14. Carr TP, Andresen CJ, Rudel LL (1993) Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Brioche 26: 39–42. [DOI] [PubMed] [Google Scholar]
- 15.Stouffer SA, Suchman EA, DaVinney LC, Star SA, Williams RM (1949) How the volumes were produced. In The American Soldier, Volume I: Adjustment to Army Life Princeton, NJ: Princeton University Press. 45 p.
- 16. Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ (2001) Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 291: 2613–2616. [DOI] [PubMed] [Google Scholar]
- 17. Abu-Elheiga L, Oh W, Kordari P, Wakil SJ (2003) Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci U S A 100: 10207–10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oh W, Abu-Elheiga L, Kordari P, Gu Z, Shaikenov T, et al. (2005) Glucose and fat metabolism in adipose tissue of acetyl-CoA carboxylase 2 knockout mice. Proc Natl Acad Sci U S A 102: 1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wakil SJ, Abu-Elheiga LA (2009) Fatty acid metabolism: target for metabolic syndrome. J Lipid Res 50 Suppl: S138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanson RL (2012) personal communication.
- 21. Das SK, Mondal AK, Elbein SC (2010) Distinct gene expression profiles characterize cellular responses to palmitate and oleate. J Lipid Res 51: 2121–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phillips CM, Goumidi L, Bertrais S, Field MR, Cupples LA, et al. (2010) ACC2 gene polymorphisms, metabolic syndrome, and gene-nutrient interactions with dietary fat. J Lipid Res 51: 3500–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhuang LN, Hu WX, Zhang ML, Xin SM, Jia WP, et al. (2011) Beta-arrestin-1 protein represses diet-induced obesity. J Biol Chem 286: 28396–28402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lenz LS, Marx J, Chamulitrat W, Kaiser I, Gröne HJ, et al. (2011) Adipocyte-specific inactivation of Acyl-CoA synthetase fatty acid transport protein 4 (Fatp4) in mice causes adipose hypertrophy and alterations in metabolism of complex lipids under high fat diet. J Biol Chem 286: 35578–35587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Z, Jiang T, Li J, Proctor G, McManaman JL, et al. (2005) Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 54: 2328–3235. [DOI] [PubMed] [Google Scholar]
- 26. Taneja D, Thompson J, Wilson P, Brandewie K, Schaefer L, et al. (2010) Reversibility of renal injury with cholesterol lowering in hyperlipidemic diabetic mice. J Lipid Res 51: 1464–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keech A, Simes RJ, Barter P, Best J, Scott R, et al. (2005) Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366: 1849–1861. [DOI] [PubMed] [Google Scholar]
- 28. Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, et al. (2010) Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 376: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park CW, Zhang Y, Zhang X, Wu J, Chen L, et al. (2006) PPARalpha agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int 69: 1511–1517. [DOI] [PubMed] [Google Scholar]
- 30. Park CW, Kim HW, Ko SH, Chung HW, Lim SW, et al. (2006) Accelerated diabetic nephropathy in mice lacking the peroxisome proliferator-activated receptor alpha. Diabetes 55: 885–893. [DOI] [PubMed] [Google Scholar]
- 31.Knowler WC (2007) personal communication.
- 32. Olson DP, Pulinilkunnil T, Cline GW, Shulman GI, Lowell BB (2010) Gene knockout of Acc2 has little effect on body weight, fat mass, or food intake. Proc Natl Acad Sci U S A 107: 7598–7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoehn KL, Turner N, Swarbrick MM, Wilks D, Preston E, et al. (2010) Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab 11: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schreurs M, van Dijk TH, Gerding A, Havinga R, Reijngoud DJ, et al. (2009) Soraphen, an inhibitor of the acetyl-CoA carboxylase system, improves peripheral insulin sensitivity in mice fed a high-fat diet. Diabetes Obes Metab 11: 987–991. [DOI] [PubMed] [Google Scholar]
- 35. Abu-Elheiga L, Wu H, Gu Z, Bressler R, Wakil SJ (2012) Acetyl-CoA Carboxylase 2−/− Mutant Mice are Protected against Fatty Liver under High-fat, High-carbohydrate Dietary and de Novo Lipogenic Conditions. J Biol Chem 287: 12578–12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BMI vs. rs2268388 in Wake Forest diabetic men and women. S1-1. BMI vs. rs2268388 in Wake Forest diabetic African Americans (AAs) (a: men BMI>30 kg/m2; b: women BMI>30 kg/m2; c: men; d: women). S1-2. BMI vs. rs2268388 in Wake Forest diabetic European Americans (EAs) (BMI>30 kg/m2) (a: men; b: women).
(JPG)
BMI distribution by age for different birth era groups in non-diabetic full-heritage Pima Indians.
(JPG)
ACACB expression in transformed lymphoblast cell lines in HapMap/Sanger gene expression data set (in CEU, JPT, &YRI).
(JPG)
Liver ACACB protein level (an example gel image for Western Blot) in Caucasians (underwent bariatric surgery: BMI>30 kg/m2).
(JPG)
Association of rs2268388(C/T) with BMI in full-heritage Pima Indian men and women.
(PDF)