Abstract
Background and Purose
Disruption of the blood-brain barrier (BBB) has been proposed to be important in vascular cognitive impairment (VCI). Increased cerebrospinal fluid (CSF) albumin and contrast-enhanced MRI provide supporting evidence, but quantification of the BBB permeability in patients with VCI is lacking. Therefore, we acquired dynamic contrast-enhanced MRI (DCEMRI) to quantify BBB permeability in VCI.
Method
We studied 60 patients with suspected VCI. They had neurological and neuropsychological testing, permeability measurements with DCEMRI and lumbar puncture to measure albumin index (Qalb). Patients were separated clinically into subcortical ischemic vascular disease (SIVD), multiple and lacunar infarcts (MI/LAC), and leukoaraiosis (LA). Twenty volunteers were controls for the DCEMRI studies, and control CSF was obtained from 20 individuals undergoing spinal anesthesia for non-neurological problems.
Results
Thirty-six patients were classified as SIVD, 8 as MI/LAC and 9 as LA. The Qalb was significantly increased in the SIVD group compared to 20 controls. Permeabilities for the VCI patients measured by DCEMRI were significantly increased over controls (p<0.05). Patient age correlated with neither the BBB permeability nor Qalb. Highest Qalb values were seen in SIVD group (p<0.05), and were significantly increased over MI/LAC. Ki values were elevated over controls in SIVD, but were similar to MI/LAC.
Conclusions
There was abnormal permeability in white matter in patients with SIVD as shown by DCEMRI and Qalb. Future studies will be needed to determine the relationship of BBB damage and development of WMHs.
Keywords: Albumin, blood-brain barrier, vascular cognitive impairment, MRI, cerebrospinal fluid
Introduction
Vascular cognitive impairment (VCI) is an important cause of intellectual decline in the elderly.1–5 Imaging and pathological studies have shown white matter hyperintensities (WMHs) on MRI in VCI patients that are suggested to be due to silent strokes.4, 6 However, an alternate hypothesis is that an inflammatory response initiates disruption of the blood-brain barrier (BBB) with subsequent damage to the white matter.7 Serial MRIs in patients with small vessel disease shows progressive growth of WMHs, suggesting a generalized abnormality of the white matter.8
Brain tissue from VCI patients shows serum-derived proteins.9 Elevated levels of CSF albumin are frequently found in patients with VCI.10, 11 Qualitative contrast-enhanced MRI studies demonstrate leakage of contrast agents.12, 13 Recently, dynamic contrast-enhanced MRI (DCEMRI), using Gadolinium-diethylenetriamine penta-acetic acid (Gd-DTPA), was used to measure BBB permeability in animals with strokes.14, 15 We adapted this method to study BBB permeability in humans, and have used it for measurements in patients with VCI.16 We compared the results of DCEMRI with CSF albumin index (Qalb), an independent measure of BBB integrity.
Subjects and Methods
Subjects
Sixty patients with cognitive complaints and abnormal white matter signal on brain MRI were entered into the study. Patients were from the University of New Mexico Hospital (UNMH) and the Albuquerque Veterans Administration Hospital (AVAH). Only those patients capable of giving informed consent for lumbar puncture were enrolled in the study. The study was approved by the UNM and AVAH Human Research Review Committees.
Patients underwent neurological and neuropsychological testing, lumbar puncture for collection of CSF, structural MRI, and BBB permeability measurements with DCEMRI. Imaging studies always preceded lumbar puncture.
Neuropsychological tests included the Mini-Mental State Examination and standardized assessments of memory (Hopkins Verbal Learning Test, Rey-Osterrieth Complex Figure Test recall), attention (Digit Span forwards, Trail making test Part A), executive function (Wisconsin Card Sort, Trail making test Part B, Digit Span backwards), and language (Boston Naming, Controlled Oral Word Association). CSF was analyzed for routine studies (cells, protein, glucose, culture) and blood was collected for calculation of a demyelinating profile and albumin index.
Twenty control subjects had an MRI with Gd-DTPA to obtain normal values for DCEMRI, and control CSF was obtained from 20 subjects without neurological diseases during spinal anesthesia.
Diagnoses
Patients had an abnormal MRI and complained of cognitive difficulties. Study neurologists (JA, EE, GR) separated patients into diagnostic subgroups based on the results of the neurological, neuropsychological, and anatomical MRI findings without knowledge of DCEMRI and CSF results: 1) suspected microvascular disease with extensive white matter involvement (SIVD); 2) large vessel multiple or small vessel lacunar infarcts limited primarily to gray matter (MI/LAC); 3) leukoaraiosis (LA) when WMHs were small and not thought to be related to VCI.4 SIVD patients had cognitive complaints, focal neurological findings, gait disturbances, and WMHs on MRI.17–19 MI/LAC was suspected in patients with stroke-like episodes that were evident on MRI. Patients with subjective complaints and abnormalities in the white matter on MRI, but who did not meet the criteria for VCI were classified as LA.20 All patients had medical evaluations to exclude other causes of white matter disease and cognitive impairment. None of the patients had a CSF demyelinating profile or clinical course compatible with multiple sclerosis.
Magnetic Resonance Data Acquisition Protocol
The method of MRI measurements of BBB permeability has been described.16 Briefly, MRI investigation was performed using a 1.5-Tesla Siemens whole-body clinical scanner with a standard eight-channel array head coil (Siemens AG, Erlangen, Germany). The MR imaging protocol consisted of anatomical and contrast-enhanced sequences with Gd-DTPA contrast (Bayer Corp.). The BBB measurement was based on a time series of 8 T1 maps acquired with a fast T1 mapping sequence with partial inversion recovery (TAPIR).21, 22 One T1 map was acquired before Gd-DTPA injection and the rest were sampled post injection resulting in a 2D time series dataset of MR images.
We used a quarter dose of Gd-DTPA, which provided sufficient washout curves. In the vicinity of this dose, there is an approximately linear relationship between T1 intensity and the concentration of Gd-DTPA with a relatively steep slope, conferring high sensitivity of T1 to Gd-DTPA concentration changes. Plasma levels of Gd-DTPA that are used in the graphical method of permeability calculation were sampled from the sagittal sinus. Gd-DTPA was injected by pump as a rapid intravenous bolus.
Dynamic Contrast-Enhanced MR Data Analysis Methods
Preprocessing and motion correction were performed before further processing of the data. After aligning the images, dynamic Kalman filtering was applied for de-noising without losing the dynamics of contrast, providing a motion-free, time series of T1-map images for analysis.23 Using time series data, we calculated the rate at which the contrast agent passed from the vascular compartment into the tissue compartment, Ki, using the Patlak formulation of tracer leakage.14, 23 Permeability measurements were made in the white matter.
We used pooled data from the healthy control subjects to obtain an upper limit and confidence intervals for normal BBB permeability coefficients. Normative permeability data came from successfully completed studies in 17 of the 20 control subjects, ranging in age from 22 to 80 years (mean ± SEM of 44 ± 4 years). The permeability data from this group were combined to generate histograms of the permeability distributions, fitting the data to a distribution function with the statistical program R (R Development Core Team, 2007). The normal value of Ki, in white matter was defined as the upper limit of the 95% range of normal value, which was 3×10−4 ml/gm-min.14, 24
Statistical Methods
Statistical analyses for between-group differences for neuropsychological tests and clinical history were done using SPSS (SPSS for Windows, 16.0.1). For the BBB and Qalb data, we determined statistical significance with nonparametric ANOVA with Kruskal-Wallis corrections for multiple comparisons, and with Spearman rank nonparametric correlations or linear correlations (Prism 5, GraphPad Software, Inc., La Jolla, CA). The data was analyzed using receiver operator characteristic (ROC) analysis with the ROC function to determine cut-points for Ki. Statistical significance was set at the p<0.05. The data was represented as mean ± standard error of the mean (SEM).
Results
Patient classification
Clinical diagnoses of the 60 VCI patients are given in Supplemental Table 1. Forty-four patients had neurological, neuropsychological and neuroimaging findings consistent with VCI-no dementia.4, 5 Thirty-six patients were classified as SIVD and 8 as MI/LAC. Nine of the patients with lesions in the white matter could not be diagnosed as VCI-no dementia. Since the diagnosis in these patients was uncertain, they were classified with the non-specific designation of LA.20 We excluded 7 patients due to technical problems related to the MRI.
Ages of the patients in the different groups were similar statistically (Supplemental Table 1) and ranged from 31 to 82 years. Average ages were not statistically different between the groups. The incidence of hypertension, diabetes and hyperreflexia were similar in the three groups. Incidence of imbalance was highest in the SIVD group (p<0.05), and hyperreflexia was highest in the MI/LAC group (Supplemental Table 2).
Neuropsychological test results were significantly different for the various diagnostic categories for language function only with the poorest performance seen in the SIVD patients and the highest in the LA group (Supplemental Table 3).
Blood-brain barrier permeability in VCI patients and controls
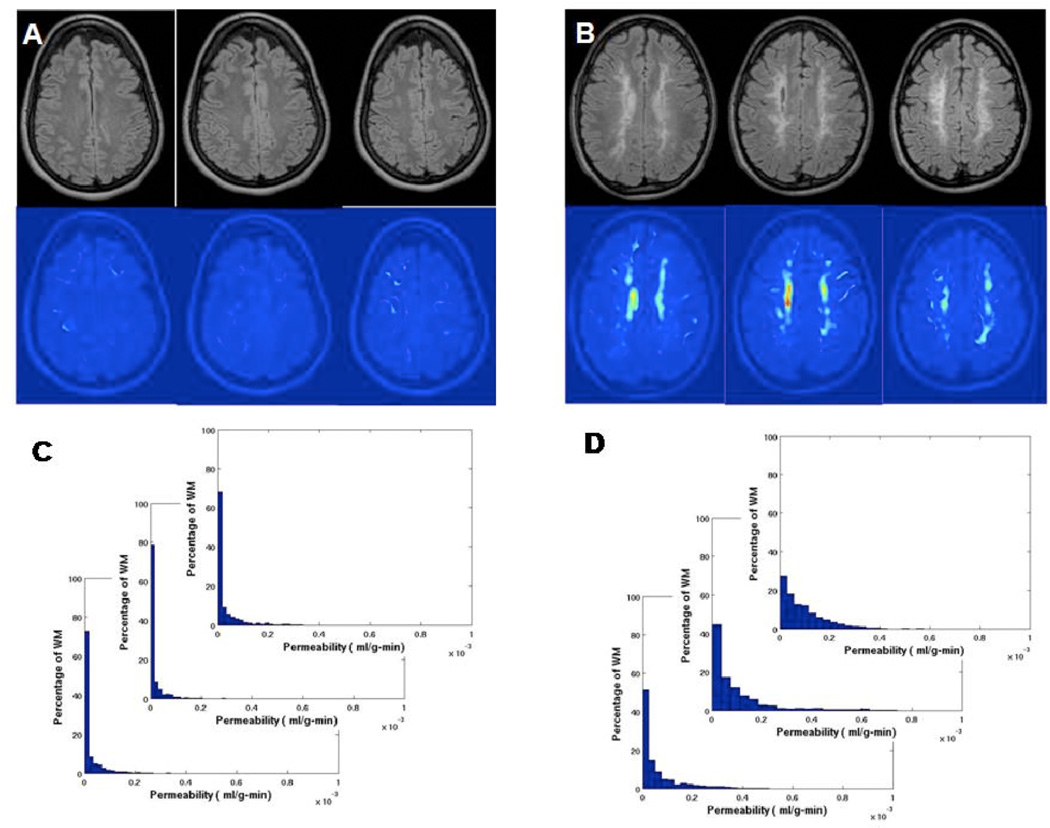
A histogram of pixels from a representative control permeability map shows no pixels with permeability statistically significant above the threshold value of 3×10−4 ml/gm-min (FIGURE 1A). In contrast, in VCI patients there were regions of permeability above the threshold value. A permeability map and corresponding histogram from a representative SIVD patient showed permeability values that were shifted to the right in the histogram (FIGURE 1B).
Figure 1.
Density distribution of permeability values for white matter (WM) voxels of a control and a VCI patient. A) The color-coded permeability map shows normal permeability, which is below the threshold of 3×10−4 ml/gm-min, which was established in 17 control subjects. C) The histogram of permeability values for the control subject shown in A. B) The permeability map of a VCI patient showing the regions of increased permeability in yellow and red. D) Permeability histogram shows the shift to the right of permeability values for patient in B.
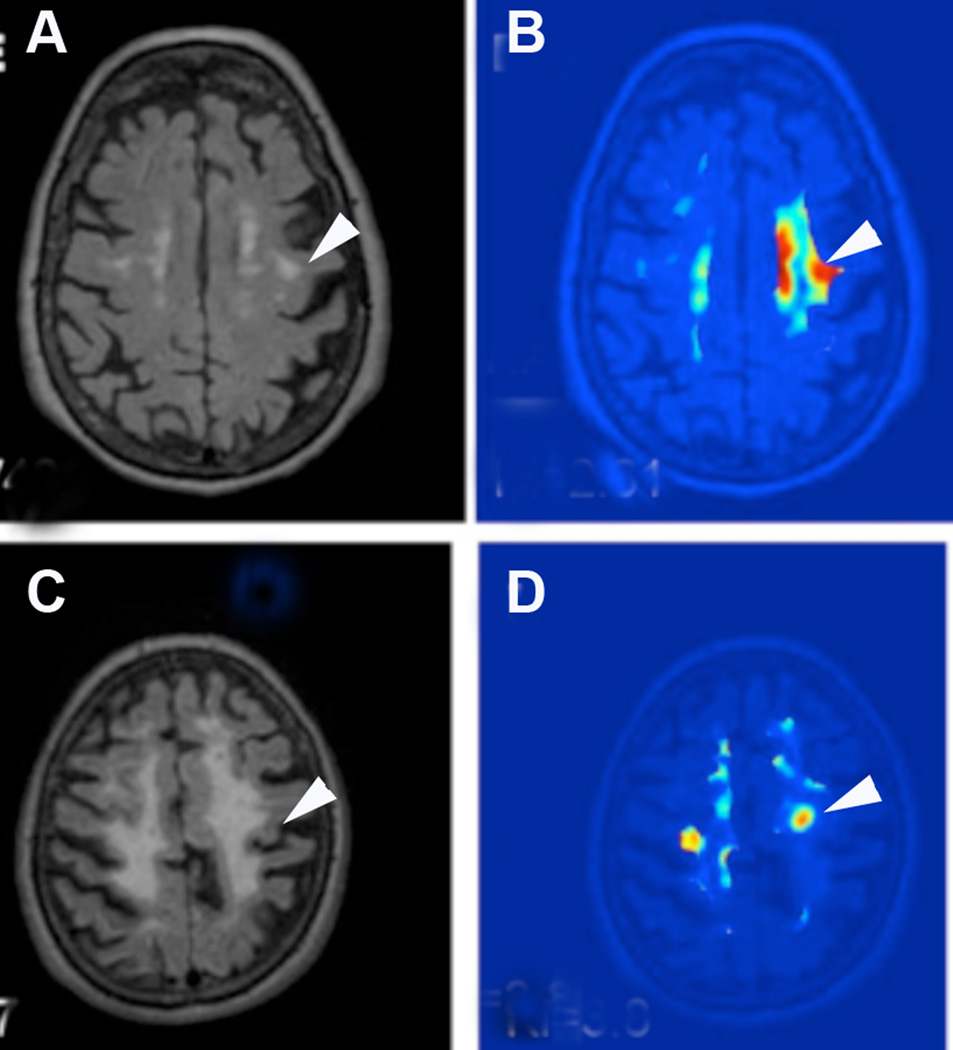
Patients classified as SIVD had large areas of WMHs. Leakage of contrast was restricted to smaller areas that were within the center of the WMHs. However, regions with elevated blood-to-brain transfer rate of Gd-DTPA could not be predicted from the FLAIR MRI as seen in a series of representative FLAIR MRI with corresponding permeability maps taken from the same slices through the brain, where the disparity between the two imaging methods is apparent (FIGURE 2).
Figure 2.
A) FLAIR MRI shows WMHs in the centrum semiovale (arrowhead) without involvement of the cortex. B) The corresponding permeability map has regions of moderately increased permeability (light blue) and high permeability (red). C) FLAIR image from another SIVD patient with larger white matter lesions (arrowhead). D) Permeability map shows increased permeability is limited to two small regions within the WMHs.
Albumin Index and MRI Permeability Coefficients
Comparison of patients’ ages with either the albumin index or the mean permeability coefficients failed to show a correlation with either parameter (FIGURE 3). In the control CSF, a correlation was found for age with Qalb (p<0.05), but DCEMRI controls failed to show a correlation with age (data not shown).
Figure 3.
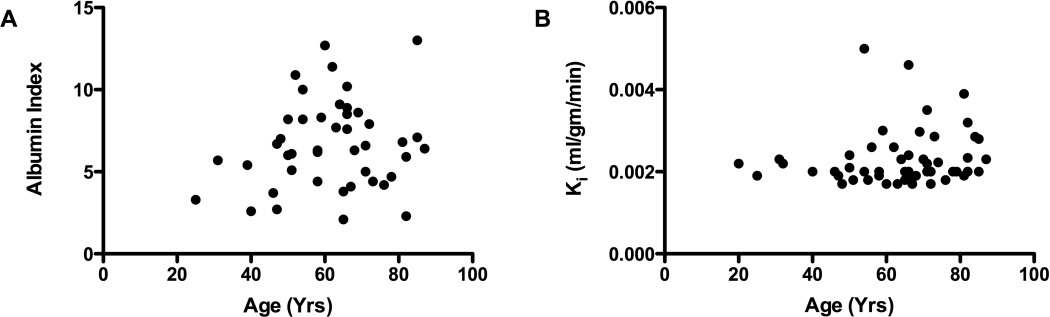
Effect of age on white matter BBB permeability in VCI and controls. A) Qalb for the VCI group plotted against age failed to show a correlation. B) BBB permeability of WM as a function of age. No correlation was found between Qalb or DCEMRI values and age.
Permeability Constants and Albumin Index in Diagnostic Groups
Albumin index was highest in the SIVD group and significantly greater than either the leukoariosis or controls (p<0.05) (FIGURE 4A). Similarly, we found the highest values for the BBB permeability in the SIVD group, which was statistically higher than the controls (FIGURE 4B). We failed to find a correlation between the levels of Ki and Qalb , possibly because the DCEMRI measures white matter permeability, while Qalb is a composite of white matter, gray matter and choroid plexus.
Figure 4.
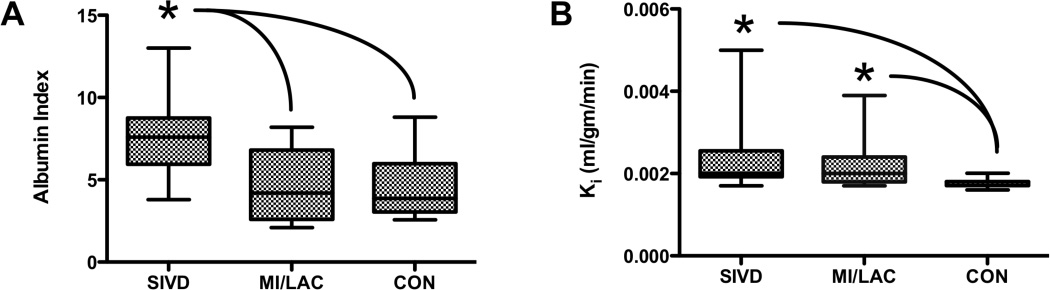
A) Qalb for the different diagnostic categories are shown; SIVD was significantly increased over MI/LAC and CON (p<0.05). B) Mean white matter permeability, Ki, was significantly higher for the SIVD and MI/LAC groups than the controls (CON), but no differences were seen between SIVD and MI/LAC. Asterisks indicate a significant difference by nonparametric ANOVA (p<0.05).
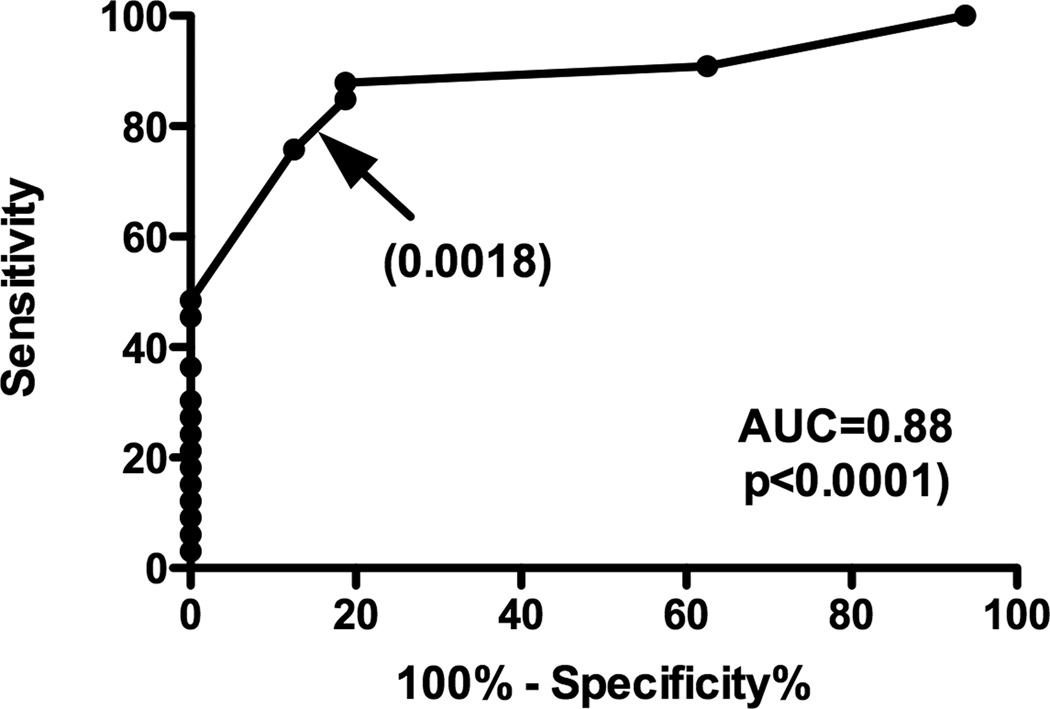
ROC analysis was performed to test the ability of the mean abnormal permeability to discriminate between SIVD and controls. The area under the ROC curve was 0.88. The cut point of 0.0018 ml/g-min provided 84% sensitivity and 81% specificity (FIGURE 5).
Figure 5.
ROC curve used for analyzing mean BBB permeability performance for classification of SIVD patients from controls. Evaluation of the discrimination of SIVD based on mean BBB permeability shows an ROC cut-point of 0.0018, which discriminates SIVD from controls with a reasonable balance in sensitivity and specificity. Area under the curve (AUC) and significance are shown.
Discussion
Blood-brain barrier permeability was abnormal in patients with VCI. This was demonstrated with increased CSF albumin as shown by others, and by a novel method to quantify BBB permeability in humans. Albumin index was greatest in the patients with SIVD and differed significantly from those with MI/LAC and controls. While DCEMRI was greater for SIVD patients compared to controls, there was no difference between SIVD and MI/LAC. Our results support the earlier studies that showed an increase in CSF albumin and contrast agent leakage, and provide the first quantitative data to support a role for BBB disruption in VCI.
Comparison of MRI FLAIR images of WMHs with regions of increased permeability showed that the areas of increased permeability were within the WMHs. We observed the highest permeability in the center of WMHs and not around the periphery. Our results provide a link between the white matter damage and the alterations in the vasculature that most likely represent vasogenic edema.
Pathological studies show extravasation of serum proteins into the white matter in SIVD.25–27 Several prior contrast-enhanced MRI studies showed abnormal BBB permeability qualitatively in Binswanger’s disease12 and lacunar strokes.13, 28 In a recent study comparing MRI with pathological findings in VCI, regions of WMHs on MRI had histological evidence of arteriolosclerotic blood vessels, corresponding to regions with demyelination.29
Increased levels of albumin in the CSF are seen in VCI, suggesting that this indicator of BBB damage can be used as a biomarker in VCI (see for review11). In surveys of patients with Alzheimer’s disease and vascular causes of dementia, patients with vascular disease have higher CSF albumin levels than seen in Alzheimer’s disease.30 When the patients were divided into diagnostic categories by clinical criteria, we found that those with SIVD had the highest values for both the mean permeability and Qalb. No relationship was observed between age and either Qalb or Ki for the patient groups. However, the controls showed a significant increase with age for the Qalb, which has been reported by others, but this was not seen in the patients.11 The Ki failed to show an increase with age in the controls (data not shown).
Quantitative measurements of BBB permeability in humans have been done with MRI and CT. Routine contrast-enhanced MRI with Gd-DTPA, which is performed with circulation of the contrast agent for several minutes, fails to show enhancement in VCI. We used a lower dose of Gd-DTPA and a longer circulation time to detect the subtle changes in BBB permeability. The measurements of permeability were obtained from an average of all sites with leakage. Using ROC plots we found a cut point for Ki values determined by DCEMRI of 0.0018 ml/min-gm, which resulted in a high sensitivity and specificity for detection of SIVD compared to controls.
Various theories have been proposed to explain the selective damage to the central white matter in patients with the small vessel form of VCI. The vasculature supplying the deep white matter is a border zone between several vascular territories, and the arteries have a long course that could be further compromised by arteriolosclerosis.31, 32 Veins in the deep white matter have increased collagen deposition, which could interfere with removal of interstitial fluid and proteins and drainage of blood.33 A recent pathological study in VCI patients showed an increase in hypoxia inducible factor-1α (HIF-1α), suggesting a chronic hypoxic state.34
The diagnoses are provisional and may be modified with long-term follow-up or at autopsy. Many of the patients have been followed for two or more years with only two that have died and undergone autopsy. As a group they had mild cognitive changes. We found that the majority of patients had the SIVD form with small vessel disease, which is consistent with other studies based on autopsies.35, 36 Many had hypertension, while fewer had diabetes. A smaller number had large vessel strokes or a single strategic stroke. Separation of SIVD from those with both Alzheimer’s disease and VCI is difficult clinically, and some of those with extensive white matter disease in the SIVD group may have mixed disease.
In conclusion, we showed disruption of the BBB in VCI using two independent measures. Both the DCEMRI method and elevated albumin in the CSF showed the greatest disruption in the patients with SIVD, suggesting a link between the extensive changes in the white matter and opening of the BBB. However, further studies will be needed to define the relationship of BBB dysfunction to the development of white matter lesions. If these results are confirmed in larger studies with long-term follow-up, DCEMRI, which is less invasive than lumbar puncture, may both aid VCI patient selection and serve as a biomarker for treatment trials.
Supplementary Material
Acknowledgments
Funding Sources: Support provided by NIH to GAR, and the University of New Mexico General Clinical Research Center Grant (M01-RR00997 NCRR/NIH), and from the Bayer Pharmaceutical Corp.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, et al. A population study on blood-brain barrier function in 85-year- olds: Relation to alzheimer's disease and vascular dementia. Neurology. 1998;50:966–971. doi: 10.1212/wnl.50.4.966. [DOI] [PubMed] [Google Scholar]
- 2.Rockwood K, Davis H, MacKnight C, Vandorpe R, Gauthier S, Guzman A, et al. The consortium to investigate vascular impairment of cognition: Methods and first findings. Can J Neurol Sci. 2003;30:237–243. doi: 10.1017/s0317167100002663. [DOI] [PubMed] [Google Scholar]
- 3.Roman GC, Sachdev P, Royall DR, Bullock RA, Orgogozo JM, Lopez-Pousa S, et al. Vascular cognitive disorder: A new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226:81–87. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 5.Bowler JV. Modern concept of vascular cognitive impairment. Br Med Bull. 2007;83:291–305. doi: 10.1093/bmb/ldm021. [DOI] [PubMed] [Google Scholar]
- 6.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: A systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt R, Scheltens P, Erkinjuntti T, Pantoni L, Markus HS, Wallin A, et al. White matter lesion progression: A surrogate endpoint for trials in cerebral small-vessel disease. Neurology. 2004;63:139–144. doi: 10.1212/01.wnl.0000132635.75819.e5. [DOI] [PubMed] [Google Scholar]
- 9.Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H. Blood-brain barrier dysfunction in binswanger's disease; an immunohistochemical study. Acta Neuropathologica. 1998;95:78–84. doi: 10.1007/s004010050768. [DOI] [PubMed] [Google Scholar]
- 10.Wallin A, Blennow K, Rosengren L. Cerebrospinal fluid markers of pathogenetic processes in vascular dementia, with special reference to the subcortical subtype. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S102–S105. S102–S105. [PubMed] [Google Scholar]
- 11.Farrall AJ, Wardlaw JM. Blood-brain barrier: Ageing and microvascular disease - systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Hanyu H, Asano T, Tanaka Y, Iwamoto T, Takasaki M, Abe K. Increased blood-brain barrier permeability in white matter lesions of binswanger's disease evaluated by contrast-enhanced mri. Dement Geriatr Cogn Disord. 2002;14:1–6. doi: 10.1159/000058326. [DOI] [PubMed] [Google Scholar]
- 13.Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Munoz Maniega S, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- 14.Ewing JR, Knight RA, Nagaraja TN, Yee JS, Nagesh V, Whitton PA, et al. Patlak plots of gd-dtpa mri data yield blood-brain transfer constants concordant with those of 14c-sucrose in areas of blood-brain opening. Magn Reson Med. 2003;50:283–292. doi: 10.1002/mrm.10524. [DOI] [PubMed] [Google Scholar]
- 15.Taheri S, Candelario-Jalil E, Estrada EY, Rosenberg GA. Spatiotemporal correlations between blood-brain barrier permeability and apparent diffusion coefficient in a rat model of ischemic stroke. PLoS One. 2009;4:e6597. doi: 10.1371/journal.pone.0006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taheri S, Gasparovic C, Shah NJ, Rosenberg GA. Quantitative measurement of blood-brain barrier permeability in human using dynamic contrast-enhanced mri with fast t1 mapping. Magn Reson in Med. doi: 10.1002/mrm.22686. published online Dec. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher CM. Binswanger's encephalopathy: A review. J Neurol. 1989;236:65–79. doi: 10.1007/BF00314400. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, Wilson RS, Gilley DW, Fox JH. Clinical diagnosis of binswanger's disease [see comments] J Neurol Neurosurg Psychiatry. 1990;53:961–965. doi: 10.1136/jnnp.53.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caplan LR. Binswanger's disease--revisited. [review] Neurology. 1995;45:626–633. doi: 10.1212/wnl.45.4.626. [DOI] [PubMed] [Google Scholar]
- 20.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Archives of Neurology. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 21.Shah NJ, Zaitsev M, Steinhoff S, Zilles K. A new method for fast multislice t(1) mapping. Neuroimage. 2001;14:1175–1185. doi: 10.1006/nimg.2001.0886. [DOI] [PubMed] [Google Scholar]
- 22.Neeb H, Zilles K, Shah NJ. A new method for fast quantitative mapping of absolute water content in vivo. Neuroimage. 2006;31:1156–1168. doi: 10.1016/j.neuroimage.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 23.Sood R, Taheri S, Estrada EY, Rosenberg GA. Quantitative evaluation of the effect of propylene glycol on bbb permeability. J Magn Reson Imaging. 2007;25:39–47. doi: 10.1002/jmri.20802. [DOI] [PubMed] [Google Scholar]
- 24.Sood RR, Taheri S, Candelario-Jalil E, Estrada EY, Rosenberg GA. Early beneficial effect of matrix metalloproteinase inhibition on blood-brain barrier permeability as measured by magnetic resonance imaging countered by impaired long-term recovery after stroke in rat brain. J Cereb Blood Flow Metab. 2008;28:431–438. doi: 10.1038/sj.jcbfm.9600534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feigin I, Popoff N. Neuropathological changes late in cerebral edema: The relationship to trauma, hypertensive disease and binswanger's encephalopathy. J Neuropathol Exp Neurol. 1963;22:500–511. doi: 10.1097/00005072-196307000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita, Nakamura S, et al. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and alzheimer's disease patients [see comments] Stroke. 1996;27:2069–2074. doi: 10.1161/01.str.27.11.2069. [DOI] [PubMed] [Google Scholar]
- 27.Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H. Blood-brain barrier dysfunction in binswanger's disease; an immunohistochemical study. Acta Neuropathol. 1998;95:78–84. doi: 10.1007/s004010050768. [DOI] [PubMed] [Google Scholar]
- 28.Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2009;81:192–197. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- 29.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–811. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- 30.Wallin A, Sjogren M, Edman A, Blennow K, Regland B. Symptoms, vascular risk factors and blood-brain barrier function in relation to ct white-matter changes in dementia. Eur Neurol. 2000;44:229–235. doi: 10.1159/000008242. [DOI] [PubMed] [Google Scholar]
- 31.De Reuck J, Crevits L, De Coster W, Sieben G, vander Eecken H. Pathogenesis of binswanger chronic progressive subcortical encephalopathy. Neurology. 1980;30:920–928. doi: 10.1212/wnl.30.9.920. [DOI] [PubMed] [Google Scholar]
- 32.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: An anatomic study. AJNR Am J Neuroradiol. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 33.Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis: Association with leukoaraiosis. Radiology. 1995;194:469–476. doi: 10.1148/radiology.194.2.7824728. [DOI] [PubMed] [Google Scholar]
- 34.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 35.Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry. 1997;63:749–753. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andin U, Gustafson L, Brun A, Passant U. Clinical manifestations in neuropathologically defined subgroups of vascular dementia. Int J Geriatr Psychiatry. 2006;21:688–697. doi: 10.1002/gps.1548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.