Abstract
Pre-erythrocytic malaria vaccines target Plasmodium during its sporozoite and liver stages, and can prevent progression to blood-stage disease, which causes a million deaths each year. Whole organism sporozoite vaccines induce sterile immunity in animals and humans and guide subunit vaccine development. A recombinant protein-in-adjuvant pre-erythrocytic vaccine called RTS,S reduces clinical malaria without preventing infection in field studies and additional antigens may be required to achieve sterile immunity. Although few vaccine antigens have progressed to human testing, new insights into parasite biology, expression profiles and immunobiology have offered new targets for intervention. Future advances require human trials of additional antigens, as well as platforms to induce the durable antibody and cellular responses including CD8+ T cells that contribute to sterile protection.
Keywords: adjuvants, liver, Plasmodium falciparum, pre-erythrocytic malaria, rodent models, sporozoite, vaccine
The pre-erythrocytic (PE)-stage Plasmodium parasite is a metabolically highly active but symptomatically silent preparatory phase of the life cycle. Intervening to kill the parasite at this stage would prevent the symptomatic blood stage of infection, and is an attractive vaccine target for several reasons: the number of infected hepatocytes is extremely low, in the range of dozens to hundreds [1]; human parasites like Plasmodium falciparum and Plasmodium vivax take nearly a week to complete development in hepatocytes [2], providing sufficient time for elimination; unlike plasmodium-infected red blood cells (RBCs), the infected hepatocyte is capable of presenting parasite antigens to immune effector cells. Despite the attractive features of PE parasites as vaccine targets, infections with sporozoites do not usually confer highly effective PE immunity in naturally infected humans or experimentally infected rodents [3,4].
Instead, attenuated PE parasites that arrest inside hepatocytes are required to induce highly effective so-called `sterile' immunity that prevents infection after sporozoite challenge, and a subunit vaccine based on the major surface protein of sporozoites reduces clinical malaria episodes but does not block infection in African children. To develop a PE vaccine that induces sterile protection of long duration, a thorough understanding of parasite development and host–parasite interactions is essential, in addition to careful vaccine designs that elicit effective immune mediators. This review considers the biology of the PE parasite and immunobiology of protective immunity, and then draws on past experience with malaria vaccines to discuss approaches to identifying and developing effective vaccine candidates that block infection.
Biology of PE stages of Plasmodium
The PE phase of Plasmodium development (Figure 1) starts when a few dozen to few hundred sporozoites are deposited in the skin by a female Anopheles mosquito during a blood meal [5]. From the site of deposition, many motile sporozoites enter blood vessels and migrate to the liver, while some remain local or pass to lymph nodes and influence the host immune response. In the liver, sporozoites cross the fenestrated layer of endothelial and Kupffer cells to enter the space of Disse, and then may traverse several hepatocytes before invading one to form a parasitophorous vacuole (PV). At this point, the sporozoite undergoes dramatic transformations – first rounding in shape, expanding in size and then undergoing successive divisions to yield tens of thousands of merozoites within 6–7 days (for human Plasmodium) or 48–50 h (for rodent Plasmodium). These merozoites emerge from hepatocytes in small pockets called merosomes and enter the blood stream, marking the end of the PE phase [6].
Figure 1. The pre-erythrocytic journey of the Plasmodium parasite in the mammalian host.
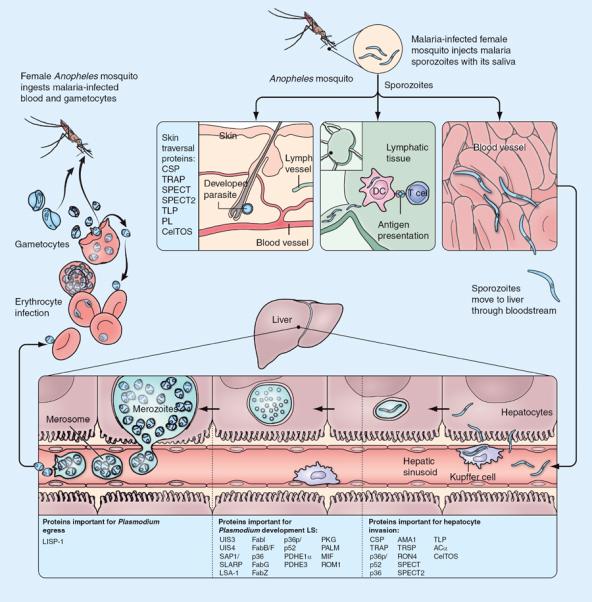
After injection into the skin by an infected female Anopheles mosquito, some sporozoites may develop in the skin, some migrate to the draining lymph node where they can be processed and presented to T cells, and some enter the bloodstream and reach the liver for complete development. After reaching the liver, sporozoites pass through the layer of Kupffer and endothelial cells to access liver parenchymal cells. A sporozoite may pass through multiple cells before forming a parasitophorous vacuole within a final hepatocyte, within which it undergoes liver stage development and gives rise to tens of thousands of merozoites. These merozoites are then released into the bloodstream as merozoite-filled packets called merosomes. DC: Dendritic cell; LS: Liver stage.
The Plasmodium genome sequences, new transfection methods, and advances in microscopy are illuminating many of the secrets of this previously inaccessible stage. New insights have challenged widely accepted aspects of the Plasmodium life cycle, for example, Plasmodium parasites can develop and produce infective merozoites in skin cells [7]. Most of the information regarding liver-stage (LS) or PE-phase development has come from rodent parasite models as well as in vitro culture systems.
Skin phase
The skin phase was previously thought to last only a few minutes, but intravital imaging reveals that sporozoites may remain in the injection site for 2–3 h [8–10]. Most sporozoites migrate to the liver, via blood vessel, but some move to draining lymph nodes and some develop in the skin, possibly in hair follicles that might give rise to infective merozoites [7,8]. While complete development of rodent Plasmodium has been demonstrated in skin cell lines, no in vivo data support this hypothesis yet, and nothing is known about how Plasmodium recognizes and selectively invades hair follicles. In the rodent model, approximately 20% of injected sporozoites go to the draining lymph nodes in the skin, where they can elicit an immune response and contribute to protection against LS parasites [8,11].
Several sporozoite proteins have been implicated in crossing the dermal cell barrier, and subsequent migration to liver sinusoid (Figure 1 & Table 1) [12], and these might be exploited for vaccine development. Recently, immunization with CelTOS, a micronemal protein and essential component of cell traversal machinery, induced humoral and cell-mediated immune responses against Plasmodium yoelii with cross-species activity against Plasmodium berghei, resulting in sterile protection of some inbred and out-bred mice against both species [13]. Other components of the cell traversal machinery, such as sporozoite protein essential for cell traversal (SPECT and SPECT2) and phospho-lipase, should be evaluated for their efficacy as vaccine candidates. Deletion or disruption of the genes encoding these proteins yields substantial but not complete reduction of liver infection (Table 1) [14–16] suggesting functional redundancy.
Table 1.
Pre-erythrocytic stage proteins.
| Protein name | Conserved | Localization | Plasmodium species studied | Functional association | Ref. |
|---|---|---|---|---|---|
| Skin to hepatocyte | |||||
| CelTOS† | Plasmodium | Microneme | Pb | Cell traversal | [160] |
| SPECT† | Plasmodium | Microneme | Pb | Cell traversal | [19] |
| SPECT2† | Plasmodium | Microneme | Pb | Cell traversal | [161] |
| TRAP† | Apicomplexa | Microneme and surface | Pb | Motility and SPZ invasion | [14,162] |
| TLP† | Apicomplexa | Surface | Pb | Motility and cell traversal | [33,163] |
| PL | Plasmodium | Surface | Pb | Cell traversal | [16] |
| CSP‡ | Plasmodium | Surface | Pb, Pf, Py, Pc and Pk | Motility, attachment, regulation of host cell gene expression | [36,164] |
| Invasion of hepatocytes | |||||
| RON4 | Apicomplexa | Rhoptry | Pb | Tight junction formation during hepatocyte invasion | [31] |
| p36p/p52‡ and p36† | Plasmodium | Microneme | Pb and Pf | Commitment to hepatocyte infection | [15,31,165] |
| AMA1 | Apicomplexa | Microneme | Pf and Pb | Invasion of hepatocyte | [166,167] |
| TRSP | Plasmodium | ND | Pb | Invasion of hepatocyte | [168] |
| Development in hepatocytes | |||||
| LSA-1 | Plasmodium | PV | Pf | LS differentiation | [169,170] |
| FabB/F and FabZ, FabG, Fabl | Plasmodium | Apicoplast | Py and Pf | LS development | [171,172] |
| SLARP/SAP1 | Plasmodium | Nucleus and cytoplasm | Pb and Py | PVM remodeling and LS development | [107,173] |
| LISP1 | Plasmodium | PVM | Pb | Merozoite release from LS | [174] |
| PDHEIα, PDHE3 | ND | Apicoplast | Py | Fatty acid biosynthesis | [175] |
| PKG | Apicomplexa | Cytosol | Pb and Pf | LS maturation | [176] |
| PALM | Plasmodium | Apicoplast | Pb | Segregation of merozoites during LS maturation | [177] |
| UIS3 | Plasmodium | PVM | Pb | LS development | [46] |
| UIS4 | Plasmodium | PVM | Pb | LS development | [47] |
| MIF | Plasmodium | Cytoplasm | Py | LS development | [178,179] |
| ROM1 | Plasmodium | Microneme | Py | PVM formation | [180] |
Important for invasion of hepafocyte.
Important for LS development.
LS: Liver stage; ND: Not determined; Pb: Plasmodium berghei; Pc: Plasmodium chabaudi; Pf: Plasmodium falaparum; Pk: Plasmodium knowlesi; PV: Parasitophorous vacuole; PVM: Parasitophorous vacuole membrane; Py: Plasmodium yoelii.
Crossing of endothelial barriers
To migrate from skin to hepatocyte, the Plasmodium sporozoite crosses endothelial cells twice; once to penetrate the blood vessel and once to pass from liver sinusoid to the space of Disse. Little is known about how a sporozoite recognizes endothelial cells. The sporozoite is thought to use its cell traversal machinery to pass through the endothelial cell lining of the blood capillaries in the dermis, but studies to examine the molecular mechanisms in skin are hindered by small parasite numbers and the brevity of the event.
The passage of the sporozoite from liver sinusoid to liver parenchyma is comparatively better studied, although also much debated. The sporozoite uses its most abundant surface protein (CSP) as well as TRAP to recognize Kupffer cells and passes through the cell within a PV [17,18]. This mode of cell traversal is quite different than the one adopted by sporozoites during their active cell traversal in the skin, but is supported by multiple lines of evidence from mutant parasites, in vitro and in vivo rodent models [19–21]. SPECT mutants and selective removal of Kupffer cells suggest that Kupffer cells are not required to cross the liver endothelial barrier [19]. Identification of novel sporozoite proteins in addition to CSP, SPECT(s) and TRAP that are involved in selective crossing of endothelial cell barrier in liver sinusoid could yield new vaccine targets.
Invasion of liver cell
Once the sporozoite exits liver sinusoid and reaches the liver parenchyma, it migrates through several hepatocytes before settling in one for its next developmental stage. Traversal through multiple hepatocytes has been observed in vitro and in vivo [22–24]. Although migration through several hepatocytes has been thought to activate sporozoites for hepatocyte invasion, infection with SPECT mutants demonstrates that cell traversal is not required before invasion [19]. Unlike migration through other cells in skin and liver parenchyma, the final hepatocyte invasion is accompanied by formation of a PV [25]. Transcriptomic studies indicate host liver cell responses following infection by rodent parasites or P. falciparum [26,27], although transcriptional changes may have occurred in cells following either migration or invasion. This might be resolved in future by assessing transcriptional changes in host cells following infection by cell traversal (SPECT or SPECT-2) mutants.
Hepatocyte invasion is complex and partially understood. A cell-adhesive domain in the CSP C-terminus binds to heparan sulfate proteoglycans on the hepatocyte membrane [28]. Merozoite invasion of RBCs is based on an AMA1–RON complex forming a tight junction [29,30], but sporozoite invasion of hepatocytes is not affected by AMA1 depletion. In contrast, knockdown of RON4 drastically reduces invasion, suggesting the important role of a rhoptry resident protein [31]. Apart from RON4, other proteins like TRAP, TREP, TLP and SPATR have roles in sporozoite motility and attachment to the hepatocyte [14,32,33]. However, no direct evidence of their involvement in host cell invasion has been documented (for a complete list, see Table 1) [34,35]. Gene deletions or mutation of essential residues of TRAP or TRAP-family proteins do not completely inhibit LS infection [35]. This raises several questions: can sporozoites complement the loss of function of one TRAP with another variant? Is there selective expression of different members of TRAP family proteins by different sporozoites? These scenarios suggest redundancy in this process, which complicates vaccine development.
Development & maturation of LS
Once inside hepatocytes, the sporozoite undergoes dramatic transformation inside the PV and develops as an exo-erythrocytic form or LS parasite. The parasite resides adjacent to the nucleus and may alter expression of host proteins by translocating CSP across the nuclear membrane and modulating gene transcription [36]. Nearly all LS data, including transcriptomic data, have been obtained from rodent malaria parasites, primarily P. berghei and P. yoelii. For P. falciparum, limited expression data are available, and then only for very early LS. Nevertheless, available transcriptomic and proteomic data identify a number of proteins important for LS development [37–42].
During LS development, Plasmodium is metabolically highly active and grows to a size that exceeds that of its host cell. The parasite synthesizes large amounts of lipids and nucleic acids needed for developing merozoites, although the means by which it meets its nutritional requirements are unclear. Targeted gene disruption and knockout studies identified several proteins involved in LS development (Table 1). Among these, LSA-1 is currently under investigation as a potential vaccine candidate [43,44]. Other proteins have been targeted to create attenuated exo-erythrocytic form parasites. Immunization of these genetically attenuated parasites can induce sterile or partial protection against Plasmodium infection in rodent models [45–47].
Once inside the hepatocyte, immune recognition of the parasite relies on antigen presentation by MHC molecules. Identification of new LS antigens recognized by CD8+ T cells from malaria-exposed individuals will constitute a repository of potential vaccine candidates. LS proteins identified thus far are either expressed inside PV or on the parasitophorus vacuole membrane, making them inaccessible to antibodies (Table 1). Identification of novel LS antigens expressed on the hepatocyte surface might be targeted by humoral immune response, yet no evidence of such proteins exists. During egress from hepatocytes to initiate BS infection, plasmodia form small merozoite-filled pockets called merosomes that are enclosed in the host hepatocyte membrane. Host cell-surface proteins including MHC class I are lost from the mero-some membrane, which may allow Plasmodium to evade detection by phagocytic cells [6,48].
Immunology of PE malaria
Mechanisms of immunity induced by whole sporozoite immunizations
The scientific rationale for a PE vaccine stems from the observation that sterile protection develops in mice, nonhuman primates and humans following immunization with radiation-attenuated sporozoites (RAS) [49–51]. These seminal studies provided impetus to develop the first PE vaccine based on CSP and the P. yoelii and P. berghei rodent models have been exploited to understand the immunobiology of RAS. While the immunological mechanisms of protection obtained from whole sporozoite immunization in humans are probably multifaceted, the plethora of information gleaned from animal models has been informative (Table 2). Immune responses against whole sporozoites may be mediated by antibodies that prevent sporozoite migration or invasion, or by T cells that recognize PE antigens on infected hepatocytes and eliminate them. RAS induce protection mediated by CD8+ T cells in mice from a variety of genetic backgrounds, as CD8 depletion following RAS immunization or immunization of β2 microglobulin−/− mice abrogates immunity [51–53]. Likewise, CD8+ T-cell depletion abrogates protection in rhesus monkeys [54].
Table 2.
Mechanisms of protective pre-erythrocytic immunity determined from rodent models.
| Vaccination strategy | Target | Rodent model | Immune response/mechanism of protection | Ref. |
|---|---|---|---|---|
| Plasmodium berghei RAS iv. | T and B cells | T and B cell-deficient C57BL/6N × BALB/c AnN F1 (BLCF1) | T cells are required for protection | [181] |
| T cell-deficient (ATX-BM-ATS) BLCF1 mice | B cells are dispensable | |||
|
| ||||
| P. berghei RAS | Thymus-derived T cells | Nude B10LP mice Thymectomized A/J mice | Thymus-derived T cells are required for the production of antisporozoite antibodies and the development of protection. | [182] |
|
| ||||
| P. berghei sporozoites by bites of irradiated mosquitoes | Antibodies against Pb44, a P. berghei sporozoite surface component | BALB/c and A/J mice | Antibodies against the Pb44 confer sterile protection against live sporozoite challenge | [68] |
|
| ||||
| P. berghei RAS | T-cell effector mechanisms | IFNγ−/−rats | CD8+ T cells, IFN-γ and antibodies are required to inhibit EEF development | [183] |
| CD4-depleted rats | CD4+ T cells are dispensable | |||
| CD8-depleted rats | ||||
|
| ||||
| Plasmodium yoelii RAS iv. | CD4+ and CD8+ T cells | CD4+ and CD8+ T-cell-depleted BALB/c | CD8+ T cells are required for protection | [52] |
| Athymic mice | CD4+ T cells are dispensable | |||
|
| ||||
| P. berghei RAS iv. | MHCI | β2m-deficient and WT C57BL/6 | Memory CD8+ T cells are required for sterile immunity | [53] |
| MHCI independent compensatory mechanisms are not required for protection | ||||
|
| ||||
| P. berghei RAS iv. | CSP-specific cytotoxic T cells | BALB/c mice | CD8+ T-cell clones specific for CSP249–260 mediate protection | [184] |
|
| ||||
| P. yoelii and P. berghei RAS iv. | CSP-expressing infected hepatocytes | BALB/cByJ B10.D2 | CSP peptide-expressing hepatocytes are direct targets for CD8+ T cells in vitro | [57] |
|
| ||||
| P. berghei RAS iv. | CSP277–288 peptide-expressing target cells | BALB/c | CD8+ T-cell clones specific for the CSP277–288 are required for sterile immunity | [55] |
|
| ||||
| P. yoelii CSP subunit vaccines ip. | CSP-specific antibodies | BALB/c | CSP subunit vaccines induce nonprotective antibodies | [185] |
| B6.D2 | P. yoelii RAS confer protection despite low titers of CPS-specific antibodies | |||
| B10.BR | No protection without CD8+ T cells | |||
|
| ||||
| P. berghei oxidized C-terminal CSP subunit vaccine sc. | CSP-specific immune mechanisms | BALB/c | Oxidized long C-terminal CSP subunit (CS242–310) vaccine induces peptide-specific high antibody titers and CTL | [186] |
| Protection is partially dependent on CS245–253-specific CD8+T cells | ||||
|
| ||||
| P. yoelii SSP2 and CSP transfected cells iv. | SSP2 and CSP-specific CTL and antibodies | BALB/c | Both SSP2 and CSP vaccinations are required for sterile protection | [187] |
| Sterile protection is mediated by CTL but not antibodies | ||||
|
| ||||
| P. berghei RAS by mosquito bite | Effector mechanisms of protection | BALB/cBYJ | Production of nitric oxide by liver cells is required for protection | [61] |
| CD8+T cells and IFN-γ are important for the production of nitric oxide | ||||
| CD4+ T cells are dispensable | ||||
|
| ||||
| Recombinant adenovirus expressing P. yoelii CSP (AdPyCS) sc. | Effector mechanisms of protection | IFN-γ−/−; IFN-γRc−/−; and WT BALB/c | CD8+ T cells are required for protection IFN-γ is not required | [117] |
|
| ||||
| Vaccinia virus expressing the SYVPSAEQI epitope of the P. yoelii CSP iv. | Effector mechanisms of protection | IFN-γ−/− and WT BALB/c Transgenic mice expressing a TCR specific to SYVPSAEQI epitope of P. yoelii CSP (CS-TCR Tg CD8+ cells) | IFN-γ secretion by CS-TCR transgenic CD8+ T cells is not required for protection | [116] |
|
| ||||
| P. yoelii and P. berghei RAS iv. | Effector mechanisms of protection | β2m−/− and WT C57BL/6 | IFN-γ from CD4+ T cells and antibodies are required for protection | [64] |
| CD8+ T cells are dispensable | ||||
|
| ||||
| P. yoelii GAS iv. | Effector mechanisms of protection | BALB/c IFN-γ KO BALB/cj | CD8+ T cells are required for protection | [63] |
| Perforin-deficient BALB/c Swiss Webster | Protection is partially dependent on IFN-γ and contact-dependent killing of infected hepatocytes | |||
|
| ||||
| P. yoelii RAS iv. or by mosquito bite | CSP-specific cytotoxic T cells | BALB/c ByJ | Cross-reactive CD8+ T cells mediate protection against both P. yeolii and P. berghei challenges | [187] |
| BALB/cAnn Nu/Nu | IFN-γ requirement for protection is antigen specific | |||
| Protection depends on the number of CD8+ T cells | ||||
|
| ||||
| P. berghei and P. yoelii RAS | Vaccination-induced CD8+ T cells | BALB/c; C57BL/6; and Swiss Webster mice | At least 8% of the CD8+ T cell compartment is required for protection, but are not always sufficient | [76] |
| The CD8+ T-cell requirement for protection varies with the genetic background | ||||
|
| ||||
| P. berghei RAS | Liver memory CD4+ and CD8+ T cells | C57BL/6 | Vaccination-induced liver lymphocytes confer protection | [188] |
| Memory CD8+ T cells persist long-term in the liver but not in the spleen of immunized mice | ||||
| Liver memory CD8+ T cells confer long-term protection | ||||
|
| ||||
| P. yoelii RAS by mosquito bite. Vaccinia virus expressing the SYVPSAEQI epitope of P. yoelii CSP | Anatomic site of CD8+ T-cell priming against liver-stage parasites | BALB/c Transgenic mice expressing a TCR specific for the SYVPSAEQI epitope of the P. yoelii CSP (CS-TCR Tg CD8 cells) CD11c-DTR mice | CD8+ T cells are primed in the skin draining lymph nodes before migrating to other tissues including the liver where they mediate protection | [11] |
Much of what the authors currently know about the immune response to liver stage malaria parasite was discovered using rodent models This table summarizes the findings that stem from the seminal observation by Ruth S Nussenzweig [49] in 1967 that vaccination with radiation-attenuated sporozoites confers sterile immunity against viable sporozoite challenges in rodents These findings include the identification of humoral and cellular immune factors that mediate protection and the anatomic sites where priming of effector functions against PE parasites occurs In addition, this table highlights some of the contradictory but insightful findings that may reflect differences between the various models An extension of this issue is the difficulty to reproduce the findings from rodent models to malaria in humans, emphasizing the uncertainty as to which model is most suitable for studying human immunity, or the possibility that the range of animal models may reflect the diversity of human experience.
CSP: Circumsporozoite protein; CS-TCR: Circumsporozoite-specific transgenic T cell receptor; CTL: Cytotoxic T lymphocyte; EEF: Exo-erythrocytic forms; iv.: Intravenous; ip.: Intrapentoneal; PE: Pre-erythrocytic; RAS: Radiation-attenuated sporozoites; sc.: Subcutaneous; WT: Wild-type.
Whether the effector mechanism is mediated by IFN-γ produced by CD8+ cells or by cytolytic perforin and granzyme through direct contact in the absence of IFN-γ is debatable, as there are publications supporting either mechanism. CD8+ cells specific for CS obtained from P. yoelii and P. berghei RAS-immunized mice have cytolytic activity in vitro [55–57]. Exogenous IFN-γ added to infected hepatocytes inhibits P. berghei development in vitro [58]. Several studies demonstrated that nitric oxide pathway is induced following RAS immunization or sporozoite infection and inhibits parasite development [59–61]. The requirement for IFN-γ varies according to genetic background of protected rodents [62], and killing of infected hepatocytes can occur by a contact-dependent mechanism involving perforin from CD8+ cells [63]. Notably, P. yoelii and P. berghei RAS-induced sterile immunity can occur in CD8+ deficient mice, where antibodies and IFN-γ derived from CD4+ T cells mediate protection [64].
Antibodies from RAS-immunized rodents and humans recognize sporozoite surface antigens and inhibit sporozoite infectivity both in vitro [65–67] and in vivo [68], inspiring the first CSP-based vaccine [69–71]. Mechanistically, antibodies can immobilize sporozoites in skin and prevent migration to the liver [72].
What is/are the immunodominant response/s to RAS? Following the observation that sporozoite antibodies were protective, CSP became the major PE vaccine target and remains the lead candidate today. An abundance of evidence supports CSP-based vaccines. Protection is diminished but not ablated in transgenic mice made tolerant of CSP, arguing for immunodominance [73]. However, immune responses to CSP do not appear to be required for protection, as immune responses against RAS lacking homologous CS protein are still protective [2,74,75]. Using a surrogate marker to identify RAS-activated parasite-specific CD8+ T cells, CSP-specific cells constitute a minority of parasite-specific CD8+ T cells in immunized mice, suggesting that many potentially protective PE antigens have yet to be discovered [76].
While humans immunized with RAS are less amenable to experimental manipulation, indirect evidence suggests that the immune mechanisms responsible for human protection are fairly concordant with those in the mouse. T cells from volunteers protected after RAS immunization respond to LS antigens by proliferating [77] and display cytolytic activity to target cells displaying CSP epitopes [78–81]. CD8+ T cells with cytolytic activity in response to SSP/TRAP have also been identified from RAS-immunized volunteers [82]. As in rodents, CD4+ T cells from humans immunized with RAS recognize CSP [83] and multiple HLA types respond to degenerate T-cell epitopes on CSP [84]. Multiple immune mechanisms have been identified as contributing to protection in reductionist experiments, and probably function in a coordinated fashion to confer maximal protection in RAS-immunized humans.
Identifying novel PE vaccine antigens
Current subunit PE vaccines in clinical development
Considering the efficacy of RAS, surprisingly few antigens among the approximately 5300 expressed genes have been assessed as vaccine candidates. Vaccines based on the sporozoite and LS antigens CSP, LSA-1, Exp-1 and SSP2/TRAP are the only PE candidates in current clinical development [201]. The most advanced malaria vaccine in development is GlaxoSmithKline's RTS,S vaccine, now in a multicenter Phase III clinical trial in sub-Saharan Africa. Based on CSP, the RTS,S vaccine is composed of 19 NANP central repeats plus the C-terminal region containing T-helper epitopes fused to the hepatitis B surface antigen. The RTS component is co-expressed with free surface antigen to form the RTS,S virus-like particle, with approximately 5% of capsomeres containing the CSP fusion protein. RTS,S is formulated with a complex adjuvant system composed of immunostimulants 3-deacylated monophosphoryl lipid A (MPL) [85] and QS21, a purified triterpene glycoside extracted from the bark of the Quillaja saponaria tree [86], prepared in oil-in-water emulsion (AS02) or with liposomes (AS01). As no analog of RTS,S exists for challenge in experimental animal malaria, the potential efficacy of GSK's vaccine cannot be assessed other than from clinical trials.
Prior to RTS,S, a number of CSP-based vaccines were found to be poorly immunogenic and minimally protective in humans. Early RTS,S trials in US malaria-naive volunteers at the Walter Reed Army Institute of Research (WRAIR; MD, USA) assessed efficacy after infective mosquito bites [87]. Preliminary evaluation of RTS,S in alum alone did not achieve sterile immunity in any of six volunteers, while formulation with MPL protected two out of eight volunteers [88]. To enhance the immunogenicity from that seen using alum, a new oil-in-water formulation containing MPL and QS21 was developed. Six out of seven (85%) volunteers who were challenged after receiving the new adjuvant were protected, compared with two out of seven who received oil-in-water alone or one out of eight who received the alum formulation [89]. Thus, a combination of complexed antigen with a novel adjuvant system appeared necessary for protection. Subsequent Phase I and II trials testing various dose sizes, immunization schedules and liquid versus lyophilized vaccine all resulted in a proportion of vaccinees protected, although none achieved the 85% that was attained initially [90–92]. Finally, the oil-in-water AS02A was tested against the new liposome based AS01B, with protection being 32 and 50%, respectively, setting the stage for advancement to field trials [93].
Efficacy of RTS,S in field trials is more difficult to assess, as exposure occurs naturally to different strains of parasites at different times and therefore it is not possible to definitively determine time from exposure to patency. For these reasons, efficacy is determined by time-to-event analyses, which compare pre-defined malaria events within a time period using Cox regression models. The first trial observed 34% efficacy in Gambian adults during a 15-week follow up period, although protection waned after 9 weeks [94]. The first efficacy trial in children found reduced incidence of malaria in Mozambique within the observed time period, but did not confer sterile protection [95]. The interim efficacy data available from the ongoing Phase III trial indicate a 50% reduction in clinical malaria episodes during a 13-month period following the first vaccination [96].
A knowledge gap that remains to be filled regarding RTS,S is the identification of immune correlate(s) of protection in the target population. A number of reports on the immunogenicity in African children support a largely antibody-mediated mechanism. FIn Phase II studies in Kenya and Tanzania, anti-CS antibodies predicted vaccine efficacy in a non-linear fashion [97]. In a Phase II study in Gabonese children, the clearest immune indicator was anti-CS IgG and associated antigen-specific B cells that persisted up to 12 months after the last dose [98,99]. As CD4+ cells against CS are known to protect in animal models and in humans immunized with RAS, a cellular correlation with efficacy has long been sought. To date, the most recent data from a Phase IIb study in Kenya involving 407 children demonstrated the clearest T-cell correlate for vaccine efficacy was TNF-α from CD4+ T cells. However, these results need confirmation from other datasets [100]. As Phase III of RTS,S progresses and immunological results are reported, the discovery of a defined correlate for efficacy hopefully may be at hand.
In RAS-immunized animals, CSP in infected hepatocytes appears to be a target for CD8+ T cells, but RTS,S is very poor at inducing CD8+ T-cell responses. To enhance CD8+ T-cell responses, a viral-vectored platform combined with RTS,S in a prime–boost regimen (Ad35.CS + RTS,S) is undergoing clinical testing. Cellular immunity provided by the virus theoretically might complement the high IgG induced by RTS,S and provide higher efficacy than either component alone. Adenovirus is highly immunogenic and has broad tissue tropism, and because viral proteins are translated in the cytoplasm, the proteins are processed and presented by MHC Class I machinery [101].
The ME-TRAP vaccine is another PE subunit vaccine with a multi-epitope construct that has the advantage of targeting multiple antigens instead of one. The multiple epitope contains CD4+ and CD8+ epitopes from six antigens (LSA1, CSP, STARP, LSA3, Exp1 and TRAP) fused to the TRAP antigen [102]. Earlier clinical studies found a DNA/MVA prime–boost regimen of ME-TRAP to be highly immunogenic in malaria-naive volunteers, with T-cell responses persisting for several months and a significant delay in parasitemia following challenge. A DNA/DNA/modified vaccinia virus Ankara (MVA) approach induced sterile protection in one out of eight volunteers [103]. A fowlpox prime–MVA boost regimen conferred sterile protection in two out of 16 volunteers [104]. However, field trials of the vaccine were less immunogenic and conferred no protection [105,106]. To increase immunogenicity, the vaccine is currently being tested in a heterologous viral vector approach composed of AdCh63 prime and MVA boost. Of note, the EP1300 polyepitope DNA vaccine, which contains epitopes from CSP, TRAP, LSA-1 and Exp-1, is delivered by electroporation and currently in Phase I trials (EP1300). No results have been published yet.
Whole organism approaches to a PE vaccine
Whole sporozoite immunization has undergone a reappraisal that began with plans to manufacture whole sporozoites (PfSPZ) under cGMPs. A Phase I clinical trial of radiation-attenuated PfSPZ vaccine injected subcutaneously or intradermally demonstrated safety but poor efficacy. An ongoing trial will determine whether PfSPZ vaccine given intravenously can induce protection. A modern approach to attenuated sporozoite vaccination uses reverse genetics to design GAP. Unlike RAS, in which genetic alterations are random, GAP are genetically identical and arrest at a defined point during LS development. Efficacy of GAP has been demonstrated in rodent models [45–47,107,108]. The mechanism of protection is concordant with that of RAS, relying on CD8-mediated killing of infected hepatocytes [109]. The current challenge for GAP vaccination is assessment of adequate attenuation of parasites [110]. Nevertheless, like RAS, the GAP approach still faces major manufacturing hurdles.
The strategy of whole sporozoite vaccination was recently expanded to include inoculation with nonattenuated sporozoites under chloroquine prophylaxis, which allows parasites to progress through LS development and establish an initial and brief infection of erythrocytes before being killed. This approach induced a long duration of sterile immunity that surpassed that from RAS immunization: sterile protection after 28 months in four out of six volunteers [111]. A notable difference between this approach and RAS immunization is the small number of mosquito bites required for protection. One possible explanation is the effective antigen load. A thousand mosquito bites that deliver an average of 450 attenuated sporozoites [112] yield 450,000 LS parasites that arrest. Forty mosquitoes bites with nonattenuated sporozoites produce 18,000 LS parasites initially that propagate approximately 20,000-fold over the next week to achieve 360 million liver merozoites. Aside from biomass, an increased repertoire of antigens expressed during late LS and BS development may result in a broader, more protective CD8+ T-cell response [113], as opposed to RAS that arrest as early LS. Finally, actively dividing parasites might provide a danger signal, a mechanism of immune detection proposed to identify replicating pathogens [114]. The reduced number of sporozoites required for protection in this model imparts a significant manufacturing advantage if this approach advances to commercial development.
The mechanisms of protection induced by nonattenuated parasites under chloroquine cover may differ from those induced by RAS. IFN-γ and IL-2 responses were detected against whole sporozoites and infected erythrocytes from CD4+ T cells and γd T cells. Of note, CD8+ T-cell responses were barely detectable, in stark contrast to published literature on RAS and GAP [115]. However, the lack of CD8+ responses might reflect in vitro re-stimulation conditions, as it is not known if whole parasite antigen can be efficiently phagocytosed and cross-presented to the MHC Class I pathway in culture. Further investigations of the immune mechanisms of protection using this immunization approach are needed.
Immunological gaps in our knowledge: immune correlates of protection
The dearth of immune correlates of protection has been a longstanding obstacle to vaccine development and testing. The most common measure of PE vaccines has been IFN-γ production from T cells, by ELISpot or intracellular cytokine staining. While IFN-γ production may be a useful indicator of `vaccine take', it may not measure the effector mechanism(s) required for parasite elimination. In mice, CD8-mediated protection can occur in the absence of IFN-γ [116,117]. Direct killing of infected hepatocytes can be contact-dependent and only partly dependent on IFN-γ and perforin [63]. Alternatively, IFN-γ from CD8+ or CD4+ T cells may indirectly kill infected cells through soluble mediators [118] such as activating reactive oxygen intermediates. Resolving these mechanistic issues and implementing the appropriate immunological measurements of protection will better guide vaccine design.
While CSP has received the greatest attention as a vaccine candidate owing to its abundance on the sporozoite surface, it is not essential for sterile protection, as demonstrated in CSP-tolerized mice [73]. The targets of effective anti-sporozoite immunity should be exposed to circulating antibody, and ideally will elicit long-lived plasma cells that produce high-affinity antibody. Additional PE antigens targeted by antibody could be combined with RTS,S most easily. Such a combination vaccine may nevertheless still require potent platforms to induce adequate and long lasting antibody, such as through co-immunization with immunostimulatory molecules [119] or in formats such as virus-like particle [120].
The universe of candidate PE antigens
The universe of potential pre-erythrocytic vaccine candidates is present in the transcriptomes and proteomes of PE parasites (Table 3). In an early study, cDNA from nine different P. falciparum life-cycle stages (seven asexual erythrocytic stages, gametocytes and salivary gland sporozoites) were hybridized to a high-density oligonucleotide array. Evidence of transcription was found for 4557 out of 5159 predicted genes in the genome. The sporozoite transcriptome included approximately 2000 genes, 41 of which were exclusive to this stage. Sporozoite-stage genes included those with known or putative roles in cell transport, cell rescue, defense and virulence, signal transduction, DNA processing, protein synthesis and metabolism, as well as cell-surface/apical organelle proteins. The proteome of salivary gland sporozoites was defined on lysates using liquid chromatography mass spectrometry multidimensional protein identification technology [121], with 1049 proteins confidently assigned to parasite origin, of which up to half are unique. Further analyses identified several potential vaccine targets, such as cell surface and organelle proteins, proteins involved in motility (such as actin and myosin) and proteins belonging to the apical complex machinery involved in invasion.
Table 3.
Transcriptome and proteome studies of pre-erythrocytic parasites.
| Parasite material | Profiling method | Major findings | Ref. |
|---|---|---|---|
| Plasmodium falciparum sporozoites; blood stage merozoites, trophozoites and gametocytes | LCMS MUDPIT | 46% (2145) of all predicted proteins were identified in the four stages analyzed | [121] |
| 1047 proteins were identified in the sporozoite stage, with 512 (49%) of the proteins being unique to this stage | |||
| Mainly cell surface and organelle proteins | |||
|
| |||
| P. falciparum salivary gland sporozoites, asexual blood stages, gametocytes | High-density oligonucleotide array of 260,000 probes (>95% of predicted genes) | 88% (4557) of predicted genes detected in total, with approximately 2000 detected in the sporozoite stage. Most sporozoite transcripts encoded hypothetical proteins, as well as proteins involved in transport, protein synthesis, transcription, cell cycle and signal transduction | [189] |
|
| |||
| Plasmodium yoelii sporozoites collected from mosquito midgut oocyst versus salivary gland | Microarray comprised of 65-mer representing 6700 ORFs | 124 genes were upregulated and 47 genes were downregulated in the infectious salivary gland sporozoites. qPCR confirmed similar transcription of 11 orthologous genes in P. falciparum sporozoites | [190] |
|
| |||
| P. falciparum blood stages, sporozoite, zygote and ookinete stages, and P. yoelii sporozoites, LS and asexual blood stages | 150,000 25-mer P. yoelii custom-designed oligonucleotide array (cDNA subtraction) | Clustering analysis and comparisons to human and yeast databases and protein-protein interaction data predicted the function of 926 uncharacterized malaria genes | [40] |
|
| |||
| P. falciparum sporozoites from midgut oocysts and salivary glands | nLC-MS/MS | The study identified a total of 728 unique proteins, among which 127 were detected in oocysts, 450 in oocyst-derived sporozoites, and 477 in salivary gland sporozoites; 250 of these 728 proteins were not detected in blood stage parasites or gametocytes | [122] |
|
| |||
| P. yoelii sporozoites grown axenically for 24 h | Sequencing of cDNA library and validation by qPCR of infected livers | Identified approximately 1000 transcripts, including LS and some blood-stage antigens. 21% of genes were not previously described | [42] |
|
| |||
| P. falciparum sporozoites exposed to hepatocytes for 1 h | DNA microarray containing 12,037 unique 70-mer oligonucleotides | 532 genes were upregulated and 79 were downregulated at least twofold. Functional characterization of four genes with differential expression patterns (see text) | [123] |
|
| |||
| P. yoelii-infected hepatocytes collected from mouse livers 40h post infection by laser capture microdissection | Sequencing of cDNA library | 621 nonredundant genes identified, including 56% expressed in blood stages and 25% unique to the LS | [41] |
|
| |||
| P. yoelii7-infected hepatocytes collected 24h, 40hand 50h post-infection; sporozoites from midgut oocysts and salivary glands; mixed blood stages | Oligonucleotide (65-mer) microarray containing annotated ORFs | Transcriptome: 1985 genes identified in LS parasites, including approximately 1000 upregulated genes and approximately 400 genes common to all timepoints | [38] |
| 1DLC/MS/MS shotgun proteomic analysis of late liver stages | Proteome: approximately 800 proteins identified, including 170 unique to LS. 50 h LS parasites were 90% identical to blood stages | ||
| 93 LS proteins had a predicted signal peptide and 73 had a predicted transmembrane domain | |||
|
| |||
| P. yoelii radiation-attenuated and wild-type parasites at salivary gland sporozoite, 24 h and48hLS | Spotted micoroarray (65 bp oligonucleotides) representing 6700 ORFs | Approximately 1600 genes were differentially expressed in comparison to mixed blood stages, including 636 in wild-type sporozoites, 889 in RAS sporozoites, 484 in 24h LS and 271 in 48h LS (161 common genes) | [191] |
LCMS: Liquid chromatography mass spectrometry; LS: Liver stage; MS: Mass spectrometry; MUDPIT: Multidimensional protein identification technology; nLC: Nano-liquid chromatography; ORF: Open reading frame; qPCR: Quantitative PCR.
A more recent transcriptome study [37] compared P. yoelii oocyst sporozoites (which are not yet infectious to mammals) with salivary gland sporozoites (which are infectious) by cDNA hybridization to 65-mer oligonucleotides. A total of 124 genes were upregulated in the infectious SPZ, and 47 genes in the oocyst SPZ; transcription of 11 gene orthologues was assessed in P. falciparum with good agreement to P. yoelii. A cDNA subtraction (i.e., host RNA depleted) hybridization analysis against a microarray containing 150,000 25-mer P. yoelii probes [40] yielded similar results (87% correlation). Tandem mass spectrometry (MS/MS) analysis of hand-dissected P. falciparum oocysts, oocyst-derived sporozoites and salivary gland sporozoites identified 728 proteins, out of which 250 were not detected in blood-stage proteome studies [122].
Intrahepatocytic parasites have proven to be least tractable, for reasons of low number and poor enrichment or purification, but recent advances moved this area forward. P. berghei and P. yoelii sporozoites can grow axenically and undergo morphological changes similar to those of intrahepatocytic parasites. Axenically grown LS parasites yielded 652 unique expressed sequence tags (ESTs), of which 87% matched a known P. yoelii gene sequence. Over-represented proteins included heat shock and chaperone proteins, possibly related to the temperature change from mosquito to mammalian host, as well as proteins involved in cell-cycle progression, metabolism and transport. Comparisons with sporozoite and blood-stage libraries revealed a shift from `sporozoite-like' to `blood stage-like' and 21% of ESTs were specific to transformed sporozoites.
Using a similar approach [123], cDNA from P. falciparum sporozoites co-cultured with human primary hepatocytes for 1 h was hybridized to 70-mer microarrays. Seventy nine genes were downregulated and 300 upregulated in comparison with salivary gland sporozoites. A total of 300 genes encode predicted proteins, including those involved in parasite invasion, metal ion homeostasis and stress responses. Genes with temporal upregulation were hypothesized to be involved in sporozoite migration and invasion, whereas those with continuous upregulation were purported to be involved in parasite development in the hepatocyte. Studies of proteins encoded by two genes with temporal upregulation (PFD0425 [SIAP1] and Pf08_0005 [SIAP2]) immunolocalized them to the sporozoite surface, and suggested roles in both traversal and hepatocyte invasion. These proteins were not detected in the hepatocyte after invasion nor in BS parasites, whereas two proteins with continuous upregulation (PFL0065w [LSAP1] and PFB0105 [LSAP2]) were both expressed in LS parasites according to immunofluorescence assay.
Laser captured microdissection (LCM) allows recovery of infected hepatocytes from mouse livers virtually free of contaminating host tissue. A cDNA library of LCM-captured schizonts yielded 623 nonredundant genes, including 25% specific to LS [41]. Later, GFP-expressing P. yoelii was purified from liver at 24 (early schizonts, LS24), 40 (late schizonts, LS40) and 50-h (merozoite formation, LS50) post-infection. Transcriptional analysis revealed over 1000 genes upregulated in LS parasites. Isolation of 40,000 LS40 and LS50 parasites allowed mass spectral analyses that identified 800 proteins, including 174 that were more abundant or unique to LS. Proteins involved in processes like translation (including ribosome structure and protein synthesis), heat shock proteins, redox metabolism, mitochondrial TCA cycle and electron transport and fatty acid synthesis were over-represented. LS parasites are highly metabolically active, which is not surprising considering the multiple cell divisions during this developmental stage [38].
Downselecting PE antigens as vaccine candidates
The existing functional genomics datasets have vastly expanded the number of PE proteins to consider as potential vaccine antigens. One approach to down-select candidates has been to screen the available datasets and define new CD4 and CD8 epitopes, although no means exists at present to associate these with protection in humans. Given the small number of PE antigens shown to confer protection in rodents and especially in humans (CSP [89] and ME-TRAP [103,104]) it is presently not possible to know which protein characteristics, such as physical properties or cellular localization, will predict effective human vaccine antigens. For example, proteins that are secreted or localized to PV membrane might be more accessible to the host hepatocyte, and thus more likely to be processed and presented to immune cells, but it has not been shown that such proteins are in fact superior vaccine targets.
Immunomics focuses on elucidating the set of antigens that interact with the host immune system and the mechanisms involved in these interactions [124]. Sera collected from humans after RAS immunization have been examined for reactivity to a microarray of 1200 P. falciparum proteins (~23% of the proteome), and the reactivity profiles examined for relationships to protective immunity. A total of 20 fragments representing 19 antigens were found to be strongly associated with RAS-induced protective immunity. Among these proteins, three were previously assessed as vaccine candidates (CSP, SSP2/TRAP and AMA-1) The average molecular weight of the antigens correlating with protection were 2.5-fold larger than the average of the ORFs in the genome (larger proteins potentially have more B-cell epitopes for antigen recognition). Export element motifs were more common in antigens associated with immunity (45 vs 27% in the total proteome). A total of 40% were hypothetical proteins, and proteins involved in or regulating DNA processing and cell cycle (20%) and protein synthesis (20%) were over-represented [125].
Cellular responses to panels of proteins have also been examined to down-select vaccine antigens, although thus far without success. A survey of 34 PE proteins with signal sequences and/or export element sequences that predict export, as well as high levels of transcription, yielded only six that were targeted by CD8+ T cells after RAS immunization. When these six were incorporated into adenovirus vectors, only three antigens (P36p[py52] [Py01340], Ag2 [PyCelTOS] and Ag5 [Py00419]) elicited CD8+ T-cell responses at a level similar to CSP (also incorporated into adenovirus) and even these three antigens failed to confer any protective benefits to mice [126].
An empiric approach has been used, by examining panels of immunogens for evidence of protection in the P. yoelii rodent model. In one study [127], 19 genes derived from a sporozoite cDNA library were prepared as DNA vaccines, and examined for protective efficacy in pools of 6–7 plasmids, with PyCSP as a benchmark of partial protection. Two pools of immunogens gave evidence of protection, but only a single antigen (Py01316) was found to be protective individually and only when delivered into the skin by gold-particle bombardment (gene gun). The authors concluded that pooling of immunogens may yield false positive results, although this may be difficult to discriminate from additive or synergistic effects of immunogen combinations. For example, in a different study, the combination of three P. yoelii antigens (Py03011/PyUIS3, Py03424 and Py03661) conferred sterile protection to a large fraction of rodents, whereas each individual antigen protected few animals [128].
Without a clear understanding of the PE immune responses that can mediate protection in humans, a strategy that prioritizes candidate antigens by their characteristics and in preclinical studies has been proposed [129]. This approach does not assume that features such as export signals, level of transcription or predicted epitopes would necessarily characterize the optimal candidates. Instead, antigens that are confirmed to be LS proteins are examined in animal and human models of immunity for the evidence that they contribute to protection. Criteria to predict vaccine potential may include protection of rodents or nonhuman primates after subunit immunization, and evidence of antigenicity in animals or humans protected by experimental or naturally acquired infection. Naturally acquired infections in humans have generally been considered to induce only suboptimal immunity to PE parasites; however, observational studies have consistently found associations between PE immune responses and end points of malaria resistance [130].
Designing efficacious PE vaccines
Vaccine platforms
Protein-in-adjuvant
Protein-in-adjuvant vaccines are based on the rationale that antibodies against the sporozoite can prevent migration and invasion. The most advanced among these, RTS,S self-assembles into virus-like particles, and is based on the GSK vaccine EngerixB™ that targets the hepatitis B virus. The hepatitis B virus surface antigen was used as a carrier matrix first by expressing it as a chimeric protein with 16 central region repeats of CSP [131], but this construct was not immunogenic in a Phase I trial [132]. For RTS,S, hepatitis B virus surface antigen is fused to a CSP fragment containing 19 NANP repeats plus the entire C-terminal flanking region. RTS,S has been formulated with different adjuvants to optimize the immune response.
Adjuvants are molecules that trigger danger signals and robustly activate the immune response, and are classified into vehicles (aluminum salts, emulsions, liposome and virosomes) that stabilize the antigen and slow its release or immunostimulatory molecules (TLR ligands and bacterial toxins). Alum/aluminum salt-based adjuvants are used as noncrystalline gels that enhance antibody response and have good safety records, but are poor stimulators of cellular immunity [133]. MF59™ (Novartis, Basel, Switzerland) is an oil (squalene)-in-water emulsion that induces antibody and strong T-helper responses but modest CD4+ Th1 responses [134]. Montanide adjuvants (e.g., ISA720) are water-in-oil emulsions that contain squalene and mannide-monoleate as an emulsifier, and are similar to incomplete Freund's adjuvant. Montanides have been used in several malaria studies and promoted strong immune responses; however, in some of these studies unacceptable reactogenicity was observed [135].
MPL is a low toxicity derivative of lipopolysaccharide endotoxin from the cell wall of Gram-negative bacteria, and stimulates Th1 responses by acting as a TLR agonist. Saponins are triterpene glycosides isolated from plants, including the most widely used derivative called Quil-A, which is extracted from the Quillaja saponaria tree. QS21, a purified component of Quil-A, enables continuous release of antigen with high adjuvant potency and low toxicity [136], yielding antibody, Th1 and cytotoxic T-cell responses.
RTS,S was initially administered with conventional Alum-based adjuvants [131,137], with or without MPL (a nontoxic derivative of LPS), that resulted in low protection rates. Among 11 novel adjuvant systems assessed in preclinical studies, AS04 (oil and MPL), AS03 (an oil [squalene]-in-water emulsion), AS02 (a squalene-in-water emulsion containing MPL and QS21, with mercury based thimerosal as a preservative), AS02A (same as AS02 but with lactose as a cryopreservatant) and AS01B (the oil-and-water emulsion of AS02 was replaced by liposomes) were further evaluated. The results of clinical trials with RTS,S are described elsewhere in this review, and the current product in Phase III trials is formulated with AS01.
Another protein-in-adjuvant malaria vaccine incorporates LSA-1, a highly conserved P. falciparum protein abundantly expressed by LS parasites. LSA-1 has no orthologue among other Plasmodium species so it cannot be assessed for protection in animal models. Immunization of mice and monkeys with the recombinant P. falciparum protein combined with AS01 or AS02 generated antibody and specific CD4+ and CD8+ responses [138,139]. The so-called NRC construct of LSA-1 (the conserved T-cell epitope in the N and C terminals and two 80-amino acid repeats out of the 17 in total) produced in Escherichia coli and formulated with AS01 or AS02 was tested in a Phase I/II trial for reactogenicity and efficacy. Overall the vaccine was well tolerated, and induced a modest increase in antibody titer against LSA-1, and low cellular responses (mainly CD4+ T cell and no detectable CD8+ T-cell responses). All the volunteers developed parasitemia after experimental infection, with no difference between the immune and nonimmune groups [43].
The virosome platform or immunopotentiating reconstituted influenza virosomes (IRIV) has been used to present peptides derived from CSP and AMA-1 anchored to a liposomal carrier. The platform has adjuvant qualities as the liposomes are derived from influenza virus and contain viral antigens. Peptides are both anchored to the surface and encapsulated in the lumen of the vesicle, and have the potential to stimulate antibody, CD4+ T-cell and CD8+ T-cell responses. IRIV containing peptides derived from AMA-1 and CSP elicited antibody that correlated with inhibition of sporozoite migration and hepatocyte invasion [140], and proliferative cellular responses to AMA-1 peptides [141]. In a Phase Ib trial among semi-immune adults and children, IRIV displaying AMA-1 and CSP peptides proved safe, immunogenic and in addition revealed a trend of protection [142].
In summary, recombinant proteins in adjuvant can induce strong antibody responses, but are poorly immunogenic for cytotoxic T cells in humans. Efforts are being made to search for adjuvants that are safe to use in humans and generate potent humoral and cellular immune responses.
DNA vaccines
DNA immunization has been appealing due to its simplicity and stability, and a vaccine expressed within host cells would appear to have advantages against an intracellular parasite such as Plasmodium. The main problem with DNA vaccines has been weak immune responses in humans, with insufficient antibody or T-cell responses to eliminate the parasite. Intramuscular immunization of mice with a DNA vaccine containing P. yoelii CSP generated low levels of antibody that poorly inhibited the development of LS parasites in vitro; however, half of animals acquired CD8-dependent sterile immunity. Human trials of P. falciparum CSP in Vical vector VR1020 induced cytolytic activity in 11 out of 20 volunteers, primarily CD8+ T cells [143], but antibody was absent [144]. Intradermal delivery improved antibody induction [144].
Co-administration of DNA vaccine constructs containing a panel of malaria PE antigens (PfCSP, PfSSP2, PfEXP1, PfLSA-1 and PfLSA-3) in the Vical vector VCL-25, together with plasmid encoding GM-CSF, yielded low cellular responses in volunteers and failed to confer protection against experimental infection [145]. More potent plasmid delivery methods, such as electroporation, are under assessment, and combinations with viral platforms in prime–boost regimens may significantly improve responses.
Viral vectors
Viral vectors induce robust levels of cytotoxic immune responses, as well as other responses that may be needed to eliminate pre-erythrocytic malaria infections. Malaria antigens are cloned into the virus genome, which delivers the gene to the target cell and facilitates intracellular expression of antigen. Processing of antigen in the cytoplasm or nucleus (depending on the virus used) of the host cell results in presentation by MHCI molecules. The virus itself has an adjuvant effect that can enhance the specific response.
The greatest concern with viral vaccines has been safety, since a malaria vaccine has healthy individuals including children as its target population. Most of these vaccines have been rendered nonreplicative with a minimum of two deletions to prevent a gain-of-function recombination event. Another disadvantage of viral vectors is pre-existing immunity to the vector, induced by prior immunization or by natural infection with the same or a related vector. This problem can be partially overcome by using non-replicating viral vectors, as well as DNA or protein-in-adjuvant vaccines for priming and/or boosting in combination with the viral vector.
Poxviruses
Poxviruses have a large (~300 kb) double-stranded DNA genome that enables cloning of up to 25 kb of foreign DNA, and expression of multiple antigens in the same vector. Vaccinia virus and fowlpoxvirus are two poxviruses utilized to deliver malaria antigens. NYVAC-Pf7 is an attenuated vaccinia virus strain containing seven P. falciparum proteins from sporozoite (CSP and PfSSP2), liver (LSA-1), blood (MSP1, SERA and AMA1) and sexual (PFs25) stages. In a Phase I/IIa trial of low (1 × 107 plaque-forming units [PFU]) and high (1 × 108 PFU) doses, antibody response was lower in individuals with prior exposure to vaccinia, although this effect was less prominent after high doses. A total of 53% of high-dose volunteers and 26% of low-dose volunteers mounted cytolytic responses to LS antigens (although to no more than one antigen by each individual), and 50% acquired proliferative responses to parasitized RBCs. Cellular immunity was not affected by pre-existing immunity to vaccinia. One volunteer in the low-dose group was completely protected after sporozoite challenge, and both low- and high-dose groups showed delay in parasitemia compared with naive controls, with protection correlating to immune responses [146].
Poxvirus family vectors have also been used to deliver TRAP antigen and other PE epitopes in a series of clinical trials [147], as described above [103–106,148,149]. The partial protection obtained with the poxviruses might be explained by strong CD4 responses in DNA/MVA and FP9/MVA ME-TRAP regimens [150,151].
Adenoviruses
In the P. berghei model, more than 1% of the peripheral blood lymphocytes must be CSP-specific memory CD8+ T cells to protect Balb/c mice, much higher than what had been achieved after human immunizations [152]. Moreover, immunization with RAS broadens the number of antigens being processed but does not lower the numbers of memory T cells required for protection, with more than 8% of the peripheral CD8+ memory cells required to be parasite-specific to protect Balb/c, while 40% were not enough to protect B6 mice [76].
Adenoviral vectors are very potent and protect mice against malaria by inducing high antibody as well as IFN-γ responses [153,154]. Adenoviruses contain linear double-stranded DNA genomes of approximately 38 kb size. Virus vectors have had two to three genes deleted to render them replication-deficient, and enabling insertion of 7–8 kb of foreign DNA. Adenovirus serotype 5 (Ad5) has been studied for malaria vaccines, although its use is hindered by pre-existing neutralizing antibodies against the vector in 35–40% of USA adults and over 90% of African adults [155]. The prevalence of anti-Ad5 immunity in young children in Africa is lower. In the STEP HIV vaccine trial, the vaccinees showed a nonsignificant trend towards increased HIV infection, and anti-vector immunity may have contributed to this adverse safety profile [156].
For malaria, a recombinant Ad5 construct expressing CSP was delivered to human volunteers, alone or in combination with the same virus vector expressing AMA-1, with high IFN-γ responses (comparable with RTS,S), but predominantly due to CD8+ T cells rather than CD4+. Antibody responses were low. Immune responses peaked after 1 month and persisted for up to 12 months. None of the volunteers were protected after sporozoite challenge, although two out of 11 showed a significant delay in parasitemia. Some of the volunteers had neutralizing antibodies to Ad5; however, there was no correlation between pre-existing immunity and generation of novel cellular or humoral immune responses [157,158].
Concerns regarding pre-existing immunity against Ad5 can be addressed by using less prevalent serotypes such as Ad35, or nonhuman adenoviruses. Human volunteers have been immunized with Chimpanzee adenoviral vector 63 (ChAd63) expressing ME-TRAP. ChAd63 induced IFN-γ responses and boosting with MVA significantly enhanced responses that persisted 3 months. Most volunteers were negative for ChAd63 neutralizing antibodies before the vaccination and more than 90% seroconverted after vaccination [159].
Expert commentary
For 40 years, scientists have known that immunity can completely block malaria infection of humans, but progress has been slow and resources sparse to achieve the goal of a completely effective pre-erythrocytic malaria vaccine. Partial protective efficacy observed in Phase III trials of the subunit vaccine RTS,S (which targets a sporozoite surface protein) has provided proof of concept, but enhanced activity is required to achieve full protection. Deeper understanding of parasite biology together with genomic, transcriptomic and proteomic data have revealed numerous PE approaches and antigens for vaccine interventions, and these should be rapidly assessed in human trials after completing preclinical evaluation. The availability of an experimental infection model of humans makes Phase I trials followed by sporozoite challenge a cost-effective means to screen PE vaccines for efficacy, but despite this, few antigens have been tested to date. Existing adjuvants and vaccine platforms may not be sufficient for the durable immune responses that would provide prolonged sterile protection and contribute to malaria elimination programs. Malaria vaccine progress depends on novel approaches, such as new adjuvants, viral vectors, nano-particle technologies and protein conjugation, which enhance and prolong immune responses. In parallel, systems immunology studies of complete protection after whole organism vaccination, or of partial protection after subunit vaccination, will probably identify immune mediators required to achieve sterile immunity.
Five-year view
Over the next 5 years, an expanded array of human and animal studies will intensively interrogate the immune responses and antigens that contribute to sterile protection induced by whole organism vaccination. In parallel, an accelerated program of clinical trials with sporozoite challenge to test subunit PE vaccines will better define the characteristics of protective antigens, including antigens that enhance the partial efficacy of the RTS,S, which is currently in Phase III testing. Advances in vaccine platforms, such as improved adjuvants or conjugation partners to prolong antibody responses and virus vectors or prime–boost regimens for durable CD8+ T-cell responses, will be needed if subunit vaccines are to achieve the level of protection induced by whole organism vaccination. The recent call for malaria elimination and eradication has intensified the impetus for PE vaccines that block human infection, which could be combined with transmission-blocking vaccines that block subsequent infection of mosquitoes, yielding bifunctional vaccines that completely interrupt malaria transmission in a community.
Key issues
The biology of pre-erythrocytic Plasmodium parasites reveals several targets for vaccine interventions, such as sporozoite migration and invasion processes targeted by antibody, or intrahepatocytic parasites targeted by CD8+ and CD4+ T cells.
Whole organism sporozoite vaccines induce sterile immunity in animals and humans, providing valuable models to guide subunit vaccine development.
Only a few pre-erythrocytic vaccine antigens have been tested in humans, although the emerging transcriptome and proteome data offer thousands of potential target antigens.
A partially effective pre-erythrocytic vaccine based on CSP reduces disease but does not prevent infection in field trials, and additional pre-erythrocytic antigens may be needed to achieve sterile immunity.
The characteristics that predict effective pre-erythrocytic malaria vaccine antigens are as yet undefined, and an accelerated program of clinical trials to test numerous novel antigens for protection against experimental infection should be undertaken to address this knowledge gap.
Current vaccine technologies may be insufficient to yield the durable antibody and cellular immune responses required for sterile protection over an extended period of time.
Long-lived sterile immunity to pre-erythrocytic malaria will be a key tool in the renewed effort to eliminate and eradicate malaria.
Acknowledgments
PE Duffy is named as a co-inventor on patents for novel pre-erythrocytic malaria vaccine antigen candidates. The authors contributing to this review are supported by funds from Division of Intramural Research, NIAID, NIH, and by a grant from the Malaria Vaccine Initiative at PATH (to PE Duffy). No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Medica DL, Sinnis P. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect. Immun. 2005;73(7):4363–4369. doi: 10.1128/IAI.73.7.4363-4369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mauduit M, Grüner AC, Tewari R, et al. A role for immune responses against non-CS components in the cross-species protection induced by immunization with irradiated malaria sporozoites. PLoS ONE. 2009;4(11):e7717. doi: 10.1371/journal.pone.0007717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman SL, Oster CN, Plowe CV, et al. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science. 1987;237(4815):639–642. doi: 10.1126/science.3299709. [DOI] [PubMed] [Google Scholar]
- 4.Belnoue E, Voza T, Costa FT, et al. Vaccination with live Plasmodium yoelii blood stage parasites under chloroquine cover induces cross-stage immunity against malaria liver stage. J. Immunol. 2008;181(12):8552–8558. doi: 10.4049/jimmunol.181.12.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderberg JP. Plasmodium berghei: quantitation of sporozoites injected by mosquitoes feeding on a rodent host. Exp. Parasitol. 1977;42(1):169–181. doi: 10.1016/0014-4894(77)90075-3. [DOI] [PubMed] [Google Scholar]
- 6.Sturm A, Amino R, van de Sand C, et al. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 2006;313(5791):1287–1290. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- 7.Gueirard P, Tavares J, Thiberge S, et al. Development of the malaria parasite in the skin of the mammalian host. Proc. Natl Acad. Sci. USA. 2010;107(43):18640–18645. doi: 10.1073/pnas.1009346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amino R, Thiberge S, Martin B, et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 2006;12(2):220–224. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- 9.Sinnis P, Zavala F. The skin stage of malaria infection: biology and relevance to the malaria vaccine effort. Future Microbiol. 2008;3(3):275–278. doi: 10.2217/17460913.3.3.275. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell. Microbiol. 2007;9(5):1215–1222. doi: 10.1111/j.1462-5822.2006.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 2007;13(9):1035–1041. doi: 10.1038/nm1628. [DOI] [PubMed] [Google Scholar]
- 12.Ejigiri I, Sinnis P. Plasmodium sporozoite-host interactions from the dermis to the hepatocyte. Curr. Opin. Microbiol. 2009;12(4):401–407. doi: 10.1016/j.mib.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Extensively reviews the journey of sporozoite from skin to liver and also highlights the important parasite proteins involved in migration and invasion events.
- 13.Bergmann-Leitner ES, Mease RM, De La Vega P, et al. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PLoS ONE. 2010;5(8):e12294. doi: 10.1371/journal.pone.0012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sultan AA, Thathy V, Frevert U, et al. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90(3):511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 15.Ishino T, Chinzei Y, Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol. Microbiol. 2005;58(5):1264–1275. doi: 10.1111/j.1365-2958.2005.04801.x. [DOI] [PubMed] [Google Scholar]
- 16.Bhanot P, Schauer K, Coppens I, Nussenzweig V. A surface phospholipase is involved in the migration of Plasmodium sporozoites through cells. J. Biol. Chem. 2005;280(8):6752–6760. doi: 10.1074/jbc.M411465200. [DOI] [PubMed] [Google Scholar]
- 17.Pradel G, Frevert U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology. 2001;33(5):1154–1165. doi: 10.1053/jhep.2001.24237. [DOI] [PubMed] [Google Scholar]
- 18.Baer K, Roosevelt M, Clarkson AB, Jr, van Rooijen N, Schnieder T, Frevert U. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell. Microbiol. 2007;9(2):397–412. doi: 10.1111/j.1462-5822.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 19.Ishino T, Yano K, Chinzei Y, Yuda M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2004;2(1):E4. doi: 10.1371/journal.pbio.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meis JF, Verhave JP, Brouwer A, Meuwissen JH. Electron microscopic studies on the interaction of rat Kupffer cells and Plasmodium berghei sporozoites. Z. Parasitenkd. 1985;71(4):473–483. doi: 10.1007/BF00928350. [DOI] [PubMed] [Google Scholar]
- 21.Verhave JP, Meis JF, De Boo TM, Meuwissen JH. The delivery of exoerythrocytic parasites of Plasmodium berghei: a hormone controlled process. Ann. Soc. Belg. Med. Trop. 1985;65(Suppl. 2):35–44. [PubMed] [Google Scholar]
- 22.Frevert U, Engelmann S, Zougbédé S, et al. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 2005;3(6):e192. doi: 10.1371/journal.pbio.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frevert U, Usynin I, Baer K, Klotz C. Plasmodium sporozoite passage across the sinusoidal cell layer. Subcell. Biochem. 2008;47:182–197. doi: 10.1007/978-0-387-78267-6_15. [DOI] [PubMed] [Google Scholar]
- 24.Mota MM, Pradel G, Vanderberg JP, et al. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291(5501):141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 25.Meis JF, Verhave JP, Jap PH, Sinden RE, Meuwissen JH. Ultrastructural observations on the infection of rat liver by Plasmodium berghei sporozoites in vivo. J. Protozool. 1983;30(2):361–366. doi: 10.1111/j.1550-7408.1983.tb02931.x. [DOI] [PubMed] [Google Scholar]
- 26.Chattopadhyay R, de la Vega P, Paik SH, et al. Early transcriptional responses of HepG2-A16 liver cells to infection by Plasmodium falciparum sporozoites. J. Biol. Chem. 2011;286(30):26396–26405. doi: 10.1074/jbc.M111.240879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albuquerque SS, Carret C, Grosso AR, et al. Host cell transcriptional profiling during malaria liver stage infection reveals a coordinated and sequential set of biological events. BMC Genomics. 2009;10:270. doi: 10.1186/1471-2164-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coppi A, Natarajan R, Pradel G, et al. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J. Exp. Med. 2011;208(2):341–356. doi: 10.1084/jem.20101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baum J, Cowman AF. Biochemistry. Revealing a parasite's invasive trick. Science. 2011;333(6041):410–411. doi: 10.1126/science.1209875. [DOI] [PubMed] [Google Scholar]
- 30.Riglar DT, Richard D, Wilson DW, et al. Cell Host Microbe. 2011;9(1):9–20. doi: 10.1016/j.chom.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Giovannini D, Späth S, Lacroix C, et al. Independent roles of apical membrane antigen 1 and rhoptry neck proteins during host cell invasion by apicomplexa. Cell Host Microbe. 2011;10(6):591–602. doi: 10.1016/j.chom.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Combe A, Moreira C, Ackerman S, Thiberge S, Templeton TJ, Ménard R. TREP, a novel protein necessary for gliding motility of the malaria sporozoite. Int. J. Parasitol. 2009;39(4):489–496. doi: 10.1016/j.ijpara.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Moreira CK, Templeton TJ, Lavazec C, et al. The Plasmodium TRAP/MIC2 family member, TRAP-Like Protein (TLP), is involved in tissue traversal by sporozoites. Cell. Microbiol. 2008;10(7):1505–1516. doi: 10.1111/j.1462-5822.2008.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bargieri D, Lagal V, Tardieux I, Ménard R. Host cell invasion by apicomplexans: what do we know? Trends Parasitol. 2012;28(4):131–135. doi: 10.1016/j.pt.2012.01.005. [DOI] [PubMed] [Google Scholar]; •• Redefines the role of the motor protein during hepatocyte invasion and discusses the different invasive stages of Plasmodium and other apicomplexa.
- 35.Morahan BJ, Wang L, Coppel RL. No TRAP, no invasion. Trends Parasitol. 2009;25(2):77–84. doi: 10.1016/j.pt.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Singh AP, Buscaglia CA, Wang Q, et al. Plasmodium circumsporozoite protein promotes the development of the liver stages of the parasite. Cell. 2007;131(3):492–504. doi: 10.1016/j.cell.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Mikolajczak SA, Silva-Rivera H, Peng X, et al. Distinct malaria parasite sporozoites reveal transcriptional changes that cause differential tissue infection competence in the mosquito vector and mammalian host. Mol. Cell. Biol. 2008;28(20):6196–6207. doi: 10.1128/MCB.00553-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarun AS, Peng X, Dumpit RF, et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc. Natl Acad. Sci. USA. 2008;105(1):305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A comprehensive proteome and transcriptome analysis of select timepoints during liver-stage development in rodent system.
- 39.Vaughan A, Chiu SY, Ramasamy G, et al. Assessment and improvement of the Plasmodium yoelii yoelii genome annotation through comparative analysis. Bioinformatics. 2008;24(13):i383–i389. doi: 10.1093/bioinformatics/btn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y, Ramachandran V, Kumar KA, et al. Evidence-based annotation of the malaria parasite's genome using comparative expression profiling. PLoS ONE. 2008;3(2):e1570. doi: 10.1371/journal.pone.0001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacci JB, Jr, Ribeiro JM, Huang F, et al. Transcriptional analysis of in vivo Plasmodium yoelii liver stage gene expression. Mol. Biochem. Parasitol. 2005;142(2):177–183. doi: 10.1016/j.molbiopara.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Brown S, Roos DS, Nussenzweig V, Bhanot P. Transcriptome of axenic liver stages of Plasmodium yoelii. Mol. Biochem. Parasitol. 2004;137(1):161–168. doi: 10.1016/j.molbiopara.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Cummings JF, Spring MD, Schwenk RJ, et al. Recombinant Liver Stage Antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-gamma/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine. 2010;28(31):5135–5144. doi: 10.1016/j.vaccine.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 44.Hillier CJ, Ware LA, Barbosa A, et al. Process development and analysis of liver-stage antigen 1, a preerythrocyte-stage protein-based vaccine for Plasmodium falciparum. Infect. Immun. 2005;73(4):2109–2115. doi: 10.1128/IAI.73.4.2109-2115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect. Immun. 2007;75(8):3758–3768. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mueller AK, Camargo N, Kaiser K, et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc. Natl Acad. Sci. USA. 2005;102(8):3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433(7022):164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 48.Graewe S, Rankin KE, Lehmann C, et al. Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress. PLoS Pathog. 2011;7(9):e1002224. doi: 10.1371/journal.ppat.1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]; • Seminal study showing that radiation-attenuated sporozoite vaccination induces sterile immunity against rodent Plasmodium parasites.
- 50.Gwadz RW, Cochrane AH, Nussenzweig V, Nussenzweig RS. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull. World Health Organ. 1979;57(Suppl. 1):165–173. [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 2002;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 52.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl Acad. Sci. USA. 1988;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White KL, Snyder HL, Krzych U. MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against. Plasmodium berghei. J. Immunol. 1996;156(9):3374–3381. [PubMed] [Google Scholar]
- 54.Weiss WR, Jiang CG. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS ONE. 2012;7(2):e31247. doi: 10.1371/journal.pone.0031247. [DOI] [PMC free article] [PubMed] [Google Scholar]; • CD8+ T cells play an essential role in sterile immunity of rhesus monkeys after attenuated sporozoite vaccination.
- 55.Rodrigues MM, Cordey AS, Arreaza G, et al. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 1991;3(6):579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 56.Bongfen SE, Torgler R, Romero JF, Renia L, Corradin G. Plasmodium berghei-infected primary hepatocytes process and present the circumsporozoite protein to specific CD8+ T cells in vitro. J. Immunol. 2007;178(11):7054–7063. doi: 10.4049/jimmunol.178.11.7054. [DOI] [PubMed] [Google Scholar]
- 57.Weiss WR, Mellouk S, Houghten RA, et al. Cytotoxic T cells recognize a peptide from the circumsporozoite protein on malaria-infected hepatocytes. J. Exp. Med. 1990;171(3):763–773. doi: 10.1084/jem.171.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mellouk S, Green SJ, Nacy CA, Hoffman SL. IFN-gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an l-arginine-dependent effector mechanism. J. Immunol. 1991;146(11):3971–3976. [PubMed] [Google Scholar]
- 59.Klotz FW, Scheller LF, Seguin MC, et al. Co-localization of inducible-nitric oxide synthase and Plasmodium berghei in hepatocytes from rats immunized with irradiated sporozoites. J. Immunol. 1995;154(7):3391–3395. [PubMed] [Google Scholar]
- 60.Nussler AK, Rénia L, Pasquetto V, Miltgen F, Matile H, Mazier D. In vivo induction of the nitric oxide pathway in hepatocytes after injection with irradiated malaria sporozoites, malaria blood parasites or adjuvants. Eur. J. Immunol. 1993;23(4):882–887. doi: 10.1002/eji.1830230417. [DOI] [PubMed] [Google Scholar]
- 61.Seguin MC, Klotz FW, Schneider I, et al. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J. Exp. Med. 1994;180(1):353–358. doi: 10.1084/jem.180.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J. Immunol. 2000;165(3):1453–1462. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 63.Trimnell A, Takagi A, Gupta M, Richie TL, Kappe SH, Wang R. Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes. J. Immunol. 2009;183(9):5870–5878. doi: 10.4049/jimmunol.0900302. [DOI] [PubMed] [Google Scholar]
- 64.Oliveira GA, Kumar KA, Calvo-Calle JM, et al. Class II-restricted protective immunity induced by malaria sporozoites. Infect. Immun. 2008;76(3):1200–1206. doi: 10.1128/IAI.00566-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 1980;207(4426):71–73. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- 66.Zavala F, Cochrane AH, Nardin EH, Nussenzweig RS, Nussenzweig V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J. Exp. Med. 1983;157(6):1947–1957. doi: 10.1084/jem.157.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stewart MJ, Nawrot RJ, Schulman S, Vanderberg JP. Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect. Immun. 1986;51(3):859–864. doi: 10.1128/iai.51.3.859-864.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potocnjak P, Yoshida N, Nussenzweig RS, Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J. Exp. Med. 1980;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nussenzweig V, Nussenzweig RS. Rationale for the development of an engineered sporozoite malaria vaccine. Adv. Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- 70.Herrington DA, Clyde DF, Losonsky G, et al. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- 71.Ballou WR, Hoffman SL, Sherwood JA, et al. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- 72.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 2004;34(9):991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 73.Kumar KA, Sano G, Boscardin S, et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444(7121):937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 74.Grüner AC, Mauduit M, Tewari R, et al. Sterile protection against malaria is independent of immune responses to the circumsporozoite protein. PLoS ONE. 2007;2(12):e1371. doi: 10.1371/journal.pone.0001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mauduit M, Tewari R, Depinay N, et al. Minimal role for the circumsporozoite protein in the induction of sterile immunity by vaccination with live rodent malaria sporozoites. Infect. Immun. 2010;78(5):2182–2188. doi: 10.1128/IAI.01415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 2010;6(7):e1000998. doi: 10.1371/journal.ppat.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krzych U, Lyon JA, Jareed T, et al. T lymphocytes from volunteers immunized with irradiated Plasmodium falciparum sporozoites recognize liver and blood stage malaria antigens. J. Immunol. 1995;155(8):4072–4077. [PubMed] [Google Scholar]
- 78.Wizel B, Houghten R, Church P, et al. HLA-A2-restricted cytotoxic T lymphocyte responses to multiple Plasmodium falciparum sporozoite surface protein 2 epitopes in sporozoite-immunized volunteers. J. Immunol. 1995;155(2):766–775. [PubMed] [Google Scholar]
- 79.Malik A, Egan JE, Houghten RA, Sadoff JC, Hoffman SL. Human cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. Proc. Natl Acad. Sci. USA. 1991;88(8):3300–3304. doi: 10.1073/pnas.88.8.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moreno A, Clavijo P, Edelman R, et al. Cytotoxic CD4+ T cells from a sporozoite-immunized volunteer recognize the Plasmodium falciparum CS protein. Int. Immunol. 1991;3(10):997–1003. doi: 10.1093/intimm/3.10.997. [DOI] [PubMed] [Google Scholar]
- 81.Nardin E, Munesinghe YD, Moreno A, et al. T cell responses to repeat and non-repeat regions of the circumsporozoite protein detected in volunteers immunized with Plasmodium falciparum sporozoites. Mem. Inst. Oswaldo Cruz. 1992;87(Suppl. 3):223–227. doi: 10.1590/s0074-02761992000700037. [DOI] [PubMed] [Google Scholar]
- 82.Wizel B, Houghten RA, Parker KC, et al. Irradiated sporozoite vaccine induces HLA-B8-restricted cytotoxic T lymphocyte responses against two overlapping epitopes of the Plasmodium falciparum sporozoite surface protein 2. J. Exp. Med. 1995;182(5):1435–1445. doi: 10.1084/jem.182.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nardin EH, Herrington DA, Davis J, et al. Conserved repetitive epitope recognized by CD4+ clones from a malaria-immunized volunteer. Science. 1989;246(4937):1603–1606. doi: 10.1126/science.2480642. [DOI] [PubMed] [Google Scholar]
- 84.Doolan DL, Hoffman SL, Southwood S, et al. Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and HLA-B supertype alleles. Immunity. 1997;7(1):97–112. doi: 10.1016/s1074-7613(00)80513-0. [DOI] [PubMed] [Google Scholar]
- 85.Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19(1):103–107. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- 86.Kensil CR, Wu JY, Soltysik S. Structural and immunological characterization of the vaccine adjuvant QS-21. Pharm. Biotechnol. 1995;6:525–541. doi: 10.1007/978-1-4615-1823-5_22. [DOI] [PubMed] [Google Scholar]
- 87.Chulay JD, Schneider I, Cosgriff TM, et al. Malaria transmitted to humans by mosquitoes infected from cultured Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1986;35(1):66–68. doi: 10.4269/ajtmh.1986.35.66. [DOI] [PubMed] [Google Scholar]
- 88.Gordon DM, McGovern TW, Krzych U, et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J. Infect. Dis. 1995;171(6):1576–1585. doi: 10.1093/infdis/171.6.1576. [DOI] [PubMed] [Google Scholar]
- 89.Stoute JA, Slaoui M, Heppner DG, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N. Engl. J. Med. 1997;336(2):86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]; • First report of clinical efficacy of RTS,S vaccine, now in multicenter Phase III trial.
- 90.Kester KE, McKinney DA, Tornieporth N, et al. RTS,S Malaria Vaccine Evaluation Group. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J. Infect. Dis. 2001;183(4):640–647. doi: 10.1086/318534. [DOI] [PubMed] [Google Scholar]
- 91.Kester KE, McKinney DA, Tornieporth N, et al. RTS,S Malaria Vaccine Evaluation Group. A Phase I/IIa safety, immunogenicity, and efficacy bridging randomized study of a two-dose regimen of liquid and lyophilized formulations of the candidate malaria vaccine RTS,S/AS02A in malaria-naive adults. Vaccine. 2007;25(29):5359–5366. doi: 10.1016/j.vaccine.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Kester KE, Cummings JF, Ockenhouse CF, et al. RTS,S Malaria Vaccine Evaluation Group. Phase 2a trial of 0, 1, and 3 month and 0, 7, and 28 day immunization schedules of malaria vaccine RTS,S/AS02 in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine. 2008;26(18):2191–2202. doi: 10.1016/j.vaccine.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 93.Kester KE, Cummings JF, Ofori-Anyinam O, et al. RTS,S Vaccine Evaluation Group. Randomized, double-blind, Phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 2009;200(3):337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 94.Bojang KA, Milligan PJ, Pinder M, et al. RTS, S Malaria Vaccine Trial Team. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358(9297):1927–1934. doi: 10.1016/S0140-6736(01)06957-4. [DOI] [PubMed] [Google Scholar]
- 95.Alonso PL, Sacarlal J, Aponte JJ, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366(9502):2012–2018. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 96.Agnandji ST, Lell B, Soulanoudjingar SS, et al. RTS,S Clinical Trials Partnership. First results of Phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 2011;365(20):1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 97.Olotu A, Lusingu J, Leach A, et al. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5–17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect. Dis. 2011;11(2):102–109. doi: 10.1016/S1473-3099(10)70262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lell B, Agnandji S, von Glasenapp I, et al. A randomized trial assessing the safety and immunogenicity of AS01 and AS02 adjuvanted RTS,S malaria vaccine candidates in children in Gabon. PLoS ONE. 2009;4(10):e7611. doi: 10.1371/journal.pone.0007611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agnandji ST, Fendel R, Mestré M, et al. Induction of Plasmodium falciparum-specific CD4+ T cells and memory B cells in Gabonese children vaccinated with RTS,S/AS01(E) and RTS,S/AS02(D) PLoS ONE. 2011;6(4):e18559. doi: 10.1371/journal.pone.0018559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Olotu A, Moris P, Mwacharo J, et al. Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P. falciparum clinical malaria. PLoS ONE. 2011;6(10):e25786. doi: 10.1371/journal.pone.0025786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Radosevic K, Rodriguez A, Lemckert AA, et al. The Th1 immune response to Plasmodium falciparum circumsporozoite protein is boosted by adenovirus vectors 35 and 26 with a homologous insert. Clin. Vaccine Immunol. 2010;17(11):1687–1694. doi: 10.1128/CVI.00311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moorthy VS, McConkey S, Roberts M, et al. Safety of DNA and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine. 2003;21(17–18):1995–2002. doi: 10.1016/s0264-410x(02)00771-5. [DOI] [PubMed] [Google Scholar]
- 103.Dunachie SJ, Walther M, Epstein JE, et al. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect. Immun. 2006;74(10):5933–5942. doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Webster DP, Dunachie S, Vuola JM, et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc. Natl Acad. Sci. USA. 2005;102(13):4836–4841. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moorthy VS, Good MF, Hill AV. Malaria vaccine developments. Lancet. 2004;363(9403):150–156. doi: 10.1016/S0140-6736(03)15267-1. [DOI] [PubMed] [Google Scholar]
- 106.Bejon P, Mwacharo J, Kai O, et al. A Phase 2b randomised trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS Clin. Trials. 2006;1(6):e29. doi: 10.1371/journal.pctr.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aly AS, Mikolajczak SA, Rivera HS, et al. Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection. Mol. Microbiol. 2008;69(1):152–163. doi: 10.1111/j.1365-2958.2008.06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Dijk MR, Douradinha B, Franke-Fayard B, et al. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc. Natl Acad. Sci. USA. 2005;102(34):12194–12199. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kumar KA, Baxter P, Tarun AS, Kappe SH, Nussenzweig V. Conserved protective mechanisms in radiation and genetically attenuated uis3(−) and uis4(−) Plasmodium sporozoites. PLoS ONE. 2009;4(2):e4480. doi: 10.1371/journal.pone.0004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Annoura T, Ploemen IH, van Schaijk BC, et al. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates. Vaccine. 2012;30(16):2662–2670. doi: 10.1016/j.vaccine.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 111.Roestenberg M, Teirlinck AC, McCall MB, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet. 2011;377(9779):1770–1776. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]; • Immunity induced by sporozoite infection of humans who are receiving chloroquine prophylaxis, confers sterile immunity for up to 2 years.
- 112.Jin Y, Kebaier C, Vanderberg J. Direct microscopic quantification of dynamics of Plasmodium berghei sporozoite transmission from mosquitoes to mice. Infect. Immun. 2007;75(11):5532–5539. doi: 10.1128/IAI.00600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe. 2011;9(6):451–462. doi: 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6(1):10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Teirlinck AC, McCall MB, Roestenberg M, et al. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog. 2011;7(12):e1002389. doi: 10.1371/journal.ppat.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chakravarty S, Baldeviano GC, Overstreet MG, Zavala F. Effector CD8+ T lymphocytes against liver stages of Plasmodium yoelii do not require gamma interferon for antiparasite activity. Infect. Immun. 2008;76(8):3628–3631. doi: 10.1128/IAI.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodrigues EG, Claassen J, Lee S, Wilson JM, Nussenzweig RS, Tsuji M. Interferon-gamma-independent CD8+ T cell-mediated protective anti-malaria immunity elicited by recombinant adenovirus. Parasite Immunol. 2000;22(3):157–160. doi: 10.1046/j.1365-3024.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 118.Renggli J, Hahne M, Matile H, Betschart B, Tschopp J, Corradin G. Elimination of P. berghei liver stages is independent of Fas (CD95/Apo-I) or perforin-mediated cytotoxicity. Parasite Immunol. 1997;19(3):145–148. doi: 10.1046/j.1365-3024.1997.d01-190.x. [DOI] [PubMed] [Google Scholar]
- 119.Coban C, Kobiyama K, Aoshi T, et al. Novel strategies to improve DNA vaccine immunogenicity. Curr. Gene Ther. 2011;11(6):479–484. doi: 10.2174/156652311798192815. [DOI] [PubMed] [Google Scholar]
- 120.Poland JD, Calisher CH, Monath TP, Downs WG, Murphy K. Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull. World Health Organ. 1981;59(6):895–900. [PMC free article] [PubMed] [Google Scholar]
- 121.Florens L, Washburn MP, Raine JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419(6906):520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]; • Early proteomic characterization of four Plasmodium falciparum stages, including sporozoites, by multidimensional protein identification technology.
- 122.Lasonder E, Janse CJ, van Gemert GJ, et al. Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathog. 2008;4(10):e1000195. doi: 10.1371/journal.ppat.1000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Siau A, Silvie O, Franetich JF, et al. Temperature shift and host cell contact up-regulate sporozoite expression of Plasmodium falciparum genes involved in hepatocyte infection. PLoS Pathog. 2008;4(8):e1000121. doi: 10.1371/journal.ppat.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rinaudo CD, Telford JL, Rappuoli R, Seib KL. Vaccinology in the genome era. J. Clin. Invest. 2009;119(9):2515–2525. doi: 10.1172/JCI38330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cardoso FC, Roddick JS, Groves P, Doolan DL. Evaluation of approaches to identify the targets of cellular immunity on a proteome-wide scale. PLoS ONE. 2011;6(11):e27666. doi: 10.1371/journal.pone.0027666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mishra S, Rai U, Shiratsuchi T, et al. Identification of non-CSP antigens bearing CD8 epitopes in mice immunized with irradiated sporozoites. Vaccine. 2011;29(43):7335–7342. doi: 10.1016/j.vaccine.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haddad D, Bilcikova E, Witney AA, et al. Novel antigen identification method for discovery of protective malaria antigens by rapid testing of DNA vaccines encoding exons from the parasite genome. Infect. Immun. 2004;72(3):1594–1602. doi: 10.1128/IAI.72.3.1594-1602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Limbach K, Aguiar J, Gowda K, et al. Identification of two new protective pre-erythrocytic malaria vaccine antigen candidates. Malar. J. 2011;10:65. doi: 10.1186/1475-2875-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Speake C, Duffy PE. Antigens for pre-erythrocytic malaria vaccines: building on success. Parasite Immunol. 2009;31(9):539–546. doi: 10.1111/j.1365-3024.2009.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kurtis JD, Hollingdale MR, Luty AJ, Lanar DE, Krzych U, Duffy PE. Preerythrocytic immunity to Plasmodium falciparum: the case for an LSA-1 vaccine. Trends Parasitol. 2001;17(5):219–223. doi: 10.1016/s0169-4758(00)01862-7. [DOI] [PubMed] [Google Scholar]
- 131.Rutgers T, Gordon D, Gathoye AM, et al. Hepatitis-B surface-antigen as carrier matrix for the repetitive epitope of the circumsporozoite protein of Plasmodium falciparum. Biotechnolgy. 1988;6(9):1065–1070. [Google Scholar]; • Described a fusion of the repetitive domain of CSP with the pre-S2 region of the surface antigen of hepatitis B virus expressed in yeast cells that assembles into virus-like particles, forming the basis for the RTS,S vaccine now in Phase III testing.
- 132.Vreden SG, Verhave JP, Oettinger T, Sauerwein RW, Meuwissen JH. Phase I clinical trial of a recombinant malaria vaccine consisting of the circumsporozoite repeat region of Plasmodium falciparum coupled to hepatitis B surface antigen. Am. J. Trop. Med. Hyg. 1991;45(5):533–538. doi: 10.4269/ajtmh.1991.45.533. [DOI] [PubMed] [Google Scholar]
- 133.Edelman R. The development and use of vaccine adjuvants. Mol. Biotechnol. 2002;21(2):129–148. doi: 10.1385/MB:21:2:129. [DOI] [PubMed] [Google Scholar]
- 134.Wack A, Baudner BC, Hilbert AK, et al. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 2008;26(4):552–561. doi: 10.1016/j.vaccine.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 135.Wu Y, Ellis RD, Shaffer D, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE. 2008;3(7):e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.White AC, Cloutier P, Coughlin RT. A purified saponin acts as an adjuvant for a T-independent antigen. Adv. Exp. Med. Biol. 1991;303:207–210. doi: 10.1007/978-1-4684-6000-1_22. [DOI] [PubMed] [Google Scholar]
- 137.Coler RN, Carter D, Friede M, Reed SG. Adjuvants for malaria vaccines. Parasite Immunol. 2009;31(9):520–528. doi: 10.1111/j.1365-3024.2009.01142.x. [DOI] [PubMed] [Google Scholar]
- 138.Brando C, Ware LA, Freyberger H, et al. Murine immune responses to liver-stage antigen 1 protein FMP011, a malaria vaccine candidate, delivered with adjuvant AS01B or AS02A. Infect. Immun. 2007;75(2):838–845. doi: 10.1128/IAI.01075-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pichyangkul S, Kum-Arb U, Yongvanitchit K, et al. Preclinical evaluation of the safety and immunogenicity of a vaccine consisting of Plasmodium falciparum liver-stage antigen 1 with adjuvant AS01B administered alone or concurrently with the RTS,S/AS01B vaccine in rhesus primates. Infect. Immun. 2008;76(1):229–238. doi: 10.1128/IAI.00977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Okitsu SL, Boato F, Mueller MS, et al. Antibodies elicited by a virosomally formulated Plasmodium falciparum serine repeat antigen-5 derived peptide detect the processed 47 kDa fragment both in sporozoites and merozoites. Peptides. 2007;28(10):2051–2060. doi: 10.1016/j.peptides.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 141.Peduzzi E, Westerfeld N, Zurbriggen R, Pluschke G, Daubenberger CA. Contribution of influenza immunity and virosomal-formulated synthetic peptide to cellular immune responses in a Phase I subunit malaria vaccine trial. Clin. Immunol. 2008;127(2):188–197. doi: 10.1016/j.clim.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 142.Cech PG, Aebi T, Abdallah MS, et al. Virosome-formulated Plasmodium falciparum AMA-1 & CSP derived peptides as malaria vaccine: randomized Phase 1b trial in semi-immune adults & children. PLoS ONE. 2011;6(7):e22273. doi: 10.1371/journal.pone.0022273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang R, Doolan DL, Le TP, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282(5388):476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 144.Le TP, Coonan KM, Hedstrom RC, et al. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine. 2000;18(18):1893–1901. doi: 10.1016/s0264-410x(99)00407-7. [DOI] [PubMed] [Google Scholar]
- 145.Wang R, Richie TL, Baraceros MF, et al. Boosting of DNA vaccine-elicited gamma interferon responses in humans by exposure to malaria parasites. Infect. Immun. 2005;73(5):2863–2872. doi: 10.1128/IAI.73.5.2863-2872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ockenhouse CF, Sun PF, Lanar DE, et al. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J. Infect. Dis. 1998;177(6):1664–1673. doi: 10.1086/515331. [DOI] [PubMed] [Google Scholar]; • First administration of attenuated vaccinia virus carrying seven P. falciparum antigens to human subjects, demonstrating the safety and immunogenicity of this platform as well as evidence of some protection after experimental malaria infection.
- 147.Gilbert SC, Schneider J, Hannan CM, et al. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime–boost immunisation regimes. Vaccine. 2002;20(7–8):1039–1045. doi: 10.1016/s0264-410x(01)00450-9. [DOI] [PubMed] [Google Scholar]
- 148.McConkey SJ, Reece WH, Moorthy VS, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 2003;9(6):729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 149.Moorthy VS, Imoukhuede EB, Milligan P, et al. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 2004;1(2):e33. doi: 10.1371/journal.pmed.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Samuel JM, William HHR, Vasee SM, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 2003;9(6):729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 151.Vuola JM, Keating S, Webster DP, et al. Differential immunogenicity of various heterologous prime–boost vaccine regimens using DNA and viral vectors in healthy volunteers. J. Immunol. 2005;174(1):449–455. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 152.Schmidt NW, Podyminogin RL, Butler NS, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc. Natl Acad. Sci. USA. 2008;105(37):14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Draper SJ, Goodman AL, Biswas S, et al. Recombinant viral vaccines expressing merozoite surface protein-1 induce antibody- and T cell-mediated multistage protection against malaria. Cell Host Microbe. 2009;5(1):95–105. doi: 10.1016/j.chom.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rodríguez A, Goudsmit J, Companjen A, et al. Impact of recombinant adenovirus serotype 35 priming versus boosting of a Plasmodium falciparum protein: characterization of T- and B-cell responses to liver-stage antigen 1. Infect. Immun. 2008;76(4):1709–1718. doi: 10.1128/IAI.01614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nwanegbo E, Vardas E, Gao W, et al. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 2004;11(2):351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Buchbinder SP, Mehrotra DV, Duerr A, et al. Step Study Protocol Team. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sedegah M, Tamminga C, McGrath S, et al. Adenovirus 5-vectored P. falciparum vaccine expressing CSP and AMA1. Part A: safety and immunogenicity in seronegative adults. PLoS ONE. 2011;6(10):e24586. doi: 10.1371/journal.pone.0024586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tamminga C, Sedegah M, Regis D, et al. Adenovirus-5-vectored P. falciparum vaccine expressing CSP and AMA1. Part B: safety, immunogenicity and protective efficacy of the CSP component. PLoS ONE. 2011;6(10):e25868. doi: 10.1371/journal.pone.0025868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.O'Hara GA, Duncan CJ, Ewer KJ, et al. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J. Infect. Dis. 2012;205(5):772–781. doi: 10.1093/infdis/jir850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kariu T, Ishino T, Yano K, Chinzei Y, Yuda M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol. Microbiol. 2006;59(5):1369–1379. doi: 10.1111/j.1365-2958.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- 161.Ishino T, Chinzei Y, Yuda M. A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell. Microbiol. 2005;7(2):199–208. doi: 10.1111/j.1462-5822.2004.00447.x. [DOI] [PubMed] [Google Scholar]
- 162.Baldacci P, Ménard R. The elusive malaria sporozoite in the mammalian host. Mol. Microbiol. 2004;54(2):298–306. doi: 10.1111/j.1365-2958.2004.04275.x. [DOI] [PubMed] [Google Scholar]
- 163.Heiss K, Nie H, Kumar S, Daly TM, Bergman LW, Matuschewski K. Functional characterization of a redundant Plasmodium TRAP family invasin, TRAP-like protein, by aldolase binding and a genetic complementation test. Eukaryotic Cell. 2008;7(6):1062–1070. doi: 10.1128/EC.00089-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Godson GN, Ellis J, Svec P, Schlesinger DH, Nussenzweig V. Identification and chemical synthesis of a tandemly repeated immunogenic region of Plasmodium knowlesi circumsporozoite protein. Nature. 1983;305(5929):29–33. doi: 10.1038/305029a0. [DOI] [PubMed] [Google Scholar]
- 165.VanBuskirk KM, O'Neill MT, De La Vega P, et al. Pre-erythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc. Natl Acad. Sci. USA. 2009;106(31):13004–13009. doi: 10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pizarro JC, Vulliez-Le Normand B, Chesne-Seck ML, et al. Crystal structure of the malaria vaccine candidate apical membrane antigen 1. Science. 2005;308(5720):408–411. doi: 10.1126/science.1107449. [DOI] [PubMed] [Google Scholar]
- 167.Silvie O, Franetich JF, Charrin S, et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J. Biol. Chem. 2004;279(10):9490–9496. doi: 10.1074/jbc.M311331200. [DOI] [PubMed] [Google Scholar]
- 168.Labaied M, Camargo N, Kappe SH. Depletion of the Plasmodium berghei thrombospondin-related sporozoite protein reveals a role in host cell entry by sporozoites. Mol. Biochem. Parasitol. 2007;153(2):158–166. doi: 10.1016/j.molbiopara.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 169.Fidock DA, Gras-Masse H, Lepers JP, et al. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J. Immunol. 1994;153(1):190–204. [PubMed] [Google Scholar]
- 170.Mikolajczak SA, Sacci JB, Jr, De La Vega P, et al. Disruption of the Plasmodium falciparum liver-stage antigen-1 locus causes a differentiation defect in late liver-stage parasites. Cell. Microbiol. 2011;13(8):1250–1260. doi: 10.1111/j.1462-5822.2011.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vaughan AM, O'Neill MT, Tarun AS, et al. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell. Microbiol. 2009;11(3):506–520. doi: 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yu M, Kumar TR, Nkrumah LJ, et al. The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe. 2008;4(6):567–578. doi: 10.1016/j.chom.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Silvie O, Goetz K, Matuschewski K. A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog. 2008;4(6):e1000086. doi: 10.1371/journal.ppat.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ishino T, Boisson B, Orito Y, et al. LISP1 is important for the egress of Plasmodium berghei parasites from liver cells. Cell. Microbiol. 2009;11(9):1329–1339. doi: 10.1111/j.1462-5822.2009.01333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pei Y, Tarun AS, Vaughan AM, et al. Plasmodium pyruvate dehydrogenase activity is only essential for the parasite's progression from liver infection to blood infection. Mol. Microbiol. 2010;75(4):957–971. doi: 10.1111/j.1365-2958.2009.07034.x. [DOI] [PubMed] [Google Scholar]
- 176.Falae A, Combe A, Amaladoss A, Carvalho T, Menard R, Bhanot P. Role of Plasmodium berghei cGMP-dependent protein kinase in late liver stage development. J. Biol. Chem. 2010;285(5):3282–3288. doi: 10.1074/jbc.M109.070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Haussig JM, Matuschewski K, Kooij TW. Inactivation of a Plasmodium apicoplast protein attenuates formation of liver merozoites. Mol. Microbiol. 2011;81(6):1511–1525. doi: 10.1111/j.1365-2958.2011.07787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Miller JL, Harupa A, Kappe SH, Mikolajczak SA. Plasmodium yoelii macrophage migration inhibitory factor is necessary for efficient liver-stage development. Infect. Immun. 2012;80(4):1399–1407. doi: 10.1128/IAI.05861-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Miller JL, Harupa A, Kappe SH, Mikolajczak SA. Plasmodium yoelii macrophage migration inhibitory factor is necessary for efficient liver-stage development. Infect. Immun. 2012;80(4):1399–1407. doi: 10.1128/IAI.05861-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vera IM, Beatty WL, Sinnis P, Kim K. Plasmodium protease ROM1 is important for proper formation of the parasitophorous vacuole. PLoS Pathog. 2011;7(9):e1002197. doi: 10.1371/journal.ppat.1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen DH, Tigelaar RE, Weinbaum FI. Immunity to sporozoite-induced malaria infeciton in mice. I. The effect of immunization of T and B cell-deficient mice. J. Immunol. 1977;118(4):1322–1327. [PubMed] [Google Scholar]
- 182.Spitalny GL, Verhave JP, Meuwissen JH, Nussenzweig RS. Plasmodium berghei: T cell dependence of sporozoite-induced immunity in rodents. Exp. Parasitol. 1977;42(1):73–81. doi: 10.1016/0014-4894(77)90063-7. [DOI] [PubMed] [Google Scholar]
- 183.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 184.Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341(6240):323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 185.Lal AA, de la Cruz VF, Good MF, et al. In vivo testing of subunit vaccines against malaria sporozoites using a rodent system. Proc. Natl Acad. Sci. USA. 1987;84(23):8647–8651. doi: 10.1073/pnas.84.23.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roggero MA, Meraldi V, López JA, et al. The synthetic, oxidized C-terminal fragment of the Plasmodium berghei circumsporozoite protein elicits a high protective response. Eur. J. Immunol. 2000;30(9):2679–2685. doi: 10.1002/1521-4141(200009)30:9<2679::AID-IMMU2679>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 187.Weiss WR, Berzofsky JA, Houghten RA, Sedegah M, Hollindale M, Hoffman SL. A T cell clone directed at the circumsporozoite protein which protects mice against both Plasmodium yoelii and Plasmodium berghei. J. Immunol. 1992;149(6):2103–2109. [PubMed] [Google Scholar]
- 188.Guebre-Xabier M, Schwenk R, Krzych U. Memory phenotype CD8(+) T cells persist in livers of mice protected against malaria by immunization with attenuated Plasmodium berghei sporozoites. Eur. J. Immunol. 1999;29(12):3978–3986. doi: 10.1002/(SICI)1521-4141(199912)29:12<3978::AID-IMMU3978>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 189.Le Roch KG, Zhou Y, Blair PL, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301(5639):1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]; • An early whole genome expression analysis of P. falciparum during selected phases of development in both human and mosquito hosts.
- 190.Mikolajczak SA, Aly AS, Dumpit RF, Vaughan AM, Kappe SH. An efficient strategy for gene targeting and phenotypic assessment in the Plasmodium yoelii rodent malaria model. Mol. Biochem. Parasitol. 2008;158(2):213–216. doi: 10.1016/j.molbiopara.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 191.Williams CT, Azad AF. Transcriptional analysis of the pre-erythrocytic stages of the rodent malaria parasite, Plasmodium yoelii. PLoS One. 2010;5(4):e10267. doi: 10.1371/journal.pone.0010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 201. [(Accessed 10 May 2012)];WHO Rainbow tables. http://www.who.int/vaccine_research/links/Rainbow/en/index.html.


