Abstract
This article represents one of five contributions focusing on the topic “Plasticity and neuroadaptive responses within the extended amygdala in response to chronic or excessive alcohol exposure” that were developed by awardees participating in the Young Investigator Award Symposium at the “Alcoholism and Stress: A Framework for Future Treatment Strategies” conference in Volterra, Italy on May 3–6, 2011 that was organized/chaired by Drs. Antonio Noronha and Fulton Crews and sponsored by the National Institute on Alcohol Abuse and Alcoholism. This review discusses the dependence-induced neuroadaptations in affective systems that provide a basis for negative reinforcement learning and presents evidence demonstrating that escalated alcohol consumption during withdrawal is a learned, plasticity-dependent process. The review concludes by identifying changes within extended amygdala dynorphin/kappa-opioid receptor systems that could serve as the foundation for the occurrence of negative reinforcement processes. While some evidence contained herein may be specific to alcohol dependence-related learning and plasticity, much of the information will be of relevance to any addictive disorder involving negative reinforcement mechanisms. Collectively, the information presented within this review provides a framework to assess the negative reinforcing effects of alcohol in a manner that distinguishes neuroadaptations produced by chronic alcohol exposure from the actual plasticity that is associated with negative reinforcement learning in dependent organisms.
Keywords: Anxiety, Dependence, Dynorphin, Extended Amygdala, Extracellular matrix, Kappa-opioid receptor, Matrix metalloproteinase, Negative reinforcement, Nucleus accumbens, Stress
Introduction
The intent of this review is to discuss the negative reinforcing effects of alcohol during withdrawal in dependent animals from a learning-based perspective. By identifying the nature of chronic alcohol-induced neuroadaptive responses in the brain that promote the development of negative affective behaviors resembling depression and anxiety, specific brain regions can be assessed for their putative contribution to the escalated alcohol consumption that is a characteristic phenotype of dependence. Most importantly, methodology will be introduced that successfully distinguishes the neuroadaptations associated with the transition to dependence from the plasticity and structural reorganization that is required for negative reinforcement learning and the associated escalation of self-administration.
Alcohol abuse and dependence afflict approximately 8% of those 12 yrs and older in the United States (Substance Abuse and Mental Health Services Administration, 2010) and cause great personal, familial and societal harm. Health problems associated with alcohol consumption have been shown to be the third leading cause of preventable death (Mokdad, Marks, Stroup, and Gerberding, 2004) and societal costs associated with alcohol use disorders have been estimated to be at least $148 billion per year (Harwood, Fountain, & Livermore, 1998). To model the multifaceted impact of alcohol on human physiology and behavior, numerous animal models have been successfully utilized. Historically, much of the scientific effort has focused on understanding the acute effects of alcohol within a variety of behavioral domains. To assess abuse-related issues, animal models such as the conditioned place preference and operant self-administration paradigms have shown that acute alcohol is both rewarding (Bozarth, 1990; Walker & Ettenberg, 2007) and reinforcing (Anderson & Thompson, 1974; Smith & Davis, 1974), respectively.
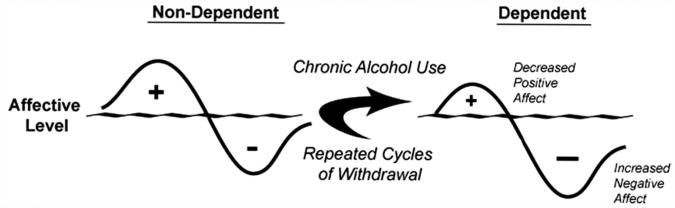
Understanding the acute neurobiological effects of alcohol is critically important because once known, it is possible to putatively predict the neuroadaptive, and resulting behavioral, impact of long-term alcohol exposure using theories such as The Opponent-Process Theory of Motivation (Solomon & Corbit, 1974). If applying this theory to alcohol abuse, in order to maintain homeostasis, an increase in hedonic state (e.g., alcohol-induced euphoria) will be followed by a compensatory decrease in hedonic state. Furthermore, after repeated alcohol exposure, the positive hedonic state is reduced while the negative component is enhanced to compensate for the continued perturbation of the affective system produced by chronic alcohol exposure (see Fig. 1). These opponent-process changes have been linked to allostatic mechanisms (Koob & Le Moal, 1997; Koob & Le Moal, 2001), which are hypothesized to reflect a new set-point from which an individual would be required to continue ingesting drugs of abuse to maintain a normal affective state that without drug is severely attenuated.
Fig. 1.
Alterations in opponent-processes in response to chronic alcohol exposure. In a non-dependent state, alcohol-induced positive affective states precede compensatory negative affective states. Following chronic alcohol exposure, the positive states are attenuated and the negative states are exacerbated.
Dependence-induced neuroadaptations and behavioral phenotypes
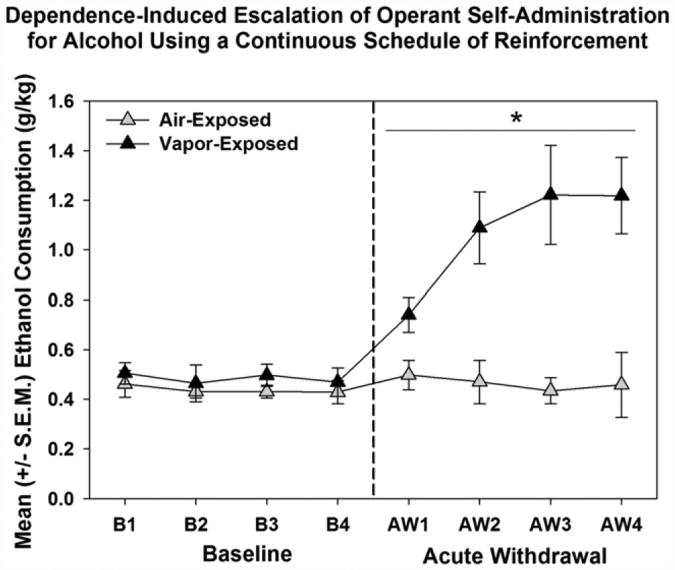
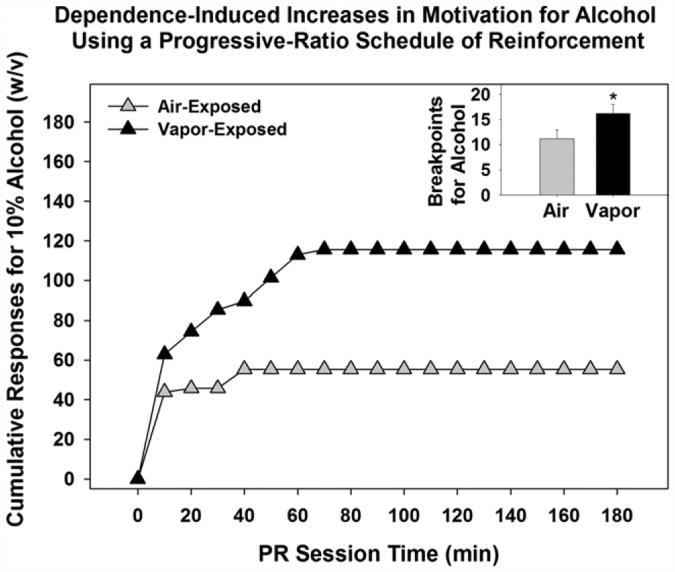
Chronic alcohol exposure induces profound neurochemical and morphological changes within the central nervous system that underlie altered motivational and affective behavior (e.g., Koob, 2009b). Animals will engage in operant self-administration to acquirealcoholand alcohol vapor exposure has been effectively used for decades to induce dependence (Rogers, Wiener, & Bloom, 1979). During acute and protracted withdrawal after alcohol vapor exposure, rats show physiological and behavioral signs of dependence-like behavior (O'Dell, Roberts, Smith, & Koob, 2004; Roberts, Cole, & Koob, 1996; Roberts, Heyser, Cole, Griffin, & Koob, 2000; Schulteis, Markou, Cole, & Koob, 1995). As seen in Fig. 2, following stable alcohol self-administration behavior when non-dependent, a subset of animals subjected to intermittent alcohol vapor exposure will show increased operant alcohol self-administration on a continuous schedule of reinforcement (Smith, Nealey, Wright, & Walker, 2011; Walker & Koob, 2008) and increased breakpoints for alcohol using a progressive ratio schedule of reinforcement (see Fig. 3; Walker & Koob, 2007).
Fig. 2.
Escalated alcohol self-Administration in response to chronic alcohol vapor exposure. Compared to baseline (left side of dashed line), the vapor-exposed animals escalated their intake of alcohol during acute withdrawal (right side of dashed line; main effect of exposure level, * =p < 0.05). Adapted from Smith et al. (2011), Neurobiology of Learning and Memory with permission of Elsevier.
Fig. 3.
Increased motivation for alcohol in alcohol-dependent animals. Dependent animals demonstrated significantly increased motivation (i.e., increased cumulative responses and increased breakpoints) for alcohol using a progressive ratio schedule of reinforcement (* =p < 0.05 when compared to air-exposed animals). Adapted from Walker and Koob (2007), Alcoholism: Clinical and Experimental Research with permission of John Wiley & Sons Inc.
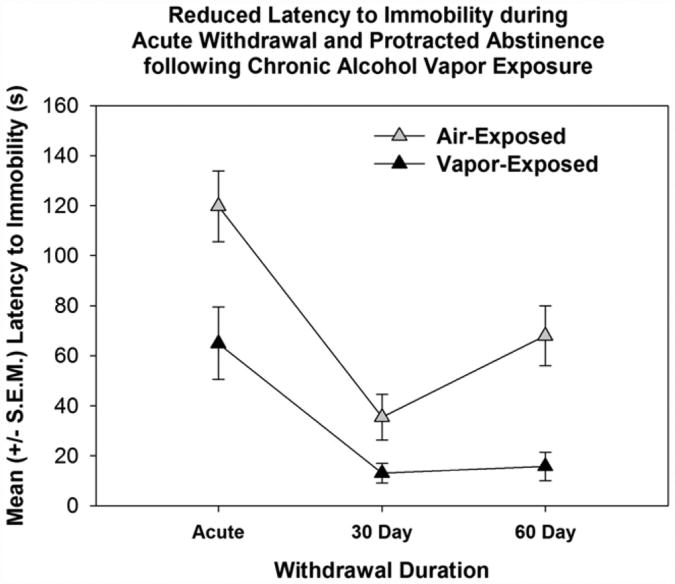
Other dependence-induced phenotypes include the development of negative affective behaviors resembling anxiety and depression. Animals display anxiety-like behavior during withdrawal from chronic alcohol exposure as measured by assays such as the social interaction test (File, Baldwin, & Hitchcott, 1989) and elevated-plus maze (Baldwin, Rassnick, Rivier, Koob, & Britton, 1991; Valdez et al., 2002). Depressive-like behavior has also been demonstrated during acute withdrawal by increased intracranial self-stimulation thresholds (Schulteis et al., 1995), decreased latency to immobility (see Fig. 4; Walker et al., 2010), and increased total immobility time in the forced swim test, as well as increased 22-kHz ultrasonic vocalizations (which are an ethologically-valid measure of negative affect in rats, see Williams et al., 2012 for a systematic evaluation).
Fig. 4.
Depressive-like behavior produced by chronic alcohol vapor exposure. Reduced latency to immobility in the forced swim test during acute and protracted withdrawal for those animals that were previously exposed to chronic intermittent alcohol vapor exposure when compared to air-exposed animals. Adapted from Walker et al. (2010), Alcohol with permission of Elsevier.
Negative reinforcement learning
The removal of dependence-induced negative affecting states via alcohol consumption would be reinforcing (termed negative reinforcement; i.e., the removal of a negative stimulus is beneficial to the organism) and this ‘self-medication’ contributes to excessive alcohol consumption and relapse (Heilig, Egli, Crabbe, & Becker, 2010; Heilig & Egli, 2006; Markou, Kosten, & Koob, 1998). While there is no confusion regarding the removal of an aversive stimulus being an event that would contribute to the welfare of an organism, it must be noted that there is more than one type of aversive state that the term ‘negative affect’ could apply to. In general, both depressive- and anxiety-like states are referred to as being representative of negative affective states and it follows that removal of either state would be considered negatively reinforcing. Taking Major Depressive Disorder as an example, one of two symptoms must be present for a diagnosis to be made, namely depressed mood or loss of pleasure. The two are distinct from each other and considered independent entities, but either would provide the foundation for a diagnosis of Major Depressive Disorder (American Psychiatric Association, 1994, 2000). It follows that, regardless of how the negative affective state is achieved, it is the removal of the aversive, negative affective, condition that is reflective of negative reinforcement processes.
Previously, it has been proposed that the neuroadaptive changes that occur in response to chronic alcohol and drug exposure can occur via within- or between-system changes in reward and anti-reward systems, respectively (Koob, 2009b; Koob & Bloom, 1988; Koob & Le Moal, 2008). There is evidence supporting both possibilities in the form of neuroadaptations that occur within classical motivational systems (Funk & Dohrman, 2007; Koob, 2004; Koob & Weiss, 1992; McBride & Li, 1998; Siggins et al., 2003; Walker & Ettenberg, 2007), as well as systems distinct from those that are involved in anhedonia and dysphoria (Funk, O'Dell, Crawford, & Koob, 2006; Nealey, Smith, Davis, Smith, & Walker, 2011; Sperling, Gomes, Sypek, Carey, & McLaughlin, 2010; Valdez et al., 2002; Walker & Koob, 2008; Walker, Zorrilla, & Koob, 2011). It follows that any actions resulting in reductions of the aversive condition would be considered negatively reinforcing and would have an increased probability of occurring again under similar conditions. The label ‘reinforcement’ is by definition, a learned behavior and the pattern of acquisition for dependence-induced escalation of alcohol self-administration is consistent with a standard learning curve, in that self-administration steadily increases with each acute withdrawal self-administration session until a plateau is reached and responding stabilizes (see Fig. 2; Smith et al., 2011).
Of critical importance is the distinction between neuroadaptations that occur during the transition to dependence and neuroadaptations associated with negative reinforcement are understood. Plasticity that occurs during the transition to dependence is posited to be a compensatory response to chronic alcohol exposure that is necessary for the development of negative affective states observed during withdrawal and provides the foundation for alcohol to be a negative reinforcer. However, as will be discussed below, such plasticity is not sufficient to induce escalation of self-administration alone, but instead requires specific forms of plasticity that occur during self-administration sessions that allow an organism to learn that the negative affective symptoms which occur during acute withdrawal can be removed by alcohol ingestion.
It should be noted that there are alternative theoretical explanations to account for escalated self-administration other than dependence-related negative reinforcement processes. Competing theories include the development of tolerance (for an excellent review, see Kalant, LeBlanc, & Gibbins, 1971) or sensitization to the discriminative stimulus, positive reinforcing or locomotor effects of alcohol (e.g., see Becker & Baros, 2006; Broadwater, Varlinskaya, & Spear, 2011; Lessov, Palmer, Quick, & Phillips, 2001). Because tolerance (e.g., tolerance to the positive reinforcing effects of alcohol) could be posited as the most parsimonious explanation for the increased responding observed in dependent animals, one might be inclined to reject theories with increased complexity based on degree of simplicity alone. Continuing with the example of tolerance to the positive reinforcing effects of alcohol as a basis for escalation, the inclusion of negative reinforcement in the explanation is more complex than simply positing that all self-administration behavior is related to positive reinforcement and nothing else. However, evidence will be presented below within the matrix metalloproteinase experimental discussion demonstrating that escalated self-administration is not consistent with the concept of tolerance. The use of Ockham's razor (plurality should not be posited without necessity) or opposing theories (e.g., Hickam's dictum) as a guiding principle for one's approach to scientific inquiry or the diagnosis of disorders is beyond the scope of this review and has been discussed previously from various theoretical positions (e.g., Gernert, 2009; Holmes & Sen, 2007; Schattner, 2009).
One additional caveat pertinent to the issue of tolerance is the proposition that tolerance and dependence are processes completely in dependent of each other. Supported by pinnacle papers within the ‘alcohol tolerance’ literature (e.g., Kalant et al., 1971), data suggests that tolerance and dependence are not independent, but intricately linked–the development of tolerance is an indicator that the drug is being administered with enough frequency to induce adaptations and suggests the transition to dependence is beginning to occur (although tolerance and dependence do not necessarily have the same substrates). This idea is further supported by data indicating the co-development of tolerance and withdrawal symptoms if use of the drug ceases (please see Kalant et al., 1971). Additional support comes from the DSM-IV (American Psychiatric Association, 2000) which includes the selection of: 1) tolerance (marked increase in amount; marked decrease in effect) or 2) characteristic withdrawal symptoms (substance taken to relieve withdrawal) among the cluster of symptoms that must be present to make a diagnosis of substance dependence.
Escalated self-administration is plasticity-dependent
A host of neuroadaptations have been associated with chronic alcohol and drugs of abuse that include morphological and intra-cellular signaling changes (Nestler, 1993; Ortiz et al., 1995). Many of these changes contribute to the development of negative affective states resembling depression and anxiety. However, other morphological changes can occur when information is learned or unlearned through processes that increase and decrease synaptic strength such as long-term potentiation (LTP) and depression (LTD; Bear & Malenka, 1994; Siegelbaum & Kandel, 1991). Of course, LTP and LTD are extremely relevant to discussions of plasticity, but due to the recent comprehensive reviews related to chronic alcohol and electrophysiological recordings in a variety of brain regions (e.g., McCool, 2011; McCool, Christian, Diaz, & Lack, 2010), this review will not attempt to duplicate those efforts. Extracellular matrix (ECM) proteins provide structural support in the nervous system and in order for synaptic plasticity (e.g., Hebbian, homeostatic and metaplasticity) to occur, the extracellular matrix must be degraded (Dityatev & Fellin, 2008; Lee, Tsang, & Birch, 2008; Wright & Harding, 2004, 2009). Furthermore, the ECM is involved in the regulation of intracellular/extracellular signaling, receptor localization in a number of neurotransmitter systems and astrocytic functions (Dityatev & Fellin, 2008). Matrix metalloproteinases (MMPs), a family of proteolytic enzymes, can cleave extracellular matrix proteins to allow for the reconfiguration of neural pathways (Ethell & Ethell, 2007; Lee et al., 2008; Wright & Harding, 2004). MMP secretion can be stimulated by growth factors and ECM-intracellular signaling pathways (Wright & Harding, 2009). MMP expression appears to be required for hippocampal-based learning and MMP inhibition interferes with LTP induction and maintenance, as well as memory tasks (Meighan et al., 2006; Meighan, Meighan, Davis, Wright, & Harding, 2007; Nagy, Bozdagi, & Huntley, 2007; Wright, Brown, & Harding, 2007).
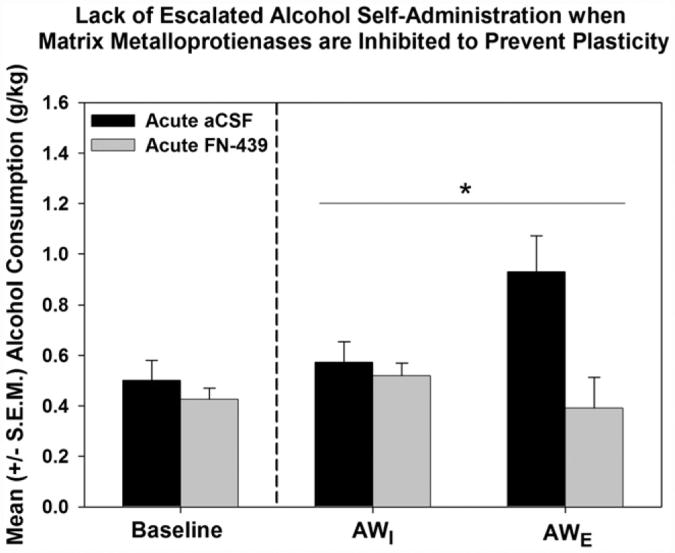
Based on their role in learning and memory, Dr. Walker conducted and presented data from a series of experiments in which MMPs were inhibited to prevent plasticity and thus, learning in alcohol-dependent animals that were allowed to self-administer in an operant paradigm (see Fig. 5 for experimental design; Smith et al., 2011). In the first experiment, animals were trained to self-administer 10% alcohol (w/v) and the MMP inhibitor FN-439 was chronically infused via an intracerebroventricular (ICV) route of administration using osmotic minipumps during a one-month alcohol vapor exposure period (to induce dependence) and during the subsequent acute withdrawal self-administration sessions. This approach assessed the presence or absence of escalated self-administration when using a dependence induction method that was shown by Dr. Walker to produce depressive- and anxiety-like behaviors in separate groups of animals (unpublished data presented by Dr. Walker during the symposium). The results indicated that chronic MMP inhibition during dependence induction and acute withdrawal prevented escalation whereas control animals treated with artificial cerebrospinal fluid (aCSF) in an identical manner as the experimental group, did demonstrate normal escalation. These results led to the second experiment in which animals were trained to self-administer alcohol and subjected to alcohol vapor exposure for one month to induce dependence. Following the dependence induction period, prior to and following the acute withdrawal self-administration sessions, FN-439 was infused ICV to test the hypothesis that response escalation indicative of negative reinforcement would only occur if the molecular mechanisms related to plasticity and structural remodeling in the CNS were intact. Indeed, the results showed that those animals receiving aCSF demonstrated normal escalation, but those receiving FN-439 did not escalate (see Fig. 6; Smith et al., 2011), indicating that the neuroadaptive changes which occur during dependence induction were, alone, insufficient to induce escalation and that an intact MMP system was required for the escalation that typifies dependence.
Fig. 5.

Experimental design to specifically assess plasticity that occurs during withdrawal. Acute and chronic FN-439 treatment following the acquisition of operant ethanol self-administration, dependence induction and post-dependence induction self-administration sessions during acute withdrawal. aCSF = artificial cerebrospinal fluid, ICV = intracerebroventricular and PDI = post-dependence induction. Adapted from Smith et al. (2011), Neurobiology of Learning and Memory with permission of Elsevier.
Fig. 6.
Blockade of plasticity during acute withdrawal prevents escalation of alcohol self-administration. Acute MMP inhibition via an intracerebroventricular route of administration prevents escalated responding for alcohol. A significant Drug Treatment × Session interaction was found (* =p ≤ 0.05) indicating that acute exposure to FN-439 attenuated the negative reinforcement learning associated with ethanol self-administration during acute withdrawal. AWI = initial post-vapor sessions and AWE = escalated post-vapor session. Adapted from Smith et al. (2011), Neurobiology of Learning and Memory with permission of Elsevier.
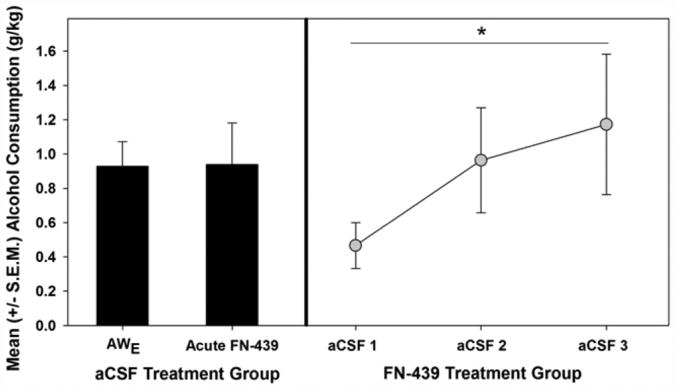
However, the aforementioned interpretation required two critical control manipulations if one were to accept it with any confidence. Namely, those animals that had received acute FN-439 (and didn't escalate) needed to demonstrate normal escalation if allowed to experience the negative reinforcing effects of alcohol when the molecular mechanisms related to CNS plasticity were not compromised. Secondarily, it was necessary to demonstrate that FN-439 was without any locomotor or non-specific effects that could account for the lack of escalation following acute administration. By using the aCSF-treated animals for the latter control condition, the construct validity of this model and the role of MMPs within learning paradigms could be assessed simultaneously. Specifically, the underlying assumption regarding MMPs and plasticity is that MMPs are produced when learning is initially occurring to promote ECM degradation and allow for synaptic plasticity. However, once learned (i.e., plasticity has occurred), MMP production should cease, the ECM should reform and MMP inhibition should have no impact on escalated responding. The results from those control manipulations are displayed in Fig. 7 and clearly indicate that those animals previously treated with aCSF during the acute withdrawal testing (which displayed normal escalation) received an acute ICV injection of FN-439, there was no change in their escalated response pattern (i.e., there was no locomotor or non-specific effects of FN-439). Furthermore, the animals previously treated with FN-439 demonstrated normal escalation when infused with vehicle (aCSF) which indicates that MMP inhibition did not impact their brain in a manner that permanently abolished that ability of the organism to learn. One inconsistency is present within the MMP literature when considering negative reinforcement learning. There is data showing MMP inhibition does not block aversive associative learning within a fear conditioning paradigm (Brown, Wilson, Cocking, & Sorg, 2009). However, in that study, FN-439 was only administered prior to the conditioning sessions and not after them which raises the possibility that consolidation could have occurred if the effects of FN-439 began to wane prior to the conclusion of plasticity-dependent processes. In the present case, FN-439 was administered prior to, and after, the self-administration session to specifically protect against that possibility in order to confirm that mechanisms related to plasticity were prevented from occurring.
Fig. 7.
Control conditions required for a learning-based explanation of withdrawal-induced escalation. Left panel: Session in which the artificial cerebrospinal fluid (aCSF) – treated animals escalated (AWE) compared to those same animals receiving subsequent acute intracerebroventricular FN-439 infusion. Right Panel: Acute aCSF infusions in animals initially treated with FN-439 that did not escalate – demonstrates that those animals are capable of learning and display a normal dependence-like phenotype in the absence of FN-439. Main effect of session (* =p ≤ 0.05). Adapted from Smith et al. (2011), Neurobiology of Learning and Memory with permission of Elsevier.
As mentioned above, other theories to account for escalated self-administration, such as tolerance to the positive reinforcing effects of alcohol, have been posited. However, the data presented in Figs. 6 and 7 argue against such alternative explanations. If one assumes that positive reinforcement is a learned process, then it follows that any learning regarding the positive reinforcing properties of alcohol had previously taken place for those animals. Furthermore, learning about a preferred level of self-administration through titration had already occurred, as the animals in that study were required to show stable responding prior to dependence induction. Therefore, if learning about the positive reinforcing effects of alcohol and about modulation of lever-pressing to achieve a desired effect had occurred, then one could posit that such learning would still be present following the one-month dependence induction procedure and, because the animals had already learned to titrate, would result in escalated self-administration during the initial acute withdrawal self-administration session. However, that pattern was not observed in the animals that were treated with FN-439 (see Fig. 6) until the treatment was terminated at which point, escalation occurred in a manner consistent with a learned response (i.e., gradually increased over a number of sessions; see Fig. 7). In addition, inhibition of MMPs did not result in the complete attenuation of self-administration behavior, with the FN-439 animals maintaining a rate of responding that was consistent with their pre-dependence induction baseline rate of responding; suggesting that the processes governing pre-dependence and post-dependence response are different from each other. Therefore, positing that escalation of self-administration in dependent animals during withdrawal can solely be explained by tolerance to the positive reinforcing effects of alcohol is inconsistent with the evidence identified in the MMP inhibition experiments. Likewise, the idea that escalation might be related to tolerance to the locomotor suppressant effects of alcohol would also be inconsistent with the data presented in the MMP experiment because such tolerance would be present at the time of the first self-administration session since the animals had been exposed to alcohol for the month prior to that first acute withdrawal test session. Collectively, the data from the MMP experiment are supportive of an explanation for escalated self-administration that does not involve mechanisms of positive reinforcement or tolerance as the sole explanation, but instead necessitate that plurality should be posited if one is to best describe and study the phenomenon of escalation.
Although FN-439 has been extensively characterized and repeatedly been shown to attenuate the effects of MMPs on the ECM and reduce various indices of associative and non-associative learning (including the underlying processes necessary for such learning to occur; Brown et al., 2007, 2009; Meighan et al., 2006; Wiediger & Wright, 2009; Wright et al., 2007), it is considered a general MMP inhibitor and does not allow for the precise specification of which MMPs are relevant to particular behaviors. While the MMP family consists of over 25 enzymes, MMP-9 and MMP-3 have been heavily implicated in systems that could contribute to escalated responding and negative reinforcement learning. MMP-9 is required for the synaptic plasticity related to hippocampal-based long-term potentiation and memory (Nagy et al., 2006) and is involved in dendritic spine enlargement through a β1-integrin pathway (Wang et al., 2008). Furthermore, MMP-9 levels are increased following cocaine-primes in a reinstatement paradigm (Brown, Forquer, Harding, Wright, & Sorg, 2008), have been shown to be altered in the hippocampus by chronic cocaine exposure (Mash et al., 2007) and are increased in mice displaying behavioral sensitization to methamphetamine (Mizoguchi et al., 2007). Interestingly, a functional polymorphism in the MMP-9 gene is associated with alcohol dependence in humans (Samochowiec et al., 2010) and, although it relates to peripheral rather than brain MMPs, serum MMP-9 concentrations are increased alcoholics compared to controls (Sillanaukee, Kalela, Seppa, Hoyhtya, & Nikkari, 2002). In addition, MMP-3 is required for spatial learning (Meighan et al., 2006), as well as passive avoidance conditioning (Olson et al., 2008) and habituation (Wright & Harding, 2009). Future studies will need to be conducted in order to identify the precise involvement of the different MMPs, as well as specific brain nuclei in which synaptic remodeling is occurring as the basis for the negative reinforcing effects of alcohol.
Extended amygdala and negative reinforcement
As mentioned, dependence-induced negative affective states could theoretically occur via either within- or between-system neuroadaptations. Although dopamine (DA) and the endogenous opioids (EOS) within the mesolimbic pathway, ventral striatum and central amygdala (CeA) are hypothesized to participate in dependence-induced within-system changes and stress-related peptides in extended amygdala (nucleus accumbens shell, CeA and bed nucleus of the stria terminalis) are hypothesized to participate in between-system changes (for one of many examples, see the excellent review by Koob, 2009a), there are numerous neuropeptides that are involve in alcohol positive and negative reinforcement. This section is not intended to be exhaustive because the other Young Investigator Awardees are each contributing to different aspects of a cohesive set of reviews that collectively focus on plasticity and neuroadaptive responses within the extended amygdala in response to chronic or excessive alcohol exposure. Consequently, this section will primarily discuss the emerging roles of KORs in the extended amygdala and their contribution negative reinforcing effects of alcohol. As mentioned above, a negative affective state is required in order for negative reinforcement processes to occur and KORs in the extended amygdala appear to be a primary mediator of the negative affective states that are associated with alcohol dependence.
Opioids in the extended amygdala
The pharmacological effects of alcohol on the central nervous system include alterations in the function of the cholinergic, dopaminergic (DA), gamma-aminobutyric acid, glutamatergic, opioidergic, and serotonergic neurotransmitter systems (for review see Eckardt et al., 1998). Because one of the three approved treatments for alcohol abuse and dependence has an opioidergic mechanism of action (Heilig & Egli, 2006), the EOS has been extensively studied under conditions of non-dependent alcohol reward and reinforcement. Selective antagonists of the μ-and δ-opioid receptor (MOR and DOR, for which the endogenous ligands are β-endorphin (βEND) and enkephalin (ENK), respectively) have been shown to reduce alcohol self-administration (Hyytia & Kiianmaa, 2001; Stromberg, Casale, Volpicelli, Volpicelli, & O'Brien, 1998), whereas antagonists selective for the κ-opioid receptor (KOR, for which dynorphin (DYN) is the endogenous ligand), generally show no effect on non-dependent alcohol self-administration (Doyon, Howard, Shippenberg, & Gonzales, 2006; Logrip, Janak, & Ron, 2008; Nealey et al., 2011; Walker et al., 2011; Walker & Koob, 2008; Williams & Woods,1998), but see Mitchell, Liang, and Fields (2005) who used a strain of rats (i.e., Lewis rats) that have dramatically altered DYN levels compared to other heterogeneous strains (Nylander, Vlaskovska, & Terenius, 1995), a fact that was not addressed in the study. Thus, the majority of evidence suggests that the MOR and DOR are viable targets to reduce the positive reinforcing effects of alcohol in non-dependent cohorts, whereas DYN/KOR systems do not appear to be involved in the positive reinforcing effects of alcohol. Conversely, DYN/KOR systems appear to contribute to the negative reinforcing effects of alcohol (Nealey et al., 2011; Walker et al., 2011; Walker & Koob, 2008) based, in part, on the pro-depressive effects of DYN/KOR system activation (Todtenkopf, Marcus, Portoghese, and Carlezon, Jr., 2004; Carlezon et al., 2006), the anti-depressant properties of KOR antagonists (Carr et al., 2010; Mague et al., 2003; Pliakas et al., 2001) and involvement of KORs with dysphoria produced by stress (Land et al., 2008; McLaughlin, Marton-Popovici, & Chavkin, 2003; Sperling et al., 2010).
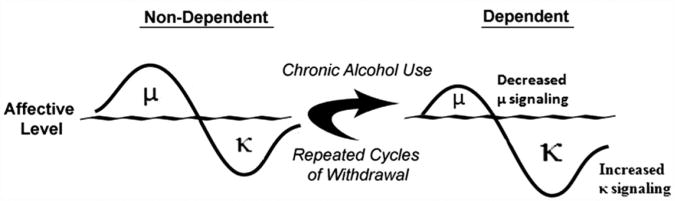
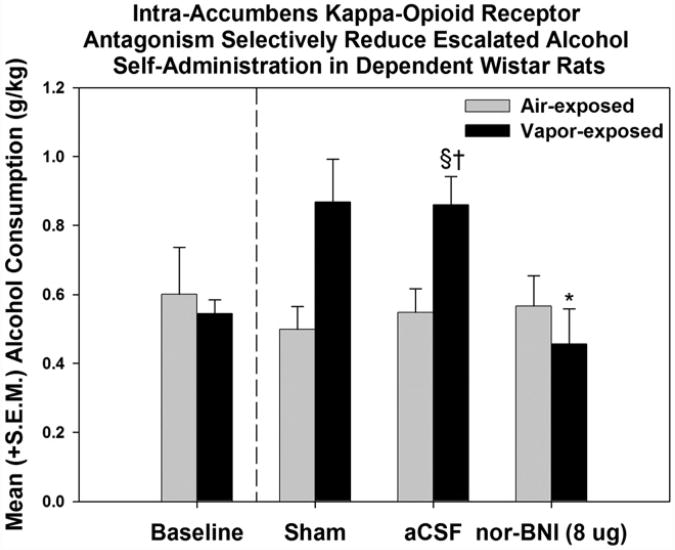
In accordance with the Opponent-Process Theory, if MOR and/or DOR stimulation produces positive hedonic states (Amalric, Cline, Martinez, Jr., Bloom, and Koob, 1987; Herz, 1997; Shippenberg, Bals-Kubik, & Herz, 1987; Shippenberg & Herz, 1986), then one putative compensatory mechanism would be an increase in DYN and/or activation of KORs, the stimulation of which produces negative hedonic states (Mucha & Herz, 1985). Recent evidence evaluating opioid release in the ventral tegmental area (VTA) and CeA strongly supports the opponent-process construct, namely that acute alcohol administration initially increases βEND within the first 30 min which is followed by a significant increase in DYN A approximately 1.5–2 h later (Jarjour, Bai, & Gianoulakis, 2009; Lam, Marinelli, Bai, & Gianoulakis, 2008) – a profile that is also observed within the nucleus accumbens (Marinelli, Lam, Bai, Quirion, & Gianoulakis, 2006; Marinelli, Quirion, & Gianoulakis, 2004). The Opponent-Process Theory predicts that chronic alcohol exposure would decrease positive affect and increase negative affect (see Fig. 8). In support of that prediction, evidence has shown that the MOR- and DOR-regulated component of the opioid peptide system shows decreased signaling in response to chronic alcohol (Chen & Lawrence, 2000; Gianoulakis, Krishnan, & Thavundayil, 1996; Saland et al., 2004; Turchan et al., 1999). Also consistent with that hypothesis, chronic alcohol–exposed animals have been shown to have increased prodynorphin mRNA levels in the nucleus accum-bens (Acb; Przewlocka, Turchan, Lason, & Przewlocki, 1997), increased expression of DYN B in the Acb (Lindholm, Ploj, Franck, & Nylander, 2000) and altered KOR mRNA expression in the Acb and VTA (Rosin, Lindholm, Franck, & Georgieva, 1999) that support the concept of an upregulated DYN system. Furthermore, intracranial self-stimulation (ICSS) thresholds are increased during acute withdrawal from chronic alcohol, reflecting anhedonia/dysphoria (Schulteis et al., 1995) and KOR agonists produce comparable changes in ICSS thresholds (Todtenkopf et al., 2004). A molecular mechanism by which DYN levels in the Acb could be increased following chronic drug treatment involves elevations of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein (for review, see Carlezon, Duman, & Nestler, 2005). Those elevated levels of CREB activity are associated with increased expression of CREB-associated target genes for various peptides (e.g., DYN) that have been associated with increased negative affect and depressive-like behaviors and are also linked to altered dopami-nergic function. The involvement of DYN in dependence-induced escalation of alcohol self-administration is highlighted by the fact that antagonists for the KOR,administered 5minor 24 h prior to self-administration sessions, selectively attenuate escalated responding in dependent animals without impacting non-dependent alcohol consumption following systemic (Walker et al., 2011), ICV (Walker & Koob, 2008), intra-accumbens shell (see Fig. 9 for example; Nealey et al., 2011) and intra-CeA infusions (unpublished data presented by Dr. Walker during the symposium). Thus, the EOS appears to contribute to negative affective states produced by dependence in a manner consistent with attenuated positive affect and exacerbated negative affect that provides a basis for alcohol to have negative reinforcing properties.
Fig. 8.
Opioidergic compensatory responses prior to and following chronic alcohol exposure. In non-dependent organisms, alcohol-induced positive affective states mediated by the μ-opioid receptor precede compensatory negative affective states expressed through the κ-opioid receptor. Following chronic alcohol exposure, μ-opioid receptor signaling is attenuated and through multiple mechanisms, κ-opioid receptor signaling is increased to produce increased negative affective states. Adapted from Walker, Valdez, McLaughlin, & Bakalkin, (2012), Alcohol with permission of Elsevier.
Fig. 9.
Intra-accumbens κ-opioid receptors mediate withdrawal-induced escalation of alcohol self-administration. Mean (+S.E.M.) responses for ethanol in non-dependent and ethanol-dependent animals during acute withdrawal following intra-accumbens nor-BNI treatment prior to self-administration sessions. Nor-BNI selectively attenuated alcohol self-administration in dependent animals († =p < 0.05 when compared to the corresponding air-exposed group, § =p < 0.05 when compared to baseline of the same exposure condition, * =p < 0.05 when compared to the vapor-exposed aCSF-treated group). From Nealey et al. (2011), Neuropharmacology with permission with permission of Elsevier.
Extended amygdala dopamine
The functional impact of increased KOR/DYN signaling involves, in part, the mesocorticolimbic DA system. This system has been implicated as a signaling system for biologically relevant information through which drugs of abuse (e.g., Di Chiara et al., 2004; Ikegami & Duvauchelle, 2004; Koob, 2000; Maldonado, 2003) and natural reinforcers (e.g., Carelli, 2002; Hull et al., 1999; Kelley et al., 2002; Kelley, Baldo, Pratt, & Will, 2005) or punishers can exert their behavioral effects due to the mesolimbic DA pathway's capacity for bidirectional signaling (i.e., ability to signal both positive and negative stimuli; Wheeler & Carelli, 2009). Within the mesocorticolimbic DA system, KORs are neuroanatomically positioned on DA terminals in the Acb shell (AcbSh) which enables them to oppose the effects of MOR agonists on DA release (Di Chiara & Imperato, 1988); KORs are also positioned on DA perikarya in the VTA (Margolis, Hjelmstad, Bonci, & Fields, 2003; Margolis et al., 2006; Svingos, Colago, & Pickel, 1999). Much research has been done to determine how KOR stimulation impacts dopaminergic neurotransmission and drug self-administration (for an excellent review, see Shippenberg et al. 1987). In essence, while the KORs positioned on the terminal regions in the AcbSh reduce DA release (Di Chiara & Imperato, 1988) in that region, KORs on VTA DA neurons do not (Margolis et al. 2006; Spanagel, Herz, & Shippenberg, 1992), but instead selectively reduce DA release in the prefrontal cortex (Margolis et al. 2006). Thus, increased signaling through the DYN/KOR system could functionally result in an attenuated dopaminergic system in both cortical and limbic circuitry. Indeed, deficiencies in dopaminergic transmission has been posited by some to be the neurobiological basis of depression (Nestler & Carlezon, 2006).
Substantial evidence supports the concept of chronic alcohol-induced attenuation of dopaminergic systems (Carroll, Rodd, Murphy, & Simon, 2006; Healey, Winder, & Kash, 2008) through multiple mechanisms. Stimulation of the KOR produces dysphoria in humans (Pfeiffer, Brantl, Herz, & Emrich, 1986) and place aversions in animals (Mucha & Herz,1985). Furthermore, increased DYN transmission has been hypothesized to induce depressive-like behavioral states in animal models of depression and negative affect (Carlezon, Jr. et al., 2006; Mague et al., 2003; Pliakas et al., 2001; Shirayama et al. 2004; Todtenkopf et al., 2004), and the DYN/KOR system has been implicated as a mediator of dysphoria through which stress-related systems can exert their effects in the basolateral amygdala (BLA; Land et al., 2008). Thus, if a compensatory response to chronic alcohol involved alterations in DYN/KOR signaling, then DYN/KOR-mediated negative affect could contribute to the increased alcohol consumption observed in dependence. Taken together, increased DYN transmission could result in attenuated dopaminergic transmission and produce depressive-like behaviors and dysphoria that are thought to involve multiple nuclei within extended amygdala circuitry.
Conclusions
One goal of this review was to identify important factors that contribute to the negative reinforcing effects of alcohol during withdrawal in dependent organisms. However, the primary purpose of this review was to distinguish the neuroadaptations associated with the transition to dependence from the plasticity and morphological reorganization that is required for negative reinforcement learning. Data were presented that highlighted the role of matrix metalloproteinases in the plasticity associated with escalated self-administration and presented the use of MMP inhibition as a technique to prevent plasticity and evaluate those factors that are necessary and sufficient for dependence-induced negative affect and/or escalated self-administration during acute withdrawal. Future work will need to be conducted to determine the precise substrates underlying negative reinforcement, but a means to do so has now been established.
Acknowledgments
Support was provided by National Institute on Alcohol Abuse and Alcoholism grants R01AA020394 to BMW and R13AA017581 to Dr. Marisa Roberto to support the “Alcoholism and Stress: A Framework for Future Treatment Strategies” conference in Volterra, Italy on May 3–6, 2011. Dr. Walker would like to thank Drs. Noronha and Crews for chairing the symposium and NIAAA for providing the young investigators with travel assistance to attend the award symposium. Additional support was provided by the Hope for Depression Research Foundation, H. Lundbeck A/S and WSU Alcohol and Drug Abuse Research Program grants according to the State of Washington Initiative Measure No. 171. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health or the State of Washington. Dr. Walker would like to thank Alexander W. Smith and Drs. Aaron Ettenberg, George Koob, John Wright, Joe Harding, Jaak Panksepp and Barbara Sorg for their intellectual contributions and Mrs. Jennifer Walker for her continued assistance with, and support of, the research endeavors within the Laboratory of Alcoholism and Addictions Neuroscience at WSU.
References
- Amalric M, Cline EJ, Martinez JL, Jr, Bloom FE, Koob GF. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology (Berlin) 1987;91:14–19. doi: 10.1007/BF00690919. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR. 4th., text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Anderson WW, Thompson T. Ethanol self-administration in water satiated rats. Pharmacology Biochemistry and Behaviour. 1974;2:447–454. doi: 10.1016/0091-3057(74)90002-1. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berlin) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Current Opinion in Neurobiology. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Becker HC, Baros AM. Effect of duration and pattern of chronic ethanol exposure on tolerance to the discriminative stimulus effects of ethanol in C57BL/6J mice. Journal of Pharmacology and Experimental Therapeutics. 2006;319:871–878. doi: 10.1124/jpet.106.108795. [DOI] [PubMed] [Google Scholar]
- Bozarth MA. Evidence for the rewarding effects of ethanol using the conditioned place preference method. Pharmacology Biochemistry and Behaviour. 1990;35:485–487. doi: 10.1016/0091-3057(90)90191-j. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcoholism: Clinical and Experimental Research. 2011;35:1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learning & Memory. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Harding JW, Wright JW, Sorg BA. Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse. 2008;62:886–889. doi: 10.1002/syn.20562. [DOI] [PubMed] [Google Scholar]
- Brown TE, Wilson AR, Cocking DL, Sorg BA. Inhibition of matrix metalloproteinase activity disrupts reconsolidation but not consolidation of a fear memory. Neurobiology of Learning and Memory. 2009;91:66–72. doi: 10.1016/j.nlm.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiology & Behaviour. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. Journal of Pharmacology and Experimental Therapeutics. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends in Neurosciences. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in wistar kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MR, Rodd ZA, Murphy JM, Simon JR. Chronic ethanol consumption increases dopamine uptake in the nucleus accumbens of high alcohol drinking rats. Alcohol. 2006;40:103–109. doi: 10.1016/j.alcohol.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Lawrence AJ. Effect of chronic ethanol and withdrawal on the mu-opioid receptor- and 5-Hydroxytryptamine(1A) receptor-stimulated binding of [(35)S]Guanosine-5′-O-(3-thio) triphosphate in the fawn-hooded rat brain: a quantitative autoradiography study. Journal of Pharmacology and Experimental Therapeutics. 2000;293:159–165. [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Fellin T. Extracellular matrix in plasticity and epileptogenesis. Neuron Glia Biology. 2008;4:235–247. doi: 10.1017/S1740925X09000118. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. Kappa-opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–496. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcoholism: Clinical and Experimental Research. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. Journal of Neuroscience Research. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- File SE, Baldwin HA, Hitchcott PK. Flumazenil but not nitrendipine reverses the increased anxiety during ethanol withdrawal in the rat. Psycho-pharmacology (Berlin) 1989;98:262–264. doi: 10.1007/BF00444702. [DOI] [PubMed] [Google Scholar]
- Funk CK, Dohrman DP. Chronic ethanol exposure inhibits dopamine release via effects on the presynaptic actin cytoskeleton in PC12 cells. Brain Research. 2007;1185:86–94. doi: 10.1016/j.brainres.2007.09.069. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. Journal of Neuroscience. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernert D. Ockham's razor and its improper use. Cognitive Systems. 2009;7:133–138. [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary beta-endorphin to ethanol in subjects at high risk of alcoholism. Archives of General Psychiatry. 1996;53:250–257. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- Harwood HJ, Fountain D, Livermore G. Economic costs of alcohol abuse and alcoholism. Recent Developments in Alcoholism. 1998;14:307–330. doi: 10.1007/0-306-47148-5_14. [DOI] [PubMed] [Google Scholar]
- Healey JC, Winder DG, Kash TL. Chronic ethanol exposure leads to divergent control of dopaminergic synapses in distinct target regions. Alcohol. 2008;42:179–190. doi: 10.1016/j.alcohol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacology & Therapeutics. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction Biology. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berlin) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Holmes MV, Sen D. Microscopic polyangiitis and myasthenia gravis: the battle of Occam and Hickam. Clinical Rheumatology. 2007;26:1981–1983. doi: 10.1007/s10067-007-0599-9. [DOI] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, et al. Hormone-neurotransmitter interactions in the control of sexual behavior. Behavioural Brain Research. 1999;105:105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcoholism: Clinical and Experimental Research. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Duvauchelle CL. Dopamine mechanisms and cocaine reward. International Review of Neurobiology. 2004;62:45–94. doi: 10.1016/S0074-7742(04)62002-2. [DOI] [PubMed] [Google Scholar]
- Jarjour S, Bai L, Gianoulakis C. Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcoholism: Clinical and Experimental Research. 2009;33:1033–1043. doi: 10.1111/j.1530-0277.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE, Gibbins RJ. Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacological Reviews. 1971;23:135–191. [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiology & Behaviour. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiology & Behaviour. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Annals of the New York Academy of Sciences. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochemical Pharmacology. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009a;42(Suppl 1):S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009b;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Weiss F. Neuropharmacology of cocaine and ethanol dependence. Recent Developments in Alcoholism. 1992;10:201–233. doi: 10.1007/978-1-4899-1648-8_11. [DOI] [PubMed] [Google Scholar]
- Lam MP, Marinelli PW, Bai L, Gianoulakis C. Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacology (Berlin) 2008;201:261–271. doi: 10.1007/s00213-008-1267-8. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. Journal of Neuroscience. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Tsang VW, Birch NP. Synaptic plasticity-associated proteases and protease inhibitors in the brain linked to the processing of extracellular matrix and cell adhesion molecules. Neuron Glia Biology. 2008;4:223–234. doi: 10.1017/S1740925X09990172. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Palmer AA, Quick EA, Phillips TJ. Voluntary ethanol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacology (Berlin) 2001;155:91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol. 2000;22:165–171. doi: 10.1016/s0741-8329(00)00118-x. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB Journal. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. Journal of Pharmacology and Experimental Therapeutics. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maldonado R. The neurobiology of addiction. Journal of Neural Transmission Supplementum. 2003:1–14. doi: 10.1007/978-3-7091-0541-2_1. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. Journal of Neuroscience. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1-8) release in the rat nucleus accumbens following alcohol administration. Alcoholism: Clinical and Experimental Research. 2006;30:982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience. 2004;127:777–784. doi: 10.1016/j.neuroscience.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Mash DC, French-Mullen J, Adi N, Qin Y, Buck A, Pablo J. Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PLoS ONE. 2007;2:e1187. doi: 10.1371/journal.pone.0001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Critical Reviews in Neurobiology. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011;61:1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Christian DT, Diaz MR, Lack AK. Glutamate plasticity in the drunken amygdala: the making of an anxious synapse. International Review of Neurobiology. 2010;91:205–233. doi: 10.1016/S0074-7742(10)91007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. Journal of Neuroscience. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan PC, Meighan SE, Davis CJ, Wright JW, Harding JW. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. Journal of Neurochemistry. 2007;102:2085–2096. doi: 10.1111/j.1471-4159.2007.04682.x. [DOI] [PubMed] [Google Scholar]
- Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, et al. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. Journal of Neurochemistry. 2006;96:1227–1241. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berlin) 2005;182:384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Niwa M, Mouri A, Mizuno T, Noda Y, et al. Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and -9-deficient mice. Journal of Neurochemistry. 2007;100:1579–1588. doi: 10.1111/j.1471-4159.2006.04288.x. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Journal of the American Medical Association. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berlin) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Huntley GW. The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learning & Memory. 2007;14:655–664. doi: 10.1101/lm.678307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. Journal of Neuroscience. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology. 2011;61:35–42. doi: 10.1016/j.neuropharm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Cellular responses to chronic treatment with drugs of abuse. Critical Reviews in Neurobiology. 1993;7:23–39. [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nylander I, Vlaskovska M, Terenius L. Brain dynorphin and enkephalin systems in Fischer and Lewis rats: effects of morphine tolerance and withdrawal. Brain Research. 1995;683:25–35. doi: 10.1016/0006-8993(95)00279-y. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical and Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Olson ML, Meighan PC, Brown TE, Asay AL, Benoist CC, Harding JW, et al. Hippocampal MMP-3 elevation is associated with passive avoidance conditioning. Regulatory Peptides. 2008;146:19–25. doi: 10.1016/j.regpep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, et al. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. Journal of Neuroscience. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewlocka B, Turchan J, Lason W, Przewlocki R. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neuroscience Letters. 1997;238:13–16. doi: 10.1016/s0304-3940(97)00829-x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcoholism: Clinical and Experimental Research. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behavioral and Neural Biology. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Rosin A, Lindholm S, Franck J, Georgieva J. Downregulation of kappa opioid receptor mRNA levels by chronic ethanol and repetitive cocaine in rat ventral tegmentum and nucleus accumbens. Neuroscience Letters. 1999;275:1–4. doi: 10.1016/s0304-3940(99)00675-8. [DOI] [PubMed] [Google Scholar]
- Saland LC, Abeyta A, Frausto S, Raymond-Stintz M, Hastings CM, Carta M, et al. Chronic ethanol consumption reduces delta-and mu-opioid receptor-stimulated G-protein coupling in rat brain. Alcoholism: Clinical and Experimental Research. 2004;28:98–104. doi: 10.1097/01.ALC.0000108658.00243.BF. [DOI] [PubMed] [Google Scholar]
- Samochowiec A, Grzywacz A, Kaczmarek L, Bienkowski P, Samochowiec J, Mierzejewski P, et al. Functional polymorphism of matrix metalloproteinase-9 (MMP-9) gene in alcohol dependence: family and case control study. Brain Research. 2010;1327:103–106. doi: 10.1016/j.brainres.2010.02.072. [DOI] [PubMed] [Google Scholar]
- Schattner A. As sharp as Occam? Lancet. 2009;373:1996. doi: 10.1016/S0140-6736(09)60696-6. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. Motivational properties of opioids: evidence that an activation of delta-receptors mediates reinforcement processes. Brain Research. 1987;436:234–239. doi: 10.1016/0006-8993(87)91667-2. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Research Monographs. 1986;75:563–566. [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. Journal of Neurochemistry. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Siegelbaum SA, Kandel ER. Learning-related synaptic plasticity: LTP and LTD. Current Opinion in Neurobiology. 1991;1:113–120. doi: 10.1016/0959-4388(91)90018-3. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De LL. Glutamatergic transmission in opiate and alcohol dependence. Annals of the New York Academy of Sciences. 2003;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- Sillanaukee P, Kalela A, Seppa K, Hoyhtya M, Nikkari ST. Matrix metalloproteinase-9 is elevated in serum of alcohol abusers. European Journal of Clinical Investigation. 2002;32:225–229. doi: 10.1046/j.1365-2362.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- Smith AW, Nealey KA, Wright JW, Walker BM. Plasticity associated with escalated operant ethanol self-administration during acute withdrawal in ethanol-dependent rats requires intact matrix metalloproteinase systems. Neurobiology of Learning and Memory. 2011;96:199–206. doi: 10.1016/j.nlm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SG, Davis WM. Intravenous alcohol self-administration in the rat. Pharmacological Research Communications. 1974;6:379–402. doi: 10.1016/s0031-6989(74)80039-1. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychological Review. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berlin) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O'Brien CP. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998;15:281–289. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2009 national survey on drug use and health. Rockville, MD: Office of Applied Studies, DHHS Publication; 2010. [Google Scholar]
- Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. Journal of Neuroscience. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berlin) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Turchan J, Przewlocka B, Toth G, Lason W, Borsodi A, Przewlocki R. The effect of repeated administration of morphine, cocaine and ethanol on mu and delta opioid receptor density in the nucleus accumbens and striatum of the rat. Neuroscience. 1999;91:971–977. doi: 10.1016/s0306-4522(98)00637-x. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcoholism: Clinical and Experimental Research. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A. Intracerebroventricular ethanol-induced conditioned place preferences are prevented by fluphenazine infusions into the nucleus accumbens of rats. Behavioral Neuroscience. 2007;121:401–410. doi: 10.1037/0735-7044.121.2.401. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcoholism: Clinical and Experimental Research. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Valdez GR, McLaughlin JP, Bakalkin G. Targeting Dynorphin/Kappa Opioid Receptor Systems to Treat Alcohol Abuse and Dependence. Alcohol. 2012 doi: 10.1016/j.alcohol.2011.10.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addiction Biology. 2011;16:116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009;56(Suppl 1):149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiediger RV, Wright JW. Influence of dorsal hippocampal lesions and MMP inhibitors on spontaneous recovery following a habituation/classical conditioning head-shake task. Neurobiology of Learning and Memory. 2009;92:504–511. doi: 10.1016/j.nlm.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE. The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in wistar rats. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2691-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Woods JH. Oral ethanol-reinforced responding in rhesus monkeys: effects of opioid antagonists selective for the mu-, kappa-, or delta-receptor. Alcoholism: Clinical and Experimental Research. 1998;22:1634–1639. doi: 10.1111/j.1530-0277.1998.tb03960.x. [DOI] [PubMed] [Google Scholar]
- Wright JW, Brown TE, Harding JW. Inhibition of hippocampal matrix metalloproteinase-3 and -9 disrupts spatial memory. Neural Plasticity. 2007;2007:73813. doi: 10.1155/2007/73813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Harding JW. The brain angiotensin system and extracellular matrix molecules in neural plasticity, learning, and memory. Progress in Neurobiology. 2004;72:263–293. doi: 10.1016/j.pneurobio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Wright JW, Harding JW. Contributions of matrix metalloproteinases to neural plasticity, habituation, associative learning and drug addiction. Neural Plasticity. 2009;2009:579382. doi: 10.1155/2009/579382. [DOI] [PMC free article] [PubMed] [Google Scholar]