Abstract
Background
Dominant biliary strictures occur commonly in patients with primary sclerosing cholangitis (PSC), who have a high risk of developing cholangiocarcinoma. The natural history and optimal management of dominant strictures remains unclear, with some reports suggesting that endoscopic interventions improve outcome.
Methods
We describe a 25 year experience in patients with PSC related dominant strictures at a single tertiary referral centre.
Results
128 patients with PSC (64% males, mean age at referral 49 years) were followed for a mean of 9.8 years. 80 patients (62.5%) with dominant biliary strictures had a median of 3 (range 0–34) interventions, compared to 0 (0–7) in the 48 without dominant strictures (p<0.001). Endoscopic interventions included: (i) stenting alone (46%), (ii) dilatation alone (20%), (iii) dilatation and stenting (17%), and (iv) none or failed intervention (17%, of whom most required percutaneous transhepatic drainage). The major complication rate for ERCP was low (1%). The mean survival of those with dominant strictures (13.7 years) was worse than for those without dominant strictures (23 years), with much of the survival difference related to a 26% risk of cholangiocarcinoma developing only in those with dominant strictures. Half of those with cholangiocarcinoma presented within four months of diagnosis of PSC, highlighting the importance of thorough evaluation of new dominant strictures.
Conclusions
Repeated endoscopic therapy in PSC patients is safe but the prognosis remains worse in the subgroup with dominant strictures. In our series, dominant strictures were associated with a high risk of developing cholangiocarcinoma.
Keywords: Cholangiocarcinoma, dominant stricture, endoscopic retrograde cholangiopancreatography, primary sclerosing cholangitis
INTRODUCTION
During the course of PSC, localised, high-grade (dominant) strictures may develop de novo or be superimposed on diffuse ductal disease, resulting in impaired biliary drainage with clinical consequences including pruritus, jaundice and recurrent cholangitis. The prevalence of dominant strictures in PSC is approximately 36–56% [1–3] Biochemical and clinical improvements have been reported after stenting and/or balloon dilatation of dominant strictures [1, 4–8]. Also, there is some evidence that relief of biliary obstruction can reverse secondary liver fibrosis [9]. However, most endoscopic studies of patients with PSC have been relatively small, non-randomised, with short-term follow-up, and other studies report a lack of biochemical response and worse survival in patients with DS who had undergone endoscopic therapy [2, 10]. It remains unproven whether endoscopic therapy improves long term outcome in patients with dominant strictures.
PSC is strongly associated with cholangiocarcinoma which often arises at the site of dominant strictures [11]. In theory, successful endoscopic therapy, by delaying the need for transplant referral, may delay diagnosis and increase the risk of developing unresectable malignancy. For this reason some have advocated early referral for transplantation in PSC patients with dominant strictures, although this remains controversial.
The aim of this retrospective review was to compare the outcome of a large series of patients with PSC with and without dominant biliary strictures managed at a single tertiary referral centre over a 25-year period, with a particular emphasis on the safety and efficacy of endoscopic therapy.
METHODS
Patient population
Data collection was conducted in accordance with local guidelines and ethics approval (06/Q0152/106). From January 1984 to March 2011, patients with a possible diagnosis of PSC attending University College London Hospitals NHS Foundation Trust were identified using computerised clinical and endoscopy databases of over 14,000 ERCPs performed during the 25 year study period. The medical records were reviewed to obtain demographic, clinical, laboratory and follow-up data.
The diagnosis of PSC was based on published clinical, laboratory and cholangiographic findings [12]. In patients who underwent liver biopsy, normal findings did not preclude the diagnosis of PSC. Patients with secondary sclerosing cholangitis and those with IgG4 related cholangitis were excluded. Those with localised strictures of the extrahepatic biliary tree or right and left hepatic ducts amenable to therapeutic intervention were included in a Dominant Stricture group. Patients with diffuse disease including intrahepatic strictures above the main right and left hepatic ducts but without tight narrowing of the main ducts were assigned to a No Dominant Stricture group. Patients with small duct PSC were rare in our referral base and were not included in the study.
Standard biochemistry and, where available CA19-9, were recorded nearest the time of PSC and/or cholangiocarcinoma diagnosis. Endoscopic retrograde cholangiopancreatography (ERCP) films and other imaging were reassessed wherever possible. Patients were reviewed in the clinic or, if not in regular contact with the hospital, the GP and/or patients contacted by telephone.
Entry into the study was recorded from the time of the first cholangiogram that showed features of PSC. Endpoints were death, liver transplantation, last clinical contact or the end of the study on 1st March 2011. Death registry data was collected via the UK General Register Office (www.gro.gov.uk).
Endoscopic techniques
All patients received prophylactic antibiotics prior to ERCP, as per departmental policy. In the early part of the series, ERCPs were performed in both symptomatic and asymptomatic patients in order to establish the diagnosis of PSC. Since 2000, magnetic resonance cholangiopancreatography (MRCP) largely replaced diagnostic ERCP, although ERCP was still performed if MRCP results were inconclusive. The presence of dominant strictures usually prompted ERCP for tissue acquisition and endoscopic therapy by stricture dilatation and/or biliary stenting, at the discretion of the endoscopist.
Most patients in the early part of our series were treated with biliary stent insertion (usually 10F straight plastic stents) replaced at approximately six-monthly intervals, or earlier if there was evidence of stent dysfunction. Prior biliary dilatation was performed where necessary to aid stent insertion using over-the-wire biliary dilating catheters up to 10Fr in diameter or using 4 to 12 mm biliary dilating balloons. Stenting was only discontinued if strictures were judged to be widely patent on repeat ERCP. Subsequent clinical symptoms and liver biochemistry were observed, with stenting repeated if jaundice or cholangitis developed. In later years, some patients were treated with biliary dilatation alone if the stricture was thought to have opened successfully following dilatation. Patients with jaundice or cholangitis in whom ERCP was unsuccessful were treated with percutaneous transhepatic drainage (PTD).
Data collection and analysis
Patients with dominant strictures were defined as above. Cholangiocarcinoma was diagnosed using a combination of suggestive clinical and radiographic findings plus confirmatory cyto/histo-pathology.
Child-Pugh, MELD, UKELD and Mayo PSC risk scores were calculated where possible using blood results closest to the time of diagnosis. Differences in patient characteristics and endoscopic findings were ordinarily assessed using the Mann-Whitney U test or Test or Student T Test as appropriate. A p value of <0.05 was considered statistically significant for all tests.
Survival was measured using the Kaplan-Meier method both from the time of PSC diagnosis and from first visit to our unit until death, and/or time to liver transplantation or study closure. Significance values for differences in survival were compared using the Log Rank test.
RESULTS
Patient characteristics
128 patients with a confirmed diagnosis of PSC were included for analysis. The median age at PSC diagnosis was 49 years (range 13 to 86 years), with men being diagnosed at a similar age to women. 64% were male and 36% female. Mean follow up was 9.8 years (median 9 years, range 0.1 to 27.4 years). Of the 92 patients (72%) with a final diagnosis of inflammatory bowel disease, ulcerative colitis was present in 86%, Crohn’s disease in 9%, and indeterminate colitis in 5%.
At the time of referral to our institution, which has a specialist interest in biliary disease and endotherapy, 96% of patients had been symptomatic, mostly with right upper quadrant pain, jaundice or cholangitis. Two percent of patients (asymptomatic at the time of referral for ERCP) did not undergo repeat ERCP during the follow-up period after the index ERCP at the referring hospital.
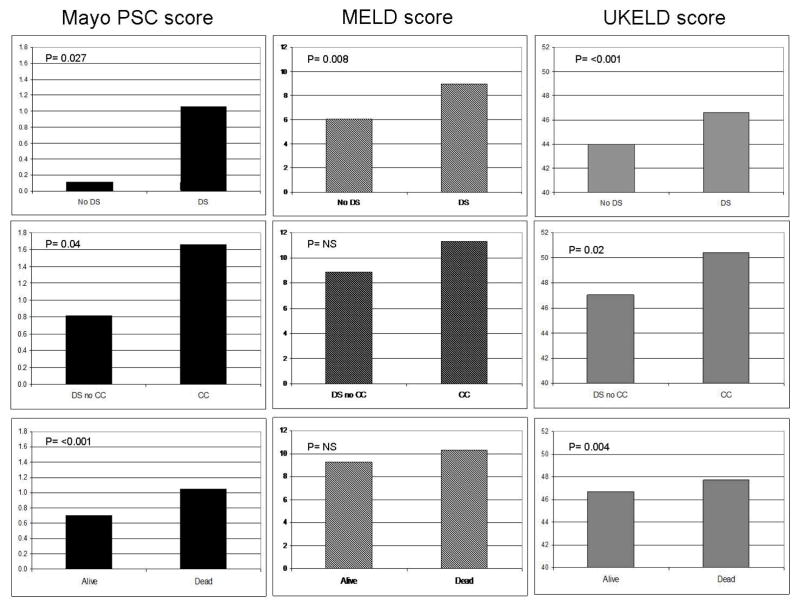
Demographic and clinical data of patients with and without dominant strictures are given in Table 1. Twenty patients (16%) had documented pancreatic duct abnormalities, primarily reported to be changes of chronic pancreatitis. Median values for the PSC Mayo risk score (1.05 v 0.12, p=0.026), MELD score (9.0 v 6.06, p=0.009) and UKELD scores (46.6 v 44.0, p=<0.001) were significantly worse in the dominant stricture group compared to the no dominant stricture group (Figure 1).
Table 1.
Patient characteristics at presentation and clinical outcome in those with PSC and dominant vs. no dominant strictures
| No dominant stricture | Dominant stricture | Benign dominant stricture (CC cases excluded) | CC | |
|---|---|---|---|---|
| No. of patients | 48 | 80 | 59 | 21 |
| Male:Female (%) | 67:33 | 63:37 | 61:39 | 67:33 |
| Median age at diagnosis PSC (range) | 46 (18 to 83) | 51 (13 to 86) | 52 (13 to 85) | 49 (23 to 86) |
| Median follow up (years) from diagnosis PSC (range) | 12.4 (0.2 to 30.7) | 8.6 (0.1 to 27.4) | 10.5 (0.2 to 23.8) | 2.4 (0.1 to 21.8) |
| Follow up (years) at UCL (median, range) | 12.3 (0.1 to 26) | 7.9 (0.1 to 27.4) | 9.7 (0.1 to 27.4) | 0.6 (0.1 to 1.5) |
| IBD | 38 (80%) | 54 (70%) | 38 (64%) | 18 (86%) |
| Bilirubin (median with range) | 14.5 (5 to 99) | 27 (5 to 476) + | 22.5 (5 to 374) NS | 97 (13 to 476) $ |
| Alkaline phosphatise (median with range) | 290 (61 to 2154) | 359 (43 to 2313) NS | 334 (43 to 2312) NS | 561 (63 to 1627) NS |
| ALT (or AST) (median with range) | 85 (23 to 454) | 63 (14 to 896) NS | 60 (14 to 527) NS | 66 (20 to 896) NS |
| Albumin (median with range) | 41 (31 to 47) | 38 (18 to 51) + | 38 (18 to 51) NS | 35.5 (26 to 44) NS |
| CA 19-9 (median with range) | 30 (2 to 97) | 109 (0 to 84287) + | 23 (0 to 2640) NS | 688 (9 to 84287) $ |
| Gallbladder stones | 23% | 25% | 29% | 14% |
| Choledocholithiasis | 9% | 9 (11%) | 13% | 3 (14%) |
| Ursodeoxycholic acid therapy | 42% | 60% NS | 69% NS | 39% NS |
| Mean ERCPs/patient at UCL | 1.3 | 4.9 | 5.7 | 3.0 |
| Median ERCPs/patient (range) | 1 (0 to 5) | 3 (0 to 24) | 4 (0 to 24) | 3 (0 to 6) |
| Median interventions/patient (range) | 0 (0 to 7) | 3 (0 to 34) | 4 (0 to 34) | 3 (0 to 12) |
| Median ERCP IHD PSC stage | 1 | 2 | 1 | 2 |
| Median ERCP EHD PSC stage | 1 | 3 | 2 | 3 |
| EHD PSC stage % (0/I/II/III/IV) | 39/44/6/11/0 | 2/10/31/36/21+ | 16/42/23/19# | 0/0/15/62/23NS |
| IHD PSC stage % (0/I/II/III) | 8.5/47/36/8.5 | 11/34/42/13 NS | 16/40/36/8 NS | 0/23/54/23$ |
| New Mayo Risk Score (median with range) | 0.12 (−1.13 to 3.1) | 1.05 (−1.73 to 4.19) + | 0.68 (−1.73 to 4.12) NS | 1.72 (−1.1. to 4.19) $ |
| MELD score (median with range) | 6.06 (0.21 to 17.8) | 9.0 (0 to 45)+ | 6.06 (0.21 to 18.8) NS | 9.36 (3.94 to 25) NS |
| UKELD score (median with range) | 44.0 (39.9 to 50.3) | 46.6 (39.2 to 62.9)+ | 45.86 (39.2 to 62.9) # | 50 (42.4 to 60) $ |
| Survival from diagnosis PSC (mean, years) | 23.0 | 13.7 + | 17.5 NS | 4.5 |
| Deaths | 14 (29%) | 43 (54%) | 37 (63%) | 21 (100%) |
| Cholangiocarcinoma | 0 | 21 (26%) | N/A | N/A |
| Liver Transplants | 5 (10%) | 14 (17.5%) | 12 (20%) | 2 (9%) |
p<0.05 for DS versus no DS
p=<0.05 for benign DS versus no DS
P=<0.05 for CCA versus benign DS
not significant
Figure 1.
Mean figures for disease model scoring systems compared between those with DS and no DS (top row), CC versus benign DS (middle row) and those patients alive or dead (bottom row).
Seventy patients (55%) had a liver biopsy. Of these 44% were reported as stage 0, 14% stage I, 23% stage II, 7% stage III and 12% stage IV. Twenty nine patients (23%) had biopsy proven and/or clinically overt cirrhosis (52% Childs A, 27% Child B and 21% Child C). Of these, 11 (9%) developed ascites and 4 patients (3%) had variceal bleeding.
Documentation of ursodeoxycholic acid (UDCA) use was available for 102 patients of whom 54% had used UDCA during the study period, mostly at a low dose of 5 to 10mg/Kg. There was a trend towards more patients in the DS group having been prescribed UDCA (60% v 42%, p=NS). There was no significant difference in survival in those treated with UDCA.
Endoscopic interventions and effect of dominant strictures
A total of 553 ERCPs were performed in the 128 patients, with a median of two ERCPs (range, 0–23) per patient. Of these, 453 (82%) were performed at our institution, of which 443 (98%) were interventional (for tissue sampling and/or endotherapy in patients with jaundice, rising LFTs and/or cholangitis).
In all, 80 patients (62.5%) were found to have dominant strictures (DS group) and underwent 474 ERCPs during the follow-up period, of which 392 procedures (83%) were performed at our centre. The remaining 48 patients were classified as not having dominant strictures (no DS group) and underwent 79 ERCPs. Dominant strictures were primarily located in the CBD in 47 (59%), CHD in 11 (14%) and the hilum in 22 patient (27%). In those who underwent liver biopsy, the stage of PSC liver disease was significantly higher in the DS group (median stage III) compared to those without DS (median stage I, p=<0.001).
Patients with dominant biliary strictures had a median of 3 (range 0–34) therapeutic endoscopic interventions, compared to 0 (range 0–7) in the 48 without dominant strictures (p<0.001). Therapeutic endoscopic interventions to strictures included: (i) stenting alone (46%), (ii) dilatation alone (20%), (iii) dilatation and stenting (17%), and (iv) no or failed intervention (17%), of whom 53% required percutaneous transhepatic drainage. Common bile duct or intrahepatic stones was an indication for endoscopic therapy in 9% and 8% of patients with and without dominant strictures (NS). Overall, cholelithiasis (ductal and/or gallbladder) affected 31 of the 128 (24%) patients, of whom 14 (11%) underwent cholecystectomy.
Complications of ERCP in the dominant stricture group included two minor (guidewire) bile duct perforations not requiring surgery, and two episodes of pancreatitis, one requiring admission for >10 days with pseudocyst intervention, and one documented episode of severe cholangitis. This corresponds to an identified serious adverse event rate of 5/453 or 1%. Several other patients will have had mild or sub-clinical cholangitis or pancreatitis but due to the routine use of antibiotics for up to five days post-ERCP, varying periods of hospitalisation for other reasons and retrospective data collection, it was not possible to determine the incidence of these complications accurately. However, no patients required blood transfusions after ERCP, and there were no deaths resulting from endoscopic interventions performed.
During the study period, 26 of the 128 patients (20%) underwent 37 percutaneous drainage procedures (range 1–3) at our centre. Of these, 21 patients (81%) were in the DS group, and required one or more PTDs for failed ERCP access or failure to adequately drain strictures. Nine (43%) of these patients had cholangiocarcinoma with complex biliary strictures. Percutaneous transhepatic choledochoscopy with or without electrohydraulic lithotripsy to fragment intraductal stones was performed in four patients with dominant strictures.
Serious PTD-related complications included the development of a hepatocutaneous fistula in a patient who had multiple PTD’s for anastamotic biliary structuring following a Whipples procedure for ampullary carcinoma complicating his PSC disease, one of which was complicated by a common bile duct perforation following PTD tract dilatation and choledochoscopy to investigate recurrent disease. Two patients had minor bile duct leaks following PTD, both of which resolved with satisfactory biliary drainage. The incidence of mild complications was difficult to quantify retrospectively but included cholangitis, minor bleeding (not requiring blood transfusion) and pain.
A multivariate analysis was performed to identify possible independent predictors of death. This confirmed predictable poor prognostic indicators such as older age, presence of CC and variceal bleeding. Interestingly, elevated alkaline phosphatase and lower haemoglobin were also independent predictors of death but the various parameters of the MELD and UKELD scores (ie bilirubin, creatinine, INR and sodium) were not. The mostly negative results from multivariate analysis are likely due to the relatively small numbers included in this study but do suggest a low predictive power of disease models as discussed later. Similarly, in view of the small numbers in each group, the fact that many patients had different endoscopic treatment modalities (eg dilatation and stenting), and retrospective nature of the study, the effect of specific endotherapies with regards survival and quality of life in those with dominant strictures or the subgroup with jaundice, cholangitis and/or pruritis, could not be accurately assessed. However, the data suggest a non significant trend towards a poorer outcome in those with benign dominant strictures, most of whom had undergone multiple endoscopic interventions directed towards stricture therapy.
Cholangiocarcinoma (CC)
Twenty one patients (14 male, 7 female) developed proven CC during the follow-up period. All occurred in patients with dominant strictures with no CC developing in those without dominant strictures (p=<0.001). The frequency of CC during follow up in the dominant stricture group was 21 out of 80 (26%). Jaundice and abnormal liver biochemistry were the presenting features in 19 (90%). Two patients (10%) presented with pain.
The median time from diagnosis of PSC to CC was 26 months (range 0 to 246 months). Ten cases (48%) presented within 4 months of diagnosis of PSC. In these cases, cholangiocarcinoma was most likely the reason for clinical presentation in previously unidentified PSC and so it is impossible to demonstrate pre-existing dominant strictures although all tumours developed stenoses in locations compatible with dominant strictures. Thereafter, the incidence of cholangiocarcinoma was approximately 1% per year. The main tumour sites were reported to be common bile duct (43%), common hepatic duct (9%) and hilum (48%). All patients had died from their disease by the end of the study. Median time from diagnosis of cholangiocarcinoma to death was 6 (1–23) months. There were no peripheral intrahepatic tumours in our series. In addition to those who developed CC, one patient developed a fatal ampullary carcinoma and another had fatal gallbladder cancer, making the overall frequency of biliary tract cancer 23/128 (18%).
Comparison was made between those with cholangiocarcinoma and those with benign dominant stricture. CA 19-9 was measured in only 43 patients (34%). Mean CA19-9 was 8236 IU/L (9-84,000 IU/ml) in those with cholangiocarcinoma and 97 IU/L (1-2640 IU/ml) with benign dominant stricture (p=0.26). Including all those with biliary tract cancer (CC, ampullary, and gallbladder cancer, n=14) compared to those with PSC and no malignancy where the CA19-9 was measured (n=29), the sensitivity and specificity of CA19-9 (cut-off 37 IU/ml) for the diagnosis of malignancy were 86% and 59% respectively. Mean scores for disease models at diagnosis of CC were significantly higher than those with benign DS for Mayo PSC risk score (1.66 v 0.82, p=0.04) and UKELD score (50.40 v 47.04, p=0.02) but not for the MELD score (11.32 v 8.82, p=0.06).
Of the patients considered for surgical resection, all were excluded due to presence of advanced PSC, peritoneal metastases at staging laparoscopy, Bismuth III or IV strictures considered unsuitable for curative surgery, or being medically unfit for surgery. One patient with potentially resectable disease was found to have a synchronous non-resectable renal cell carcinoma.
Survival
Overall, 57 of the 128 patients (45%) died during follow-up, at a median age of 65 (range, 23–93) years and a median of 57 (0 to 316) months after PSC diagnosis. Overall predicted survival by the Kaplan Meier method was 17.7 years (95% confidence intervals 15.3 to 20.1 years) (Figure 2).
Figure 2.
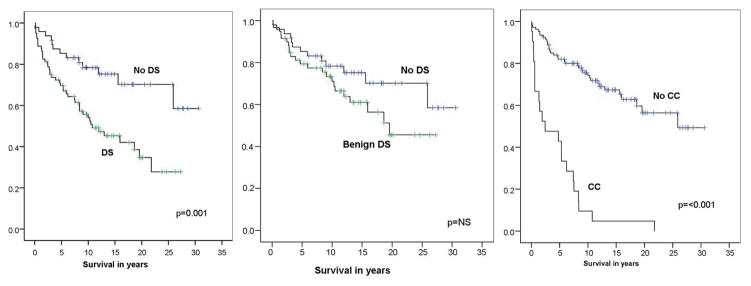
Kaplan-Meier curves for patients with and without dominant strictures and those with cholangiocaricnoma. Note that patients with DS have a significantly worse outcome than those without DS (left plot) but the significance of this difference is lost when those with cholangiocaricnoma are removed from analysis (middle plot).
Models of disease stage were significantly higher for deceased patients using the Mayo risk score (median 1.39 v 0.02 (p<0.001)) and UKELD score (median 44.79 v 47.6 (p=0.04)) but were not significantly different using the MELD score (6.1 v 8.5 (p=0.06)) which tended to be higher in those patients who remained alive (Figure 1).
The causes of death were; CC (37%), liver failure (21%), variceal bleeding (5%), other cancers (12%), ischaemic heart disease (4%), other causes (12%) and not known (9%). 43 deaths (75%) were in those with DS. Survival was significantly worse in those with dominant strictures compared to those without dominant stricture (13.7 v 23.0 years, p=0.01). However, after exclusion of those who developed CC, the trend in worse outcome for those with benign dominant stricture compared to those without dominant stricture was no longer significant (17.5 years v 23.0 years, p=0.15).
Deceased patients had a higher median bilirubin (34 μmol/L) and ALP (494 IU/ml) compared to those still alive (bilirubin 16 μmol/L, p=0.002: ALP 269 IU/ml, p=<0.001). Other simple laboratory parameters were similar between the two groups. There was no significant difference in rates of those taking UDCA in either group (49% dead; 58% alive).
Deceased patients had more ERCP interventions than surviving patients (median 3 versus 1, p=0.009) despite significantly shorter follow up times (median 38 (1 to 289) versus 152 (21 to 333) months, p=<0.001).
Eight patients developed other notable cancers; two colorectal cancers, pancreatic cancer, ampullary cancer, gallbladder cancer, hepatocellular carcinoma, non-small cell lung cancer and adenocarcinoma of unknown primary.
19 patients were referred for liver transplant assessment, of whom 6 were transplanted. Of the remaining 13 patients, one patient declined, 4 patients were considered too well for transplantation (all alive at the end of the study period) and 8 were considered unsuitable (co-morbidity n=6, coexistent malignancy n=2) and died within a year of referral for transplantation.
DISCUSSION
The results of several non-randomised studies have indicated that patients with dominant PSC strictures benefit from endoscopic intervention with five-year liver transplantation-free survival rates of 81% to 94%, compared to 65% to 78% predicted by the Mayo Clinic model [1, 7–8]. In an early prospective study of 32 PSC patients with dominant strictures treated with short-term stent insertion, pruritis improved in 83% of patients, serum bilirubin returned to normal in 12 of the 14 patients (86%) who were initially jaundiced, and only 40% of patients required additional endoscopic intervention during three years follow-up [13]. In a retrospective study of 71 patients, balloon dilatation without stenting was associated with fewer intervention-related complications or cholangitis than percutaneous or endoscopic stenting, whilst there was no significant difference between the two groups with respect to cholestasis [6]. An extension of a prospective study in Germany included 171 patients with PSC treated with UDCA followed for a median of 7 years. A subset of 96 patients with major duct stenoses was subjected to repeated endoscopic dilatation with almost no biliary stents inserted. The observed long-term survival of this subset was significantly higher than that predicted with the Mayo model or historical controls (mostly undergoing stent insertion) suggesting a possible benefit of endoscopic therapy with balloon dilatation [1, 8]. A smaller earlier study from Indianapolis (n=63) in which dominant stenoses were also treated primarily by repeated endoscopic balloon dilatation, reported very similar actuarial five-year survival rates free of liver transplantation were 83% compared with survival rates predicted by the Mayo Clinic model of 65% [7–8]. These results indicate that endoscopic therapy is beneficial in patients with dominant biliary strictures. The optimal frequency and type of intervention, or the degree to which endoscopic intervention delays or obviates the need for liver transplantation, remains unclear.
Despite the lack of well powered randomised controlled trials, based on current accepted practice, it may be considered unethical to allow patients with dominant biliary strictures to be left untreated. However, randomised trials of dilatation versus short term stenting are ethical and feasible. A multi-centre European study to address this question has recently started (www.clinicaltrials.gov, NCT01398917). Current European and American guidelines advise stricture dilatation should be undertaken as the treatment of choice for dominant strictures [14–15].
The present study suggests that patients with dominant strictures, who have undergone repeated therapeutic biliary intervention, have worse outcome than those without dominant strictures. The predominant reason for this appears to be an increased incidence of CC in this group, with the trend in poorer outcome in the dominant stricture group becoming non significant after exclusion of those who developed CC. Another retrospective study of 125 patients also failed to show improved outcome or liver biochemistry in patients with dominant stricture undergoing endoscopic therapy [2]. A prospective study, which included patients with dominant strictures who had undergone endoscopic therapy to dilate strictures, also reported a significantly worse transplant free survival in those with dominant strictures, compared to those without dominant strictures (25 v 73% p=0.011) [10]. These studies demonstrate a poorer outcome in patients with dominant strictures despite repeated endoscopic therapy.
There are a number of potential reasons for the differences in outcomes between trials. This may reflect a lack of significant clinical or long term benefit from endoscopic therapy with outcomes of small low powered studies simply reflecting expected statistical variation. There may have been a referral bias related to our specialist interest in endoscopic therapy whereas those with more advanced parenchymal disease may have been referred direct to a transplant centre. Other reasons include variations in treatment interventions between centres with some studies suggesting better outcomes with stricture dilatation without long term stenting whereas in our series, we have historically treated the majority of patients with routine stent insertion (6)]. Microbiological studies of bile from patients undergoing liver transplantation report a 58% positive culture rate, with an inverse relationship between infection rate and time from last ERCP and high rates of bactobilia (98%) in those with a biliary stent in situ compared to 55% in those without a stent [16–17]. More recent studies suggest that rates of clinically overt cholangitis may also be lower in those treated with biliary dilatation without stent insertion [1]. However, the most likely reason is that patients with dominant strictures have more severe parenchymal liver damage which cannot be entirely prevented or reversed by endoscopic therapy, diminishing any clinical benefit of endoscopic therapy.
An obvious weakness of our study is that it is a descriptive retrospective study rather than a randomised controlled trial. Not all of our cholangiograms were available for review. Also, descriptions of extent and severity of biliary strictures vary and few clinicians report these in terms of proposed systematic classifications [18]. Consequently, the nature and severity of strictures reported in our series is partly subjective.
Accurate reporting of complications based on historical data is difficult but our data suggest that complications of ERCP are fewer than that often quoted to patients based on guidelines and published literature, including a prospective study addressing the question of interventional ERCP in patients with PSC suggesting a complication rate as high as 14% [19]. ERCP in our experience had a major complication rate of only 1% which is comparable to the overall complication rate (mild to severe) of 5% in a large UK wide prospective audit of all general ERCP practice [20]. This concurs with the relatively low ERCP complication rate reported in a recent large German series [1]
Many published studies compared observed outcomes to those predicted by historical controls using the Mayo PSC model. Advances in medical and endoscopic therapy are likely to lead to a bias in treatment and clinical outcomes towards an improved outcome with modern medical care. Therefore, the study groups are not directly comparable to the data predicted by the Mayo model. Also, our data suggest that scoring systems such as the Mayo PSC score may be indicators of outcome in patients with PSC but are weak indicators of outcome when comparing subgroups with and without dominant strictures, suggesting that impaired parenchymal liver function is more important than the presence of complex dominant strictures when predicting outcome. Scoring systems, such as the Mayo PSC score, MELD and UKELD models, should be reserved for monitoring of disease progression and assisting with timing of referral for liver transplantation.
The frequency of CC noted in our cohort (approximately 16%) is similar to that reported by others. There is a striking difference in incidence of CC developing in areas with and without dominant strictures (26% and 0% respectively). This pattern was noted by others [10]. As with other series, we report a high rate of CC identified within a year of diagnosis of PSC, and in fact half of these were identified within 4 months of PSC diagnosis. The incidence thereafter was approximately 1% per year in those with dominant strictures. The high rate of CC (particularly at presentation) highlights the difficult clinical problem of differentiating malignant from benign dominant biliary strictures and demonstrates the need for improved diagnostic tests such as genomic studies and fluorescence in situ hybridisation (FISH) analysis which has been shown to improve the diagnostic accuracy of biliary cytology sampling [21–22]. Those with new presentations of PSC with evidence of a dominant stricture require thorough evaluation in order to exclude the presence of super-imposed CC, with consideration of a full range of diagnostic tests at the time of ERCP, including exfoliative cytology, intraductal biopsy and cholangioscopy
In general, the risk of cancer in any premalignant condition increases with the duration of the underlying condition and retrospective series of patients undergoing transplantation for PSC report background dysplasia in 36% of patients without cancer rising to 83% of those with coexistent CC in distant areas of the liver [23]. If endoscopic therapy were to improve the survival of patients with PSC, then it may carry the potential to increase the risk of patients developing CC before being considered for liver transplantation, although a multicentre, case-control study did not identify previous endoscopic therapy to be a risk factor for developing CC in patients with PSC [7].
In summary, our data indicate that endoscopic therapy is safe in patients with PSC but, despite repeated therapeutic endoscopic interventions, the outcome of patients with dominant strictures remains poor and the risk of developing cholangiocarcinoma in this subgroup is high.
Acknowledgments
Thank you to Dipok Dhar and Robert Muswell for their helpful assistance with statistical analysis.
Financial support
This work was supported by the British Liver Trust (with thanks to the Brian Mercer Trust) and the National Institutes of Health (grant PO1CA84203), and undertaken at UCLH/UCL which receive a proportion of funding from the Department of Health’s National Institute for Health Research (NIHR) Biomedical Research Centres funding scheme.
Abbreviations used in this paper
- CC
cholangiocarcinoma
- DS
dominant stricture
- ERCP
endoscopic retrograde cholangiopancreatography
- IBD
inflammatory bowel disease
- MRCP
magnetic resonance cholangiopancreatography
- PSC
primary sclerosing cholangitis
- PTC
percutaneous transhepatic cholangiography
- PTD
percutaneous transhepatic drain
- UDCA
Ursodeoxycholic acid
Footnotes
Author contributions:
MC, SB and JW collected the data which was analysed by MC. SP, GW, GJ and MC undertake clinical care of patients with PSC. All authors contributed to the writing and editing of the manuscript and approved the final version.
Conflicts of interest
None of the authors have conflicts of interest to declare.
References
- 1.Gotthardt DN, Rudolph G, Kloters-Plachky P, Kulaksiz H, Stiehl A. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointestinal endoscopy. 2010;71(3):527–34. doi: 10.1016/j.gie.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 2.Bjornsson E, Lindqvist-Ottosson J, Asztely M, Olsson R. Dominant strictures in patients with primary sclerosing cholangitis. The American journal of gastroenterology. 2004;99(3):502–8. doi: 10.1111/j.1572-0241.2004.04106.x. [DOI] [PubMed] [Google Scholar]
- 3.Tischendorf JJ, Hecker H, Kruger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. The American journal of gastroenterology. 2007;102(1):107–14. doi: 10.1111/j.1572-0241.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 4.May GR, Bender CE, LaRusso NF, Wiesner RH. Nonoperative dilatation of dominant strictures in primary sclerosing cholangitis. Ajr. 1985;145(5):1061–4. doi: 10.2214/ajr.145.5.1061. [DOI] [PubMed] [Google Scholar]
- 5.van Milligen de Wit AW, Rauws EA, van Bracht J, Mulder CJ, Jones EA, Tytgat GN, et al. Lack of complications following short-term stent therapy for extrahepatic bile duct strictures in primary sclerosing cholangitis. Gastrointestinal endoscopy. 1997;46(4):344–7. doi: 10.1016/s0016-5107(97)70123-8. [DOI] [PubMed] [Google Scholar]
- 6.Kaya M, Petersen BT, Angulo P, Baron TH, Andrews JC, Gostout CJ, et al. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. The American journal of gastroenterology. 2001;96(4):1059–66. doi: 10.1111/j.1572-0241.2001.03690.x. [DOI] [PubMed] [Google Scholar]
- 7.Baluyut AR, Sherman S, Lehman GA, Hoen H, Chalasani N. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. Gastrointestinal endoscopy. 2001;53(3):308–12. doi: 10.1016/s0016-5107(01)70403-8. [DOI] [PubMed] [Google Scholar]
- 8.Stiehl A, Rudolph G, Kloters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. Journal of hepatology. 2002;36(2):151–6. doi: 10.1016/s0168-8278(01)00251-3. [DOI] [PubMed] [Google Scholar]
- 9.Hammel P, Couvelard A, O’Toole D, Ratouis A, Sauvanet A, Flejou JF, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. The New England journal of medicine. 2001;344(6):418–23. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- 10.Rudolph G, Gotthardt D, Kloters-Plachky P, Kulaksiz H, Rost D, Stiehl A. Influence of dominant bile duct stenoses and biliary infections on outcome in primary sclerosing cholangitis. Journal of hepatology. 2009 doi: 10.1016/j.jhep.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Loof L, Danielsson A, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. Journal of hepatology. 2002;36(3):321–7. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 12.Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, et al. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21(10):870–7. doi: 10.1136/gut.21.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponsioen CY, Lam K, van Milligen de Wit AW, Huibregtse K, Tytgat GN. Four years experience with short term stenting in primary sclerosing cholangitis. The American journal of gastroenterology. 1999;94(9):2403–7. doi: 10.1111/j.1572-0241.1999.01364.x. [DOI] [PubMed] [Google Scholar]
- 14.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology (Baltimore, Md. 2010;51(2):660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 15.EASL Clinical Practice Guidelines: management of cholestatic liver diseases. Journal of hepatology. 2009;51(2):237–67. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Olsson R, Bjornsson E, Backman L, Friman S, Hockerstedt K, Kaijser B, et al. Bile duct bacterial isolates in primary sclerosing cholangitis: a study of explanted livers. Journal of hepatology. 1998;28(3):426–32. doi: 10.1016/s0168-8278(98)80316-4. [DOI] [PubMed] [Google Scholar]
- 17.Rerknimitr R, Fogel EL, Kalayci C, Esber E, Lehman GA, Sherman S. Microbiology of bile in patients with cholangitis or cholestasis with and without plastic biliary endoprosthesis. Gastrointestinal endoscopy. 2002;56(6):885–9. doi: 10.1067/mge.2002.129604. [DOI] [PubMed] [Google Scholar]
- 18.Ponsioen CY, Vrouenraets SM, Prawirodirdjo W, Rajaram R, Rauws EA, Mulder CJ, et al. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut. 2002;51(4):562–6. doi: 10.1136/gut.51.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Hazel SJ, Wolfhagen EH, van Buuren HR, van de Meeberg PC, Van Leeuwen DJ. Prospective risk assessment of endoscopic retrograde cholangiography in patients with primary sclerosing cholangitis. Dutch PSC Study Group. Endoscopy. 2000;32(10):779–82. doi: 10.1055/s-2000-7708. [DOI] [PubMed] [Google Scholar]
- 20.Williams EJ, Taylor S, Fairclough P, Hamlyn A, Logan RF, Martin D, et al. Risk factors for complication following ERCP; results of a large-scale, prospective multicenter study. Endoscopy. 2007;39(9):793–801. doi: 10.1055/s-2007-966723. [DOI] [PubMed] [Google Scholar]
- 21.Bangarulingam SY, Bjornsson E, Enders F, Barr Fritcher EG, Gores G, Halling KC, et al. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology (Baltimore, Md. 2010;51(1):174–80. doi: 10.1002/hep.23277. [DOI] [PubMed] [Google Scholar]
- 22.Chapman MH, Tidswell R, Dooley JS, Sandanayake NS, Cerec V, Deheragoda M, et al. Whole genome RNA expression profiling of endoscopic biliary brushings provides data suitable for biomarker discovery in cholangiocarcinoma. Journal of hepatology. 2012;56(4):877–85. doi: 10.1016/j.jhep.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC. Precancerous bile duct pathology in end-stage primary sclerosing cholangitis, with and without cholangiocarcinoma. The American journal of surgical pathology. 2010;34(1):27–34. doi: 10.1097/PAS.0b013e3181bc96f9. [DOI] [PubMed] [Google Scholar]