Abstract
Gene therapy has the potential to provide minimally invasive and long-term treatment for many inherited disorders that otherwise have poor prognoses and limited treatment options. The sustained therapeutic correction of genetic disease by viral gene transfer has been accomplished in patients with severe immune deficiencies, or by the transduction of an immune privileged site for the treatment of ocular disease. For other diseases and target tissues, immune responses to vectors or transgene products often present major obstacles for therapy. Innate and adaptive immunity, sometimes including preexisting or memory responses, may contribute by varying degrees to immune-mediated rejection and immunotoxicity. This review provides an overview of the immune responses to in vivo gene transfer with the most commonly used viral gene therapy vectors, and discusses strategies and protocols employed in evading the immune system in order to provide optimal gene therapy.
Keywords: Gene therapy, immune tolerance, AAV, adenovirus, lentivirus, immunity
Introduction
After the early hope and preclinical success of gene therapy were tempered by clinical setbacks, the field is beginning to witness the emergence of promising data in not only animal studies, but also in clinical trials such as those for Leber congenital amaurosis, adenosine deaminase deficiency and one type of muscular dystrophy [1–3]. Progress has been made to overcome the major technical limitations of gene therapy, such as low expression of the therapeutic gene and limited tropism, but some barriers to success still remain. One of the primary hurdles is the immune system. Mammals have evolved complex mechanisms to protect themselves against invading pathogens, including those that many viral gene-transfer vectors are derived from. Viral vectors can invoke an innate immune response via several pathways, such as the sensing of pathogen-associated molecular patterns on vector particles or in the vector genome [4]. The activation of downstream pathways can elicit antiviral and proinflammatory signals that recruit effector lymphocytes, inhibit viral transduction and stimulate the elimination of transduced cells by the priming of an adaptive immune response. Even if these initial barriers against the vector are overcome, the therapeutic transgene product may be either altered or completely absent in many of the monogenetic diseases that are typically targeted by gene therapy. For example, the de novo expression of a wild-type protein may trigger an adaptive immune response and the release of antibodies (which may be T-cell-dependent) or CTLs, or both, that mediate the destruction of transgene-expressing cells. Figure 1 outlines the sequence of interactions between the gene transfer vector, the gene products it encodes and the immune system. Although non-viral vectors, while beyond the scope of this review, may avoid some of the immune responses triggered by viral vectors, many barriers still have to be overcome with regard to efficiency of cell transduction, transgene expression and toxicity; non-viral vectors may also stimulate the innate and adaptive arm of the immune system [5]. This review highlights the immune responses to some of the most common vectors used in gene transfer protocols. In addition, concepts and strategies to circumvent or block these responses upon in vivo gene transfer are discussed (see Figures 2 and 3 for an overview), and specific examples are provided to illustrate their practical implementation.
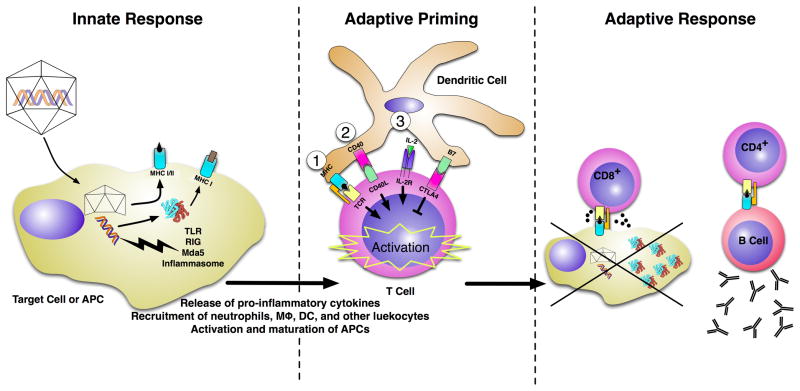
Figure 1. The immune response to viral vectors.
The immune response to viral vectors occurs in three stages: innate response (A), adaptive priming (B) and adaptive response (C).
(A) Innate response. Upon infection of the target cell or an APC, the virus uncoats and releases its genome. At this stage, the viral genome can be recognized by TLRs, Rig-1, Mda-5 or the inflammasome. The activation of these proteins results in the release of proinflammatory cytokines, which recruit other leukocytes (including neutrophils, macrophages and dendritic cells) to the area and cause an upregulation of costimulatory molecules on APCs, leading to APC activation and maturation. Meanwhile, the capsid can be degraded and presented on MHC class I (via cross-presentation) to mark the cell for future destruction by CD8+ T-cells. Capsid epitopes can also be presented by MHC class II on infected professional APCs to initiate an antibody response that is mediated by CD4+ T-cells. The transgene protein product can also flag the cell for destruction by CD8+ T-cells via classical MHC class I presentation. APCs that have been activated by the innate response induce adaptive immunity by presenting the antigens to T-cells in the context of costimulation. (B) Adaptive priming. T-cells typically require three signals for activation and proliferation: (1) the antigen-specific recognition of self-MHC:peptide on the APC by the T-cell receptor (TCR); (2) costimulatory molecule binding at the surface of both APC and T-cells (eg, CD28:CD80/86, B7:CD28 and CD40:CD40-ligand [CD40L]); and (3) stimulation by growth factors, such as the cytokines IL-15, IL-12 or IL-2. Cell-surface-molecule interactions can also lead to inhibition; for example, CTL antigen 4 (CTLA4) binds with high affinity to B7 on the APC and sends an inhibitory signal to the T-cell. (C) Adaptive response. Activated effector T-cells proliferate and carry out their respective effector function. CD8+ T-cells recognize infected cells via MHC:TCR and kill them via release of cytotoxic granules containing perforin and granzymes.. CD4+ T-cells can activate plasma B-cells to produce neutralizing antibodies against the transgene product or the virus capsid.
IL-2R IL-2 receptor
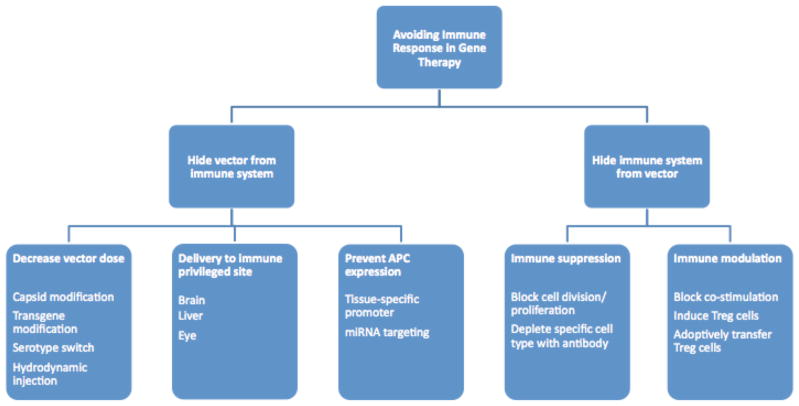
Figure 2. Strategies to avoid immune responses in gene therapy.
Attempts to avoid an immune response in gene therapy can be divided into two categories: those that hide the vector and its transgene product from the immune system, and those that hide the immune system from the vector/transgene product. In either scenario, depending on the tissue and protocol, it may be possible to ultimately present such antigens to the immune system without inflammatory signals, thereby promoting immune tolerance.
miRNA microRNA, Treg regulatory T-cell
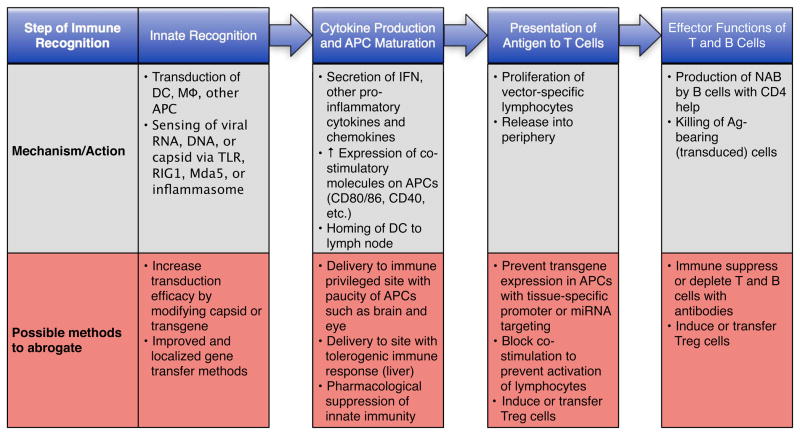
Figure 3. Steps in immune recognition and methods of intervention.
The first contact the vector has with the immune system is through innate immunity, which typically results in a cytokine response within an hour. The most prolific producers of antiviral cytokines are APCs, such as plasmacytoid dendritic cells (DCs), conventional DCs, macrophages (Mφ), and B-cells. The sensing of the viral vector can occur through TLRs, Rig-1, Mda-5 or the inflammasome. These sensors, which are located in the cytoplasm, the endosome or on the cell surface, detect the vector by recognition of the viral capsid/envelope, DNA or RNA. Cytokines and other signals that result from the ligation of these sensors induce upregulation of costimulatory molecules on the APCs, a process termed maturation. Mature DCs then home to lymphoid tissues where they present viral antigens to naïve effector T-cells (the activation requirements for APCs may be reduced in a secondary immune response as memory lymphocytes are more easily activated). T-cells that recognize a particular viral antigen become activated and proliferate, before they migrate into the periphery. These activated T-cells can then perform their respective effector function. The MHC class II presentation of peptides by B-cells to CD4+ T-cells can result in B-cell activation, which may subsequently produce antibodies against the vector or transgene product. CD8+ T-cells will specifically kill cells that present antigens via MHC class I recognition. For each step, potential countermeasures are listed.
Ag antigen, miRNA microRNA, NAB neutralizing antibody
Immune responses to viral vectors
Adenovirus (Ad) vectors were initially attractive for gene therapy applications because of their large packaging capacity, ability to efficiently transduce many non-dividing cell types and ease of production [6]. However, Ad vectors provoke a robust innate immune response via complement activation, and both TLR-dependent and TLR-independent mechanisms [4]. In the liver, which is the tissue most abundantly transduced following systemic administration, Ad immediately causes an increase in the expression of several proinflammatory chemokines, such as RANTES, IP-10, MIP-1β, MIP-2 and MCP-1, followed by the infiltration of neutrophils and other CD11b+ cells into the liver [7]. In addition to provoking innate immunity, pre-existing neutralizing antibodies (NABs) against the commonly used AdHu5 vector may further restrict the efficiency of gene therapy. A gutted helper-dependent Ad vector that contains no viral genes has been developed in an attempt to reduce immunogenicity; however, this vector still evokes the upregulation of many proinflammatory genes, as well as a prominent type 1 IFN response within 1 h of injection [8]. These observations from animal models were demonstrated to be accurate, if not underestimated, in 2002 in a clinical trial for the treatment of partial ornithine transcarbamylase deficiency [9]. This trial used doses of Ad deemed safe in non-human primates (NHPs) that resulted in the development of fever, myalgia, nausea, hepatotoxicity and subsequently death in 1 of the 18 patients treated [9].
Lentiviruses belong to the retrovirus family, and lentiviral-derived vectors also represent an attractive delivery platform for gene therapy because of their large packaging capacity, stable long-term transgene expression and an ability to transduce non-dividing cells. Although the low expression levels and limited tropism associated with these viruses have generally been overcome, cells transduced by lentivirus are usually eliminated within 4 to 6 weeks [10]. When injected intravenously into mice, vesicular stomatitis virus-psuedotyped lentivirus caused an increase in the expression of IFNα/β in the liver and spleen, accompanied by a rise in serum IFNα levels and followed by a decline in proviral DNA levels by 72 h post-injection [11]. In addition, IFNα/β receptor knockout mice demonstrated a 3-fold increase in transduction as well as improved persistence of lentiviral vector DNA compared with strain-matched control animals, indicating the importance of innate immunity in clearing the vector [11].
Adeno-associated virus (AAV), although limited in its packaging capacity, demonstrates a milder and more transient immune profile than other viruses used for gene therapy applications [7,8]. Immune responses to AAV vectors are known to be TLR3-independent and are at least partially dependent on Kupffer cells in the liver [12], while humoral responses against the AAV capsid are enhanced by the presence of complement [7,12,13]. In a clinical trial of an AAV-based gene therapy in patients with severe hemophilia B, two of the seven patients treated developed what appeared to be a CTL response against the AAV capsid, as evidenced by a transient rise in the levels of liver enzymes [14]. Capsid-specific CTLs are suggested to cause the destruction of transduced hepatocytes [14,15]. This clinical observation was not predicted by any animal model and has yet to be successfully replicated in the laboratory, making the exploration of possible strategies to avoid this type of immune response challenging [16,17]. However, Li et al recently demonstrated the CTL-mediated killing of hepatocytes following the liver-directed gene transfer of an AAV type 2 (AAV2) vector, carrying an immunodominant epitope for chicken albumin on its surface, into mice pre-immunized with the same antigen [18]. While these data may prove useful for studying the level of CTL response elicited by different serotypes of AAV, the direct implications for the clinic may be limited as the altered transgene expression and liver toxicity fail to match that demonstrated in a hemophilia B clinical trial [14,18]. In addition, the results from two patients in this clinical trial, receiving identical doses of vector, suggested a relationship between a high titer of pre-existing NABs to the AAV2 capsid and a decrease in transduction efficiency [14]. This theory has been validated in a mouse model using pooled human serum that contained NABs to AAV2 [19]. As NABs directed against AAV2 demonstrate high levels of cross-reactivity to other AAV serotypes, and because approximately 80% of the worldwide population has circulating antibodies against AAV2 as a result of natural infection, the presence of pre-existing NABs will serve as a major hurdle in the systemic administration of AAV in the clinic [15,20].
Lowering vector doses to reduce immunogenicity
The simplest approach to restrict the interaction between a viral vector and the immune system is to increase the efficiency of the vector for gene delivery so that the amount of administered virus can be reduced. This approach can have the added benefit of producing more of the therapeutic protein, which may be beneficial for tolerance induction, particularly in the liver (see section on Hepatic gene transfer) [21]. One potential pitfall of this approach may be that low vector doses could be particularly susceptible to neutralization, even by low levels of NABs, an obstacle that AAV is vulnerable to given the high frequency of pre-existing immunity in the general population [20]. Improving gene therapy efficiency can be as simple as selecting an appropriate serotype for the target tissue, such as AAV8 for murine liver transduction [22]. However, interspecies differences must also be examined, as improved gene transfer with AAV8 in mice has not consistently transferred to large animal models [23].
One interesting approach has been to use balloon occlusion catheters to prevent hepatic blood outflow prior to the delivery of Ad [24]. This strategy, performed successfully in NHPs, has the benefit of localizing in vivo administration, while the increased hepatic pressure enhances the efficiency of vector delivery [24]. Other approaches to improving vector gene transfer have typically focused on modifying the virus capsid or genome; however, strategies to improve vector design could result in an increase in immunogenicity rather than the desired decrease.
Capsid modifications
The entry of Ad into a cell is mainly dependent on its binding to the coxsackie-adenovirus receptor (CAR), which has limited expression on the surface of many therapeutically relevant tissues. To improve the efficiency of gene transfer to tissues that lack CARs, the capsid of the Ad has been modified extensively by the genetic insertion of specific motifs, the attachment of adapter molecules and the chemical addition of polymers attached to ligands [25]. A unique combination of these methods includes polyethylene glycosylation of the Ad capsid and the attachment of an antibody that homes the vector to cells expressing E-selectin [26]. E-selectin is specifically expressed on endothelial cells that are inflamed or undergoing rapid angiogenesis, making this approach attractive for the treatment of arthritis or cancer [26].
Maheshri et al have developed a directed evolution system for the AAV capsid that can potentially be used to improve vector transduction efficiency for several applications [27]. In this method, the capsid sequence is randomly mutated by PCR to produce a plasmid library of recombinant AAV capsids. These plasmids are then used to create a diverse array of AAV capsid variants that can be screened for infectivity in a desired condition, such as for the infection of resistant cell types. This method to was used to engineer an AAV capsid capable of transduction in the presence of NABs directed against AAV2 [27].
Several surface-exposed tyrosine residues on the AAV capsid were identified that were capable of being phosphorylated, leading to ubiquitination and subsequent degradation of the virus before the transgene was able to enter the cell nucleus [28]. Mutation of these tyrosine residues to phenylalanine resulted in enhanced translocation of the AAV2 vector to the nucleus, thereby avoiding proteasomal degradation in the cytoplasm. In several strains of mice, the administration of a therapeutic gene (in this case the gene for the Factor [F]IX protein), in a tyrosine-mutated vector resulted in an 8- to 17-fold increase in the expression of the FIX protein compared with a vector with a wild-type capsid [28].
Improving transgene expression
Ad vectors display robust expression, but are cleared or have reduced efficacy by pre-existing immunity, particularly when delivered systemically. An improved vector backbone (known as C4AFO) was created that contained noncoding Ad sequences, as well as human-derived ‘stuffer’ sequences [29]. Moreover, an Ad vector containing the C4AFO backbone was able to transduce muscle in mice at a increased efficiency and for prolonged periods compared with previous generation Ad vectors, even in the presence of pre-existing immunity to Ad [30].
Basic lentiviral vectors developed for gene therapy have required extensive transgene modification to achieve acceptable levels of expression; this requirement is likely to be a result of their more complex life cycle, which involves reverse transcription of the RNA genome and trafficking of the resulting DNA to the nucleus. In one study, the re-introduction of a 188bp segment of the wild-type HIV central polypurine tract, upstream of the promoter, increased transgene expression by enhancing the translocation of reverse-transcribed DNA into the nucleus [31]. Other modifications, such as the insertion of an Igκ matrix attachment region, an upstream poly-adenylation (poly-A) enhancer or post-transcriptional regulatory elements, have yielded additional improvements in transgene expression [32–34]. Lentivirus transgene expression was also improved by the insertion of an internal poly-A sequence for the transgene protein cDNA; this approach led to a 2- to 3-fold increase in transgene expression, but also reduced the viral titer [35].
AAV vectors contain a ssDNA genome that is converted to dsDNA in the nucleus, in a rate-limiting process known as second-strand synthesis. This step can be bypassed either by using a double-stranded genome or by co-expressing a phosphatase, such as PTP or PP5, that specifically inactivates FKBP52, a nuclear chaperone protein responsible for blocking second-strand synthesis [36,37].
Avoiding expression in APCs
Given the diverse tropism of most viral vectors, the imprecision of in vivo delivery and the wide distribution of APCs, that the transduction of APCs with gene therapy vectors is also likely. Expression of the transgene in APCs can be detrimental to the efficacy of gene therapy, as these cells sample and present intracellular contents at the cell surface via MHC class I, and can prime an immune response against a foreign transgene product. This response has been demonstrated in murine models, where dendritic cells transduced with Ad-lacZ ex vivo could direct the T-cell-mediated destruction of AAV-lacZ transduced muscle cells following their adoptive transfer [38]. Furthermore, a ubiquitous or an APC-specific promoter that was used to drive the expression of a circulating protein gave rise to immune responses, whereas a liver-specific promoter demonstrated no immune response following Ad gene therapy [39].
The use of a tissue-specific promoter can avoid immunity resulting from transgene expression in APCs. Tissue-specific promoters not only reduce the immune response to the transgene product, but can also increase expression levels when compared with ubiquitous promoters. This approach has been used to modify Ad, lentivirus and AAV vectors to direct transgene expression in multiple tissues, including the liver and muscle, and for the expression of both intracellular and extracellular proteins [40–45]. However, one study demonstrated that even with the use of tissue-specific promoters, some off-target expression remained that could initiate an immune response following lentiviral gene therapy [40]. Brown et al employed an alternative strategy to direct expression away from APCs, by using microRNA (miRNA) to eliminate transgene expression in APCs [46]. An miRNA target sequence, complementary to an hematopoietic cell-specific miRNA target sequence, was incorporated downstream of FIX in a lentiviral vector to abolish expression in any professional, bone-marrow-derived APCs. Using this method, the stable expression of FIX in hemophilic mice during an extended period of time was demonstrated, without the development of NABs [46].
Gene therapy to immune privileged sites
Several locations within the body are known for their unique immune status and segregation from traditional immune surveillance. For example, the brain and the eye are largely isolated from the systemic circulation by tight endothelial barriers, and therefore lack the traditional lymphatics of the periphery. The liver, while not isolated from the circulation, demonstrates a relatively tolerant response to foreign antigens compared with other tissues [21,40].
(Sub) Gene therapy in the brain
The brain parenchyma is isolated from the circulation by the tight endothelial junctions of the blood-brain barrier. This barrier is advantageous for gene therapy, as it prevents antigens from escaping into the peripheral lymphatics where an adaptive immune response can be generated. Both Ad and lentivirus have been demonstrated to effectively escape an immune response in the brain below a certain dose threshold [47]. However, at higher doses, these vectors, as well as AAV, evoked an innate inflammatory response that was deleterious to gene transfer [47,48].
While pre-existing humoral immunity to the viral vector is an impediment to gene therapy in the periphery, this response should be of little consequence in the brain given its isolation from the systemic circulation. For both Ad and helper-dependent Ad, transgene expression in the brain was uninhibited in the presence of pre-existing humoral immunity to the virus [49,50]. However, following intrastriatal injection of AAV, circulating antibodies against the AAV capsid were able to completely block transduction in one study in a serotype-dependent manner [51]. Furthermore, a second injection of AAV in the opposite hemisphere of the brain increased inflammation and reduced transgene expression [47,51,52]. In addition to viral vector immunity, peripheral immunity to the transgene product can either prevent or clear transgene expression in the brain when using Ad or lentivirus vectors; this process may involve CTLs [47,53,54]. These data have translated into clinical trials with some accuracy. For example, in a phase I clinical trial of AAV gene therapy for the treatment of Canavan disease in the brain, a dose-dependent NAB response to AAV was demonstrated [55]. However, in another phase I trial for Parkinson’s disease, no correlation between transgene expression and pre-existing NABs to AAV was observed [56].
Ocular gene transfer
The eye is another immune privileged site that is characterized by a blood-tissue barrier similar to that of the brain, a lack of lymphatics, a paucity of APCs, low levels of cellular MHC class I and II expression, and an in situ immunosuppressive environment [57]. In addition, antigens delivered to the eye have also been demonstrated to promote peripheral tolerance to the antigen via regulatory T-cells (Tregs) [57]. Preclinical studies of gene therapy targeted to the subretinal space of the eye using AAV demonstrate minimal inflammation, with any tissue damage resulting from the physical trauma of injection [58,59]. These studies were conducted in animal models of Leber Congenital Amaurosis, a form of blindness that results from the loss of function of the RPE65 gene. The absence of an immune response in these studies coupled with large gains in vision prompted the initiation of three clinical trials, in which AAV-RPE65 injection into the subretinal space of one eye was well tolerated with no serious adverse effects [1,60,61]. The patients in these trials also exhibited a significant improvement in visual function [1,60,61].
A recent study demonstrated that the delivery of AAV to the retina at doses that did not elicit NABs to the vector, had no effect on the subsequent transduction of the opposite eye [62]. However, an initial injection at higher doses that resulted in the production of NABs demonstrated a negative, though variable, effect on transduction of the other eye [62]. These observations, combined with the clinical safety profile of appropriately dosed subretinal AAV gene therapy, are promising for the treatment of inherited blindness disorders.
Hepatic gene transfer
The eye and the brain owe their immune privilege, at least in part, to their relative isolation from the circulation; the liver, however, may owe its immune-privileged status to a high level of circulation. The liver receives large amounts of foreign antigens and bacterial particles because this organ is directly downstream of the blood flow from the digestive tract. Most of these antigenic insults are ignored, which may explain the tolerogenic environment of the liver [63,64]. Liver-directed gene therapy has yielded long-term expression and immunological tolerance to the transgene product in animal models using Ad, lentivirus and AAV vectors, as reviewed in LoDuca et al [65]. The induction of transgene product-specific CD4+CD25+ Tregs, which limit antigen-specific effector T-cell functions through cell contact, cytokine-mediated and other mechanisms, is a crucial component of tolerance induction. Furthermore, Tregs induced in response to the transgene product express FoxP3 and are phenotypically comparable to naturally occurring Tregs. In addition, some transgene product-specific T-cells may be deleted or become anergic [21,66,67].
Interestingly, once tolerance had been established by the delivery of a FIX-expressing AAV (AAV-FIX) to the liver, supplementing the circulating FIX levels by vector administration to a more immunogenic site, such as skeletal muscle, was possible [68]. Similarly, tolerance to myelin basic protein, induced by gene transfer to the liver, prevented the development of experimental autoimmune encephalomyelitis in a mouse model of multiple sclerosis [69]. This concept has particular advantages in lysosomal storage diseases, where the disease manifests in both the CNS and the viscera. In a mouse model of Niemann Pick Disorder, AAV8 delivery of the therapeutic gene to the liver, followed by intracranial delivery of the same transgene, not only avoided the humoral immune response observed with brain-only injections, but also demonstrated improved disease outcomes compared with single-site injections administered systemically or intracranially [70].
Immune suppression
Thus far, the review has focused on evading the immune system by modifying aspects of the viral vector itself or the delivery system. However, instead of hiding the vector from the immune system, another possibility is to effectively hide the immune system from the vector. This outcome can be achieved by immune suppression or by modulating the immune response away from immunity and toward tolerance.
Many of the drugs used for immunosuppression in gene therapy protocols are employed in organ transplantation. These agents function by inhibiting DNA synthesis, inhibiting cell signaling required for lymphocyte activation and proliferation, or depleting or inactivating antibodies directed against specific cell types. Typically, transient immune suppression with these drugs is preferable. For example, transient immune suppression with the glucocortocoid dexamethosone decreased the cytokine storm of the innate immune reaction, as well as the adaptive responses that generally follow the systemic administration of Ad vectors [71]. Even with the less immunogenic AAV vector, immune suppression still has benefits. For example, in canines with hemophilia, treatment with cyclophosphamide before the injection of AAV-FIX (im) yielded partial phenotypic correction and prevented an antibody response against FIX [72]. Alternatively, specific tolerance to FIX was achieved by the coadministration of the antigen, rapamycin and IL-10 prior to gene transfer [73]. A combination of the immunosuppressants mycophenolate mofetil (MMF), cyclosporin (CSA) and antithymocyte globulin in a canine model of Duchenne muscular dystrophy, demonstrated long-term expression of the therapeutic transgene, but also required immune suppression regimen of 18 weeks [74]. In this example, the aggressiveness of immune suppression may have been necessary because the muscle tissue in this disease was already inflamed, suggesting that the inflammatory state of the target tissue is important. Furthermore, care must be taken when selecting appropriate agents that do not reduce the numbers of beneficial lymphocytes, such as Tregs. For example, the use of daclizumab in an NHP study resulted in an increase in the antibody response to an AAV vector and the transgene product; this finding is likely to have been caused by daclizumab-induced depletion of tolerance-inducing CD4+CD25+ Tregs [75].
The immune response against the AAV capsid observed in the hemophilia B clinical trial prompted research into whether or not immune suppression was a viable option for human gene therapy [14]. One study using MMF and tacrolimus in NHPs [23] and a clinical trial using MMF and CSA treatment [76] suggest that the use of immune suppression has no negative impact on AAV transduction efficacy, may reduce the anti-AAV response and, at least up to a certain vector dose, may prevent CD8+ T-cell responses to the capsid [23,76]. Interestingly, the administration of rituximab plus CSA abolished antibodies against FIX resulting from AAV-FIX gene transfer in NHPs [77]. This finding could prove to be clinically relevant, as NABs to FIX may not only reduce the effectiveness of repeated administration of the gene therapy, but may also have a negative impact on the treatment of the patient.
Another potential target of immune suppression is costimulation, the interaction between an APC and a lymphocyte via a variety of cell-surface molecules that directs changes in the maturation status of one or both cells. The binding of the costimulatory molecules CD40 and CD40-ligand (CD40L) results in the maturation of the APC, allowing the APC to prime an adaptive immune response. CTLA4 (CTL antigen 4) is a costimulatory molecule expressed on T-helper cells that sends an inhibitory signal to APCs. The administration of a soluble CTLA4-Ig fusion protein at high doses, or during an extended period, prevented immune responses following the retrovirus-mediated gene transfer of a reporter gene and a therapeutic gene in a mouse model of mucopolysaccharidosis type I (MPSI) [78,79]. Transient immune suppression with a combination of anti-CD40L and CTLA4-Ig has also been effective in generating long-term transgene expression in mouse models of MPSI and hemophilia A [79,80]. Other immune suppressive approaches include the targeting of ICOS (inducible T-cell costimulator) with mAbs and the partial blockage of signaling to T-cells with non-Fc (fragment crystallizable) receptor binding anti-CD3 molecules [81,82].
Alternative methods of immune suppression
Another interesting method of circumventing an immune response is the inclusion of certain sequences in the transgene, such as the guanine-adenine repeats of the gene encoding the EBNA-1 protein from EBV, that prevent the protein product from being displayed as an epitope [83].
The manipulation and exploitation of Tregs is also an attractive method for inducing tolerance in gene therapy. In one study, the transfer of antigen-specific Tregs mitigated the T-cell-mediated destruction of muscle cells transduced with an AAV vector expressing the highly immunogenic hemagglutinin protein [84]. The same investigators successfully grafted male mouse bone marrow cells into female recipients by the cotransfer of Tregs specific for the male antigen DBY [85]. Interestingly, the recipient mouse was not only tolerant to DBY, but also to minor antigens present on the donor bone marrow cells, including a foreign antigen expressed within the bone marrow cell, in this case EGFP (enhanced green fluorescent protein) [85].
In addition, exposure to an antigen via the mucosal route (nasal or oral) can induce tolerance to the antigen by the recruitment of Tregs, which may prove beneficial as a method to tolerize individuals prior to gene transfer [86,87]. Finally, the developing immune system may offer an environment favorable to tolerance following gene transfer either in utero or to the neonate [88,89].
Conclusion
Much progress remains to be made in order to be able to provide optimal gene therapy, including fine-tuning the interaction between the immune system and the viral vector used for gene transfer. Each vector, transgene product and disease present unique immunological challenges, and one approach is unlikely to be omnipotent. While animal models provide excellent preliminary data, the differences in the physiology and immunology between humans and animals necessitate that some questions must be answered in clinical trials. Which vector will prove to be the most successful in these trials remains to be determined. The immunogenicity of Ad suggests this vector may be more suitable as a vaccine or anticancer vector [90], while AAV vectors have demonstrated preclinical and some clinical success, and is considered the most viable vector for in vivo use in humans. However, lentivirus vectors have also demonstrated positive clinical results as an ex vivo gene therapy tool, and may be most useful in disease models where this approach is applicable. However, through careful study, taming or eluding the immune system sufficiently to enhance the therapeutic benefits of gene therapy seems reasonable given the vast array of emerging tools.
Acknowledgments
Research by the authors is supported by NIH grants PThe au01 HL078810 (Project 3) and R01 AI/HL51390 awarded to RWH. BKS is supported by a University of Florida Alumni Fellowship.
References
•• of outstanding interest
• of special interest
- 1••.Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, Flotte TR, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105(39):15112–15117. doi: 10.1073/pnas.0807027105. One of three clinical trials for Leber congenital amaurosis using AAV-mediated gene therapy administered to the subretinal space; demonstrated positive safety profiles and encouraging clinical outcomes (for the other clinical trials see references [60,61]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, Scaramuzza S, Andolfi G, Mirolo M, Brigida I, Tabucchi A, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 3.Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, Craenen JM, Lewis S, Malik V, Shilling C, Byrne BJ, et al. LGMD 2D gene therapy restores α-sarcoglycan and associated proteins. Annals Neurol. 2009 doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang X, Yang Y. Innate immune recognition of viruses and viral vectors. Hum Gene Ther. 2009;20(4):293–301. doi: 10.1089/hum.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao X, Kim KS, Liu D. Nonviral gene delivery: What we know and what is next. AAPS J. 2007;9(1):E92–E104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334(18):1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- 7.Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76(9):4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCaffrey AP, Fawcett P, Nakai H, McCaffrey RL, Ehrhardt A, Pham TT, Pandey K, Xu H, Feuss S, Storm TA, Kay MA. The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol Ther. 2008;16(5):931–941. doi: 10.1038/mt.2008.37. [DOI] [PubMed] [Google Scholar]
- 9.Raper SE, Yudkoff M, Chirmule N, Gao GP, Nunes F, Haskal ZJ, Furth EE, Propert KJ, Robinson MB, Magosin S, Simoes H, et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther. 2002;13(1):163–175. doi: 10.1089/10430340152712719. [DOI] [PubMed] [Google Scholar]
- 10.Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12 (5):585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 11.Brown BD, Sitia G, Annoni A, Hauben E, Sergi LS, Zingale A, Roncarolo MG, Guidotti LG, Naldini L. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood. 2007;109(7):2797–2805. doi: 10.1182/blood-2006-10-049312. [DOI] [PubMed] [Google Scholar]
- 12.Cao O, Herzog RW. TLR3 signaling does not affect organ-specific immune responses to Factor IX in AAV gene therapy. Blood. 2008;112(3):910–911. doi: 10.1182/blood-2008-02-137992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaiss AK, Cotter MJ, White LR, Clark SA, Wong NC, Holers VM, Bartlett JS, Muruve DA. Complement is an essential component of the immune response to adeno-associated virus vectors. J Virol. 2008;82(6):2727–2740. doi: 10.1128/JVI.01990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 15.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, Ragni MV, Manno CS, Sommer J, Jiang H, Pierce GF, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13(4):419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Figueredo J, Calcedo R, Lin J, Wilson JM. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther. 2007;18(3):185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Hirsch M, Asokan A, Zeithaml B, Ma H, Kafri T, Samulski RJ. Adeno-associated virus type 2 (AAV2) capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J Virol. 2007;81(14):7540–7547. doi: 10.1128/JVI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Hirsch M, DiPrimio N, Asokan A, Goudy K, Tisch R, Samulski RJ. Cytotoxic-T-lymphocyte-mediated elimination of target cells transduced with engineered adeno-associated virus type 2 vector in vivo. J Virol. 2009;83(13):6817–6824. doi: 10.1128/JVI.00278-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S, Couto LB, Pierce GF. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107(5):1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 20.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199(3):381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, Arruda VR, High KA, Herzog RW. Induction of immune tolerance to coagulation Factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111(9):1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang TP, Jin DY, Wardrop RM, 3rd, Gui T, Maile R, Frelinger JA, Stafford DW, Monahan PE. Transgene expression levels and kinetics determine risk of humoral immune response modeled in Factor IX knockout and missense mutant mice. Gene Ther. 2007;14(5):429–440. doi: 10.1038/sj.gt.3302881. [DOI] [PubMed] [Google Scholar]
- 23.Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, Zhou S, Scallan CD, Sommer J, Vijay S, Mingozzi F, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108(10):3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Brunetti-Pierri N, Stapleton GE, Palmer DJ, Zuo Y, Mane VP, Finegold MJ, Beaudet AL, Leland MM, Mullins CE, Ng P. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol Ther. 2007;15(4):732–740. doi: 10.1038/sj.mt.6300102. The use of balloon catheter occlusion in NHPs to prevent hepatic blood outflow mimics the rise in intrahepatic blood pressure seen in mice following hydrodynamic injection, allowing greater permissiveness of Ad infection and resulting in long-term gene expression. [DOI] [PubMed] [Google Scholar]
- 25.Mizuguchi H, Hayakawa T. Targeted adenovirus vectors. Hum Gene Ther. 2004;15(11):1034–1044. doi: 10.1089/hum.2004.15.1034. [DOI] [PubMed] [Google Scholar]
- 26•.Ogawara K, Rots MG, Kok RJ, Moorlag HE, Van Loenen AM, Meijer DK, Haisma HJ, Molema G. A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum Gene Ther. 2004;15(5):433–443. doi: 10.1089/10430340460745766. Creative Ad capsid modification to target inflamed tissues or tissues undergoing angiogenesis, such as arthritic joints and cancer. [DOI] [PubMed] [Google Scholar]
- 27••.Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotechnol. 2006;24(2):198–204. doi: 10.1038/nbt1182. Describes a novel method for generating mutant AAV capsids by random mutation and selection for desirable traits, in this case for infection in the presence of NABs. [DOI] [PubMed] [Google Scholar]
- 28•.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH, Jr, Weigel-Van Aken KA, Hobbs JA, et al. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105(22):7827–7832. doi: 10.1073/pnas.0802866105. The mutation of exposed tyrosine residues on the AAV capsid leads to enhanced transduction efficiency, as a result of decreased ubiquitin-dependent degradation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandig V, Youil R, Bett AJ, Franlin LL, Oshima M, Maione D, Wang F, Metzker ML, Savino R, Caskey CT. Optimization of the helper-dependent adenovirus system for production and potency in vivo. Proc Natl Acad Sci USA. 2000;97(3):1002–1007. doi: 10.1073/pnas.97.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maione D, Della Rocca C, Giannetti P, D’Arrigo R, Liberatoscioli L, Franlin LL, Sandig V, Ciliberto G, La Monica N, Savino R. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc Natl Acad Sci USA. 2001;98(11):5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25(2):217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 32.Park F, Kay MA. Modified HIV-1 based lentiviral vectors have an effect on viral transduction efficiency and gene expression in vitro and in vivo. Mol Ther. 2001;4(3):164–173. doi: 10.1006/mthe.2001.0450. [DOI] [PubMed] [Google Scholar]
- 33.Schambach A, Galla M, Maetzig T, Loew R, Baum C. Improving transcriptional termination of self-inactivating γ-retroviral and lentiviral vectors. Mol Ther. 2007;15(6):1167–1173. doi: 10.1038/sj.mt.6300152. [DOI] [PubMed] [Google Scholar]
- 34.Hlavaty J, Schittmayer M, Stracke A, Jandl G, Knapp E, Felber BK, Salmons B, Gunzburg WH, Renner M. Effect of posttranscriptional regulatory elements on transgene expression and virus production in the context of retrovirus vectors. Virology. 2005;341(1):1–11. doi: 10.1016/j.virol.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 35.Hager S, Frame FM, Collins AT, Burns JE, Maitland NJ. An internal polyadenylation signal substantially increases expression levels of lentivirus-delivered transgenes but has the potential to reduce viral titer in a promoter-dependent manner. Hum Gene Ther. 2008;19(8):840–850. doi: 10.1089/hum.2007.165. [DOI] [PubMed] [Google Scholar]
- 36.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10(26):2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 37.Jayandharan GR, Zhong L, Li B, Kachniarz B, Srivastava A. Strategies for improving the transduction efficiency of single-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2008;15(18):1287–1293. doi: 10.1038/gt.2008.89. [DOI] [PubMed] [Google Scholar]
- 38.Jooss K, Yang Y, Fisher KJ, Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72(5):4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Geest BR, Van Linthout SA, Collen D. Humoral immune response in mice against a circulating antigen induced by adenoviral transfer is strictly dependent on expression in antigen-presenting cells. Blood. 2003;101(7):2551–2556. doi: 10.1182/blood-2002-07-2146. [DOI] [PubMed] [Google Scholar]
- 40.Follenzi A, Battaglia M, Lombardo A, Annoni A, Roncarolo MG, Naldini L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic Factor IX in mice. Blood. 2004;103(10):3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- 41.Kang Y, Xie L, Tran DT, Stein CS, Hickey M, Davidson BL, McCray PB., Jr Persistent expression of Factor VIII in vivo following nonprimate lentiviral gene transfer. Blood. 2005;106(5):1552–1558. doi: 10.1182/blood-2004-11-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cordier L, Gao GP, Hack AA, McNally EM, Wilson JM, Chirmule N, Sweeney HL. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum Gene Ther. 2001;12(2):205–215. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler RJ, Lonning SM, Armentano D, Li C, Souza DW, Cherry M, Ford C, Barbon CM, Desnick RJ, Gao G, Wilson JM, et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of α-galactosidase A and the induction of immune tolerance in Fabry mice. Mol Ther. 2004;9(2):231–240. doi: 10.1016/j.ymthe.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Pastore L, Morral N, Zhou H, Garcia R, Parks RJ, Kochanek S, Graham FL, Lee B, Beaudet AL. Use of a liver-specific promoter reduces immune response to the transgene in adenoviral vectors. Hum Gene Ther. 1999;10(11):1773–1781. doi: 10.1089/10430349950017455. [DOI] [PubMed] [Google Scholar]
- 45.Cerullo V, McCormack W, Seiler M, Mane V, Cela R, Clarke C, Rodgers JR, Lee B. Antigen-specific tolerance of human α1-antitrypsin induced by helper-dependent adenovirus. Hum Gene Ther. 2007;18(12):1215–1224. doi: 10.1089/hum.2006.036. [DOI] [PubMed] [Google Scholar]
- 46••.Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P, D’Angelo A, Naldini L. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 2007;110(13):4144–4152. doi: 10.1182/blood-2007-03-078493. Prolonged and stable expression of a therapeutic protein by using miRNA to overcome off-target expression of the vector transgene in hematopoietic cells. [DOI] [PubMed] [Google Scholar]
- 47.Lowenstein P, Mandel R, Xiong W, Kroeger K, Castro M. Immune responses to adenoviral, adeno-associated viral, and lentiviral vectors used for gene therapy of brain diseases. In: Herzog RW, editor. Gene therapy immunology. John Wiley & Sons Inc; Hoboken, NJ, USA: 2009. pp. 167–198. [Google Scholar]
- 48.Reimsnider S, Manfredsson FP, Muzyczka N, Mandel RJ. Time course of transgene expression after intrastriatal pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol Ther. 2007;15(8):1504–1511. doi: 10.1038/sj.mt.6300227. [DOI] [PubMed] [Google Scholar]
- 49.Barcia C, Gerdes C, Xiong WD, Thomas CE, Liu C, Kroeger KM, Castro MG, Lowenstein PR. Immunological thresholds in neurological gene therapy: Highly efficient elimination of transduced cells might be related to the specific formation of immunological synapses between T cells and virus-infected brain cells. Neuron Glia Biol. 2006;2(4):309–322. doi: 10.1017/S1740925X07000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barcia C, Jimenez-Dalmaroni M, Kroeger KM, Puntel M, Rapaport AJ, Larocque D, King GD, Johnson SA, Liu C, Xiong W, Candolfi M, et al. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: Clinical implications. Mol Ther. 2007;15(12):2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peden CS, Burger C, Muzyczka N, Mandel RJ. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004;78(12):6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mastakov MY, Baer K, Symes CW, Leichtlein CB, Kotin RM, During MJ. Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain. J Virol. 2002;76(16):8446–8454. doi: 10.1128/JVI.76.16.8446-8454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abordo-Adesida E, Follenzi A, Barcia C, Sciascia S, Castro MG, Naldini L, Lowenstein PR. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum Gene Ther. 2005;16(6):741–751. doi: 10.1089/hum.2005.16.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiong W, Candolfi M, Kroeger KM, Puntel M, Mondkar S, Larocque D, Liu C, Curtin JF, Palmer D, Ng P, Lowenstein PR, et al. Immunization against the transgene but not the TetON switch reduces expression from gutless adenoviral vectors in the brain. Mol Ther. 2008;16(2):343–351. doi: 10.1038/sj.mt.6300375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J, Shera D, Lioutermann L, Feely M, Freese A, Leone P. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med. 2006;8(5):577–588. doi: 10.1002/jgm.885. Demonstrates the safety of intracranial AAV administration. An absence of NAB to AAV was observed in patients receiving a low dose of virus, whereas high doses yielded detectable NAB in 2/3 patients in the high dose group. [DOI] [PubMed] [Google Scholar]
- 56••.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: An open label, phase I trial. Lancet. 2007;369(9579):2097–2105. doi: 10.1016/S0140-6736(07)60982-9. Clinical trial investigating the safety of AAV in the brain. Patients with Parkinson’s disease received subthalamic injections of AAV carrying a therapeutic GAD gene. An improvement in objective measurements of disease was demonstrated for 10 of the 12 patients, while none of the 12 patients demonstrated a change in antibodies against AAV. Also, pre-existing antibodies had no effect on efficacy. [DOI] [PubMed] [Google Scholar]
- 57.Streilein JW. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3(11):879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 58.Jacobson SG, Acland GM, Aguirre GD, Aleman TS, Schwartz SB, Cideciyan AV, Zeiss CJ, Komaromy AM, Kaushal S, Roman AJ, Windsor EA, et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol Ther. 2006;13(6):1074–1084. doi: 10.1016/j.ymthe.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, Mendes-Madeira A, Provost N, Péréon Y, Cherel Y, Ali RR, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14(4):292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- 60••.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. One of three clinical trials for Leber congenital amaurosis that used AAV-mediated gene therapy to the subretinal space, and demonstrated positive safety profiles and encouraging clinical outcomes (for the other clinical trials see references [1,61]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61••.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. One of three clinical trials for Leber congenital amaurosis that used AAV-mediated gene therapy to the subretinal space, and demonstrated positive safety profiles and encouraging clinical outcomes (for the other clinical trials see references [1,60]) [DOI] [PubMed] [Google Scholar]
- 62.Barker SE, Broderick CA, Robbie SJ, Duran Y, Natkunarajah M, Buch P, Balaggan KS, Maclaren RE, Bainbridge JW, Smith AJ, Ali RR. Subretinal delivery of adeno-associated virus serotype 2 results in minimal immune responses that allow repeat vector administration in immunocompetent mice. J Gene Med. 2009;11(6):486–497. doi: 10.1002/jgm.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3(1):51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 64.Mackay IR. Hepatoimmunology: A perspective. Immunol Cell Biol. 2002;80(1):36–44. doi: 10.1046/j.1440-1711.2002.01063.x. [DOI] [PubMed] [Google Scholar]
- 65.Loduca PA, Hoffman BE, Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther. 2009;9(2):104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dobrzynski E, Mingozzi F, Liu YL, Bendo E, Cao O, Wang L, Herzog RW. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104(4):969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- 67.Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, Herzog RW. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110(4):1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffman BE, Dobrzynski E, Wang L, Hirao L, Mingozzi F, Cao O, Herzog RW. Muscle as a target for supplementary Factor IX gene transfer. Hum Gene Ther. 2007;18(7):603–613. doi: 10.1089/hum.2007.042. [DOI] [PubMed] [Google Scholar]
- 69.Lüth S, Huber S, Schramm C, Buch T, Zander S, Stadelmann C, Brück W, Wraith DC, Herkel J, Lohse AW. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest. 2008;118(10):3403–3410. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Passini MA, Bu J, Fidler JA, Ziegler RJ, Foley JW, Dodge JC, Yang WW, Clarke J, Taksir TV, Griffiths DA, Zhao MA, et al. Combination brain and systemic injections of AAV provide maximal functional and survival benefits in the Niemann-Pick mouse. Proc Natl Acad Sci USA. 2007;104(22):9505–9510. doi: 10.1073/pnas.0703509104. Tolerance induction by liver-directed AAV gene therapy prevented immune response upon gene transfer to the brain, thereby enhancing overall therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seregin SS, Appledorn DM, McBride AJ, Schuldt NJ, Aldhamen YA, Voss T, Wei J, Bujold M, Nance W, Godbehere S, Amalfitano A. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol Ther. 2009;17(4):685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4(3):192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 73.Nayak S, Cao O, Hoffman BE, Cooper M, Zhou S, Atkinson MA, Herzog RW. Prophylactic immune tolerance induced by changing the ratio of antigen-specific effector to regulatory T cells. J Thromb Haemost. 2009;7(9):1523–1532. doi: 10.1111/j.1538-7836.2009.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, Tapscott SJ, Storb R. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15(6):1160–1166. doi: 10.1038/sj.mt.6300161. Demonstrates the utility of immune suppression for gene therapy, even for gene transfer to highly inflamed tissues where immune responses are expected to be more robust than in normal tissues. [DOI] [PubMed] [Google Scholar]
- 75.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, Zhou S, Wright JF, Jiang H, Pierce GF, Arruda VR, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110(7):2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mingozzi F, Meulenberg JJ, Hui DJ, Besner-Tschakarjan E, Hasbrouck NC, Edmonson SA, Hutnick NA, Betts MR, Kastelein JJ, Stroes ED, High KA. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114(10):2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77•.Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, Tuddenham EG, Kemball-Cook G, McIntosh J, Boon-Spijker M, Mertens K, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human Factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107(7):2653–2661. doi: 10.1182/blood-2005-10-4035. Established that inhibitory antibodies against FIX could be abolished with rituximab plus CSA immune suppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Puppi J, Guillonneau C, Pichard V, Bellodi-Privato M, Cuturi MC, Anegon I, Ferry N. Long term transgene expression by hepatocytes transduced with retroviral vectors requires induction of immune tolerance to the transgene. J Hepatol. 2004;41(2):222–228. doi: 10.1016/j.jhep.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 79.Ma X, Liu Y, Tittiger M, Hennig A, Kovacs A, Popelka S, Wang B, Herati R, Bigg M, Ponder KP. Improvements in mucopolysaccharidosis I mice after adult retroviral vector-mediated gene therapy with immunomodulation. Mol Ther. 2007;15(5):889–902. doi: 10.1038/sj.mt.6300112. [DOI] [PubMed] [Google Scholar]
- 80.Miao CH, Ye P, Thompson AR, Rawlings DJ, Ochs HD. Immunomodulation of transgene responses following naked DNA transfer of human Factor VIII into hemophilia A mice. Blood. 2006;108(1):19–27. doi: 10.1182/blood-2005-11-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng B, Ye P, Blazar BR, Freeman GJ, Rawlings DJ, Ochs HD, Miao CH. Transient blockade of the inducible costimulator pathway generates long-term tolerance to Factor VIII after nonviral gene transfer into hemophilia A mice. Blood. 2008;112(5):1662–1672. doi: 10.1182/blood-2008-01-128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Waters B, Qadura M, Burnett E, Chegeni R, Labelle A, Thompson P, Hough C, Lillicrap D. Anti-CD3 prevents Factor VIII inhibitor development in hemophilia A mice by a regulatory CD4+CD25+-dependent mechanism and by shifting cytokine production to favor a Th1 response. Blood. 2009;113(1):193–203. doi: 10.1182/blood-2008-04-151597. [DOI] [PubMed] [Google Scholar]
- 83.Ossevoort M, Visser BM, van den Wollenberg DJ, van der Voort EI, Offringa R, Melief CJ, Toes RE, Hoeben RC. Creation of immune ‘stealth’ genes for gene therapy through fusion with the Gly-Ala repeat of EBNA-1. Gene Ther. 2003;10(24):2020–2028. doi: 10.1038/sj.gt.3302098. [DOI] [PubMed] [Google Scholar]
- 84.Gross DA, Leboeuf M, Gjata B, Danos O, Davoust J. CD4+CD25+ regulatory T cells inhibit immune-mediated transgene rejection. Blood. 2003;102(13):4326–4328. doi: 10.1182/blood-2003-05-1454. [DOI] [PubMed] [Google Scholar]
- 85•.Gross DA, Chappert P, Leboeuf M, Monteilhet V, Van Wittenberghe L, Danos O, Davoust J. Simple conditioning with monospecific CD4+CD25+ regulatory T cells for bone marrow engraftment and tolerance to multiple gene products. Blood. 2006;108(6):1841–1848. doi: 10.1182/blood-2006-02-011981. Demonstrates that when Tregs specific for a male mouse antigen were cotransferred with male mouse bone marrow cells to a female recipient tolerance could be induced, including to a foreign gene product. [DOI] [PubMed] [Google Scholar]
- 86.Cao O, Armstrong E, Schlachterman A, Wang L, Okita DK, Conti-Fine B, High KA, Herzog RW. Immune deviation by mucosal antigen administration suppresses gene-transfer-induced inhibitor formation to Factor IX. Blood. 2006;108(2):480–486. doi: 10.1182/blood-2005-11-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ponder KP. Immunology of neonatal gene transfer. Curr Gene Ther. 2007;7(5):403–410. doi: 10.2174/156652307782151434. [DOI] [PubMed] [Google Scholar]
- 89.Waddington S, Buckley S, Nivsarkar M, Coutelle C. Immune responses after in utero and neonatal gene transfer. In: Herzog RW, editor. Gene therapy immunology. John Wiley & Sons Inc; Hoboken, NJ, USA: 2009. pp. 125–141. [Google Scholar]
- 90.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10(4):616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]